Abstract
The immunopathology of paucibacillary and multibacillary sheep paratuberculosis is characterized by inflammatory T cell and macrophage responses respectively. IL-23 and IL-25 are key to the development of these responses by interaction with their complex receptors, IL-23R/IL-12RB1 and IL-17RA/IL-17RB. In humans, variations in structure, sequence and/or expression of these genes have been implicated in the different pathological forms of tuberculosis and leprosy, and in gastrointestinal inflammatory disorders such as Crohn’s disease. Sequencing has identified multiple transcript variants of sheep IL23R, IL12RB1 and IL17RB and a single IL17RA transcript. RT-qPCR assays were developed for all the identified variants and used to compare expression in the ileo-caecal lymph node of sheep with paucibacillary or multibacillary paratuberculosis and uninfected animals. With IL-23 receptor, only the IL12RB1v3 variant, which lacks the receptor activation motif was differentially expressed and was significantly increased in multibacillary disease; this may contribute to high Th2 responses. Of the IL17RB variants only full length IL17RB was differentially expressed and was significantly increased in multibacillary pathology; which may also contribute to Th2 polarization. IL17RA expression was significantly increased in paucibacillary disease. The contrast between the IL17RA and IL17RB results may indicate that, in addition to Th1 cells, Th17 T cells are also involved in paucibacillary pathology.
Electronic supplementary material
The online version of this article (doi:10.1186/s13567-016-0314-4) contains supplementary material, which is available to authorized users.
Introduction
Paratuberculosis (Johne’s disease) is an endemic enteric disease of ruminants caused by Mycobacterium avium subspecies paratuberculosis (MAP) [1]; and like human mycobacterial infections, clinical paratuberculosis develops in a minority of infected animals [2]. In sheep, approximately one-third of the diseased animals develop paucibacillary or tuberculoid pathology and two-thirds develop multibacillary or lepromatous disease [2]; with lesions localized largely in the lamina propria of the terminal ileum [2]. Paucibacillary pathology is characterized by lymphocyte infiltration, granulomatous inflammation and few bacteria. In contrast multibacillary lesions are composed largely of heavily infected macrophages [2]. There is progression from sub-clinically infected to paucibacillary disease and terminal multibacillary pathology [3]; however progression occurs less frequently in sheep than in cattle and paucibacillary disease is usually fatal in infected sheep [2, 4, 5].
The immunology of paratuberculosis in sheep is also similar to tuberculoid and lepromatous forms of the human mycobacterial diseases, tuberculosis and leprosy [6, 7]. Paucibacillary disease is associated with a highly polarized Th1/Th17 response, characterized by high levels of IL-12, IL-17A and IFNγ [8–10]; and multibacillary paratuberculosis is linked with a strong Th2 response with high levels of IL-5 and IL-10 [8–11]. MAP has also been implicated as an aetiological agent of inflammatory intestinal diseases in humans because of the apparent similarity between the pathology of paucibacillary paratuberculosis and Crohn’s disease [12]. Like the human mycobacterial diseases, the epidemiology of paratuberculosis strongly suggests a host genetic susceptibility to disease severity and pathological form [13, 14]. Many of the genes associated with severity of human disease and pathology belong to the pathways that control differential T cell activation [13]; and genes in these pathways are also linked to gastrointestinal inflammatory diseases including ulcerative colitis and Crohn’s disease [12–15].
Of particular interest in this study are genes for the cytokines and cytokine receptors that control the expression of Th1, Th17 and Th2 subsets associated with the different forms of chronic gastrointestinal inflammation [15, 16] and are also important in the pathogenesis of tuberculosis and leprosy [7–13]. The activation of Th1/Th17 T cells is regulated by the interaction of the related heterodimeric cytokines IL-12 and IL-23 with their respective receptor complexes, IL-12RB1/IL-12RB2 and IL-12RB1/IL-23R [17]. Macrophages play a critical role in the presentation of antigen to T cells and their downstream activation and polarization, and are the target cells for MAP infection [18]. Mycobacterial infection of macrophages affects the expression of IL-12 and IL-23 [8, 19, 20] and therefore potentially influences the polarization of the immune response and consequently disease pathology. In contrast, the activation of Th2 T cells is influenced by IL-25 [21], which is produced by cells such as intestinal epithelium and innate lymphoid cells 2 (ILC2) and functions by interacting with its complex receptor IL-17RA/IL-17RB on T cells [22].
Variations in gene structure, sequence or expression levels of these cytokines and their receptors have been implicated in human mycobacterial pathogenesis and/or gastrointestinal and respiratory inflammatory diseases [13, 23]. Genome wide association studies have identified the association of single nucleotide polymorphisms (SNPs) of IL12B and IL23R with susceptibility to leprosy and tuberculosis [13, 24] and with Crohn’s disease [16]. In addition, specific splice variants of IL12B and IL23R have been linked to disseminated BCG infection [25] and with inflammatory bowel disease [26]. Studies on IL-25 and its receptor complex have found similar associations with inflammation; SNP analysis of IL17RA has linked three alleles associated with aspirin exacerbated respiratory disease [27] and IL17RB polymorphisms with asthma [28].
The aims of this study were to characterize the different sequence and transcript variants of the sheep IL-23 and IL-25 receptor complexes associated with Th1/Th17 and Th2 T cell polarization within the ileo-caecal lymph node (ICLN), the major immune inductive site for pathogens infecting the terminal ileum [29]; and to assess the expression of the different transcript variants in relation to defined paucibacillary and multibacillary pathology of sheep paratuberculosis.
Materials and methods
Animals, disease diagnosis and tissue collection
Animals with clinical paratuberculosis were out-bred Blackface or Blackface cross female sheep with naturally acquired MAP infection sourced from six farms; uninfected controls were from a single source with no history of paratuberculosis. The pathology of paratuberculosis was confirmed in clinically-affected sheep at post-mortem, by haematoxylin and eosin and Ziehl–Neelsen histopathology of the terminal ileum, MLN and ICLN. Infection was confirmed in affected sheep by IS900 PCR; all control sheep were IS900 negative (Table 1). There were six animals in each of three groups. Tissue was ICLN, removed immediately post-mortem, cut into blocks of ~0.5 g and placed in five volumes of RNAlater (Ambion, Huntingdon, UK), which were then incubated overnight at 4 °C and stored at −80 °C. No animals were euthanized specifically for this study; the animals were humanely culled for clinical reasons of paratuberculosis disease or for reasons unrelated to this study (uninfected controls).
Table 1.
Breed, age, histopathology and disease diagnosis of sheep
| Sheep ID | Breed | Origina | Age (years) | SGIb | AFBc | Tissue specific lesion graded | IS900 e | Diagnosisf |
|---|---|---|---|---|---|---|---|---|
| SH.139 | Blackface | A | 3 | 5 | 4 | Severe | +, + | Multibacillary |
| SH.140 | Blackface | A | 3 | 7 | 4 | Severe | +, + | Multibacillary |
| SH.146 | Blackface x | B | 2.5 | 5 | 4 | Severe | +, + | Multibacillary |
| SH.190 | Blackface | C | 3 | 5 | 4 | Severe | +, + | Multibacillary |
| SH.199 | Blackface | C | 2 | 6 | 4 | Severe | +, + | Multibacillary |
| SH.204 | Blackface | D | 1.5 | 6 | 4 | Severe | +, + | Multibacillary |
| SH.107 | Blackface x | E | 2.5 | 2.5 | 0 | Mild | +, + | Paucibacillary |
| SH.147 | Blackface x | B | 2 | 3 | 0 | Mild | +, + | Paucibacillary |
| SH.155 | Blackface | F | 3 | 2 | 0 | Mild | +, + | Paucibacillary |
| SH.160 | Blackface x | B | 3 | 2.5 | 0 | Mild | +, + | Paucibacillary |
| SH.188 | Blackface x | B | 4 | 4 | 1 | Moderate | +, + | Paucibacillary |
| SH.205 | Blackface | D | 4 | 2 | 0 | Mild | +, + | Paucibacillary |
| K207 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
| K208 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
| K213 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
| K224 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
| K227 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
| K229 | Blackface | G | 2.5 | 0 | 0 | ND | −, − | Control |
aSource farms.
bSeverity of granulomatous inflammation (SGI) grading: based on total number of epithelioid macrophages and leukocyte distribution patterns of the terminal ileum [5].
cAcid fast bacteria (AFB) -; grading: grades 0–2 were defined as paucibacillary; grades 3–4 were defined as multibacillary observed in terminal ileum tissue [5].
dTissue specific lesion grading: for each terminal ileum histological section, the sum of the points (SGI + AFB) was used to determine tissue-specific lesion grades. A severity of “none” was assigned to those with a lesion grade of 0, “mild” was assigned to a lesion grade of ≥2 and ≤3, “moderate” was assigned to those with a lesion grade of >3 and ≤5 and “severe” was assigned to a lesion grade of 6–11.
e IS900 PCR result using each of the two primer sets.
fDiagnosis: based on histopathological observations.
IS900 quantitative PCR
ICLN of all animals was tested for the presence of MAP by PCR for insertion sequence IS900. Two independent primer sets were used [30, 31]; set 1 generated an amplicon of 99 bp (for: GTTCGGGGCCGTCGCTTAGG; rev: GCGGGCGGCCAATCTCCTT) and set 2 generated a product of 314 bp (for: CTGGCTACCAAACTCCCGA; rev: GAACTCAGCGCCCAGGAT). Genomic DNA (gDNA) was purified using the Wizard® Genomic DNA Purification Kit (Promega, UK). All reactions used FastStart Taq DNA Polymerase (Roche Diagnostics, UK) following the manufacturer’s instructions, with 500 ng of gDNA and 0.2 μM of each primer. PCRs were performed using a Veriti® Thermal Cycler (Applied Biosystems) with four separate reactions performed for each primer set at different annealing temperatures. PCR reactions consisted of a heat activation step for 5 min at 95 °C, 35 cycles of 15 s at 95 °C, 15 s at 55, 58, 60 and 62 °C, and 30 s at 72 °C, with a final elongation step of 10 min at 72 °C.
PCR products were analysed on a 2% agarose gel electrophoresis, purified with a MinElute Gel Extraction Kit (Qiagen, UK) according to the manufacturer’s instructions; cloned using a TOPO TA cloning kit for sequencing (Invitrogen) and sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit and 3730 DNA Analyser (Applied Biosystems). The presence of an amplicon, confirmed by sequencing, from either primer set was taken as a positive result for the presence of MAP; the absence of an amplicon in all PCR reactions was confirmation of absence of MAP infection. All multibacillary and paucibacillary animals were positive and all uninfected, control animals were negative for IS900.
Cloning of ovine gene fragments
RNA was isolated from ~20 mg of ICLN using the Ribopure Kit (Ambion, UK) according to the manufacturers’ instructions. Genomic DNA was removed by On-column PureLink® DNase I treatment (Ambion). RNA quantity, quality and integrity were determined using a Labtech NanoDrop ND-1000 spectrophotometer and Agilent 2200 TapeStation system; samples had an RNA Integrity Number of 7.4–9. cDNA was synthesised from 1.0 μg RNA using SuperScript™ II RT with RNaseOUT (Invitrogen, UK) and oligo-dT(15) primer (Promega, UK), in 20 μL final volume. The predicted sequences of the sheep genes were obtained by NCBI-BLAST of the bovine (or if bovine sequences were not available, human and/or mouse) sequences against the Oar v3.1 sheep genome assembly [32]. Primers were selected using Primer-BLAST [33] and reanalysed using Net Primer [34]. The primers were used to amplify overlapping sections of genes, with at least 100 bp overlap of amplicons; each primer set was used in RT-PCR using FastStart Taq (Roche, UK) as per manufacturer’s instructions. New primers were selected (Additional file 1) from these assembled sequences to obtain full length transcripts using Platinum®Taq DNA Polymerase High Fidelity (Invitrogen). PCR products were purified using a MinElute PCR Purification Kit (Qiagen), ligated into pGEM®-T Easy Vector (Promega). Plasmids were extracted using QIAprep Spin MiniPrep Kit (Qiagen) following the manufacturers’ protocol. At least three clones from all diseased sheep, for each insert, were sequenced using T7 and SP6 primers, with BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, UK). The GeneRacer™ Kit for full-length, RNA ligase-mediated rapid amplification of 5′ and 3′ cDNA ends (Invitrogen) was used to sequence the 5′ and 3′ untranslated region (UTR).
Quantitative real-time RT-PCR analysis
Primers for quantitative real-time RT-PCR (RT-qPCR) were selected and optimized for all genes and variants (Additional file 2). Primers were designed overlapping exon/exon boundaries to ensure specificity for variants; these were amplified and sequenced using end-point RT-PCR to ensure specificity before performing RT-qPCR. Reactions contained 2 μL template cDNA (diluted 1/10–1/40), 7.5 μL FastStart Universal SYBR Green Master (Rox) 2 × concentration (Roche, UK), 0.2–1.0 μL of each primer at 10 mM and nuclease-free water to 15 μL. All reactions were prepared using a CAS-1200™ Precision Liquid Handling System and performed on the Rotor-Gene™ 3000 or Rotor-Gene Q (Qiagen). The amplification profile used was the same for each gene except for the annealing temperature; 5 min at 94 °C, followed by 40 cycles of 20 s at 94 °C, 20 s at optimized annealing temperature for each primer set (Additional file 2) and 20 s at 72 °C, followed by dissociation curve analysis to confirm a single gene product; amplicons were sequenced to confirm primer specificity. Relative expression levels were quantified in duplicate in three separate RT-qPCR runs, each time using cDNA from a different RT reaction, a no-template control was included in all runs. Linearity and efficiency of PCR amplification was determined for each primer pair using a standard curve generated by a dilution series of a pool of sample cDNA. All reactions had an efficiency of >90% and correlation coefficients were R2 > 0.98 and a single peak melt curve.
Relative gene expression levels were calculated in GenEx 5 Standard Programme (MultiD Analyses AB, Sweden) using the comparative 2-(ΔΔ Cq) method and normalized to the geometric mean of YWHAZ and SDHA. Fold changes were calculated from ΔCq values using GenEx. The expression level in the three groups for each variant was analysed by one-way ANOVA; the difference between group means for each variant were analysed using Tukey’s multiple comparison test within one-way ANOVA, with a significance threshold of p ≤ 0.05.
Results
Cloning and sequencing identified six transcript variants of IL23R, five variants of IL12RB1, a single IL17RA transcript and four IL17RB variants (Table 2).
Table 2.
Genbank accession numbers for the nucleotide and protein sequences of sheep IL12RB1, IL17RA, IL17RB and IL23R
| Gene | Nucleotide_id | Protein_id |
|---|---|---|
| IL23R | LN868336 | CRX77112.1 |
| IL23Rv1 | LN868337 | CRX77113.1 |
| IL23Rv2 | LN868338 | CRX77114.1 |
| IL23Rv3 | LN868339 | CRX77115.1 |
| IL23Rv4 | LN868340 | CRX77116.1 |
| IL23Rv5 | LN868341 | CRX77117.1 |
| IL12RB1 | LN878970 | CUH82712.1 |
| IL12RB1v1 | LN878971 | CUH82713.1 |
| IL12RB1v2 | LN878972 | CUH82714.1 |
| IL12RB1v3 | LN878973 | CUH82715.1 |
| IL12RB1v4 | LN878974 | CUH82716.1 |
| IL17RA | LN878979 | CUH82721.1 |
| IL17RB | LN878975 | CUH82717.1 |
| IL17RBv1 | LN878976 | CUH82718.1 |
| IL17RBv2 | LN878977 | CUH82719.1 |
| IL17RBv3 | LN878978 | CUH82720.1 |
Characterization of sheep IL23R and IL12RB1
Sheep IL23R is encoded by 11 exons on the plus strand of chromosome 1 (NC_019458.1) and encodes a mature protein of 627 amino acids. IL23Rv1 has a 29 bp insertion in the 5′ UTR (Chr1: 42 464 555–42 464 584) but has an identical predicted protein sequence to full length IL-23R (Additional file 3). IL23Rv2 has a 21 bp deletion (530–551) in exon 4 (Chr1: 42 476 073–42 476 094) that results in the loss of 7 amino acids (YVVYVKS) at amino acid positions 158–164 (151–157 in IL-23Rv2), and a 2 bp insertion that causes a frame shift and a premature stop codon resulting in a protein of 287 amino acids (Additional file 3). IL23Rv3 has a 28 bp insertion (878–906) in exon 6 (Chr1: 42 492 950–42 492 977) and is predicted to encode a truncated protein of 268 amino acids. IL23Rv4 does not contain exon 8 (Chr1: 42 512 417–42 512 506) and encodes a 597 amino acid protein as a consequence of a 30 amino acid deletion (VPQVTMKSFQHDTQNSGLLIASIFKKHLTS); this causes the excision of the extracellular region. IL23v5 has a deletion of exon 9 (Chr1: 42 515 314–42 515 419) and is predicted to encode a truncated protein of 356 amino acids.
Sheep IL12RB1 is encoded by 16 exons on the plus strand of chromosome 5 (NC_019462.1) and is predicted to encode a protein of 730 amino acids. The 5′ UTR is at the 5′ end of exon 1, the extracellular region spans exons 1–13, the transmembrane region is encoded by exon 14 and the 3′ UTR is located at the 3′ end of exon 16. IL12RB1v1 contains a deletion from the 3′ end of exon 5 to exon 8 (Chr5: 4 857 176–4 859 356), which leads to a frame shift and a truncated protein of 185 amino acids. IL12RB1v2 contains a 651 bp deletion spanning the 3′ end of exon 1 to the 5′ end of exon 7 (Chr5: 4 853 183–4 858 549); this results in a 217 amino acid deletion at position 16–233 in the extracellular region. IL12RB1v3 contains a 528 bp deletion from exon 3 to exon 7 (Chr5: 4 855 325–4 858 535) that causes a 176 amino acid deletion at position 51–226 within the extracellular region (Additional file 3). IL12RB1v4 contains a deletion from the 3′ end of exon 4 to the 5′ end of exon 9 (Chr5: 4 855 845–4 860 357) causing a frame shift and a truncated protein of 163 amino acids. All four IL12RB1 splice variants are predicted to encode proteins containing the extracellular domain only.
Characterization of sheep IL17RA and IL17RB
Only one sheep IL17RA transcript was identified (Table 1). This is a partial sequence; the 5′ 1306 bp mapped to chromosome 3 of Oar v3.1 (NC_019460.1) with >99% identity; however the 3′ 581 bp did not map, as this region of Oar v3.1 genome assembly is incomplete. Comparison with human IL17RA transcript variants 1 and 2 (NM_014339.6 and NM_001289905.1) shows that the sheep gene is orthologous to human variant 1 as it contains the region encoded in humans by exon 11 (Chr3: 212 818 526–212 818 627). The RT-qPCR primers used to quantify sheep IL17RA transcript expression were situated in exons 4 and 5.
Sheep IL17RB is encoded by 11 exons on the minus strand of chromosome 19 (NC_019476.1) and is predicted to encode a mature protein of 497 amino acids. The splice variant IL17RBv1 has a deletion of 96 bp (975–1071) that results in a 32 amino acid deletion at position 311 causing a truncated intracellular domain and the loss of the conserved TRAF6 binding motif. IL17RBv2 has a complete deletion of exon 4 (Chr19: 47 056 667–47 054 190) that causes a frame shift and a predicted protein of only 96 amino acids. IL17RBv3 has a deletion of exon 4 and a 177 bp insertion (651–828) at the 5′ end of exon 8 (Chr19: 47 050 145–47 049 971); this also results in a premature stop codon and a predicted protein of 96 amino acids.
Quantification of receptor expression
RT-qPCR assays were developed and expression levels of the four cytokine receptor transcripts were quantified in the paucibacillary, multibacillary and uninfected, control animals. However only IL23R, IL12RB1 and IL12RB1v3 and v4, IL17RA, IL17RB and IL17RBv2 and v3 could be accurately quantified; expression levels of all the others were too low for accurate measurement. Positive control assays, using a template sample known to contain the individual variants were used to confirm the reliability of the assay.
Of the IL23R and IL12RB1 variants only IL12RB1v3 showed evidence of differential expression (Figure 1) with a significant 2.1-fold (p = 0.04) increase in expression in the multibacillary sheep in comparison to paucibacillary animals. Of the receptors for IL-25 (Figure 2), IL17RA was significantly higher (Table 3) in paucibacillary sheep when compared to both multibacillary (2.0-fold, p = 0.001) and control sheep (2.2-fold, p = 0.02). Full length IL17RB was significantly higher in the multibacillary versus control comparison (2.49-fold, p = 0.04) but not in the other comparisons (although it was 2.08-fold higher in the multibacillary vs. paucibacillary comparison, but p = 0.08). No significant differential expression was seen with IL17RBv2 or v3.
Figure 1.
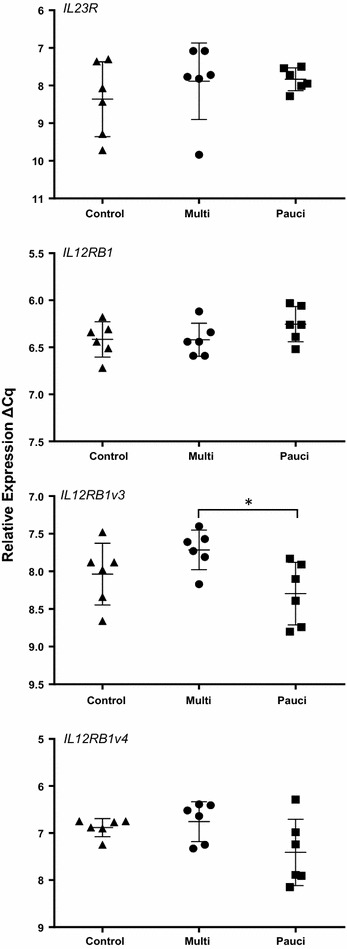
Relative expression of IL23R and IL12RB1 transcript variants. RT-qPCR analysis of IL23R and IL12RB1 transcript variants in the ICLN of paucibacillary, multibacillary and uninfected control sheep. Results are expressed as ΔCq replicate means of individual animals; error bars are ±SD of the group. *p = ≤ 0.05.
Figure 2.
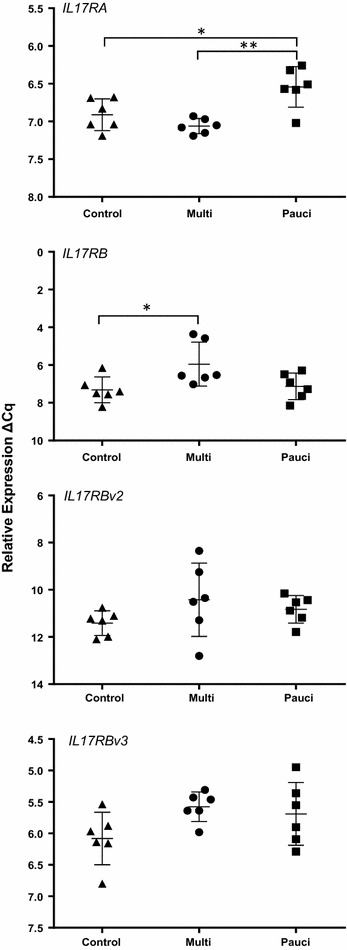
Relative expression of IL17RA and IL17RB transcript variants. RT-qPCR analysis of IL17RA and IL17RB transcript variants in the ICLN of paucibacillary, multibacillary and uninfected control sheep. Results are expressed as ΔCq replicate means of individual animals; error bars are ±SD of the group. *p = ≤ 0.05, **p = ≤ 0.01.
Table 3.
Relative expression of cytokine receptor expression within the ICLN
| Gene | ANOVA p value |
Multi vs. control | Pauci vs. control | Multi vs. pauci | |||
|---|---|---|---|---|---|---|---|
| FCa | p value | FC | p value | FC | p value | ||
| IL23R | 0.501 | 0.6 | 0.97 | 1.35 | 0.54 | 0.98 | 0.99 |
| IL12RB1 | 0.232 | 0.97 | 0.99 | 1.57 | 0.28 | 0.62 | 0.28 |
| IL12RB1v3 | 0.049 | 1.42 | 0.32 | −1.49 | 0.47 | 2.1 | 0.04 |
| IL12RB1v4 | 0.079 | 1.1 | 0.89 | −1.72 | 0.18 | 1.87 | 0.08 |
| IL17RA | 0.0016 | 0.46 | 0.43 | 2.2 | 0.02 | −2.0 | 0.001 |
| IL17RB | 0.035 | 2.49 | 0.04 | 1.19 | 0.93 | 2.08 | 0.08 |
| IL17RBv2 | 0.262 | 1.70 | 0.24 | 1.40 | 0.58 | 1.20 | 0.77 |
| IL17RBv3 | 0.104 | 1.60 | 0.12 | 1.52 | 0.24 | 1.09 | 0.88 |
Italics p ≤ 0.05.
aFold change (ΔΔCq values).
Discussion
This study is the first investigation into structural, sequence and quantitative variation of expression of genes of the IL-23 and IL-25 receptor complexes, in relation to the different pathological forms of sheep paratuberculosis. Sequencing cDNA from the ICLN of paratuberculosis-diseased sheep identified multiple transcript variants of all genes except IL17RA.
Splice variation is a common feature of these genes in many species including humans. Ensembl 82 [35] reports four transcript variants and three protein-coding isoforms of human IL23R (ENSG00000162594). However up to 24 human isoforms have been identified [36] and similar patterns can be recognised in the sheep and human variants, with four sheep variants (IL23Rv2–v5) predicted to encode truncated proteins. IL23Rv2, v3 and v5 lack the transmembrane and intracellular domains and are likely not to encode cell receptors but to act as antagonists as has been described for human IL23R variants [36]. IL23Rv5 specifically lacks exon 10 (exon 9 in human IL23R) and is therefore equivalent to human IL23RΔ9, which is strongly linked to human IBD [26]. IL23Rv4 encodes a protein with a truncated extracellular domain, although as with all the variants IL23Rv4 retains the conserved WSXWS class 1 cytokine receptor activation motif [37]. However, none of the IL23R isoforms, including the full length form, was differential expressed in paratuberculosis. This is consistent with paratuberculosis in red deer [38], where IL23R showed no difference in expression in jejunal lymph nodes of animals with minimal (largely paucibacillary) or severe disease (multibacillary). Interestingly the ligand for the IL-23 receptor (IL-23A) is significantly increased in paucibacillary lesions but not in the draining lymph nodes [19]. In humans, the R318Q SNP protects against a number of immune-mediated diseases by reducing IL-23-mediated Th17 function [39]; this SNP was identified in the protein sequence translated from IL23R, IL23Rv1 and v4 nucleotide sequences but no correlation with paratuberculosis pathology was identified.
All four IL12RB1 splice variants lack the WSXWS motif encoded within exon 7, suggesting that IL12RB1v1–v4 are non-functional or that they can act as modulators of IL-12/IL-23 activity by ligand binding without signalling. A truncated IL12RB1Δtm variant lacking these domains has been identified in humans and mice [40]. In mice it is induced in dendritic cells (DC) after Mycobacterium tuberculosis infection [41]; expression promotes DC migration and may act to up-regulate IL-12RB1-dependent DC function and the immune response to mycobacteria. Mendelian susceptibility to mycobacterial diseases (immunodeficiency 30) can partly be explained by IL12RB1 deficiency; some of these patients express 5′-truncated IL12RB1 that encodes a non-functional protein incapable of binding IL-12 or IL-23 [42]. In the paratuberculosis studies only IL12RB1v3 was differentially expressed with a significant increase in multibacillary vs. paucibacillary sheep. This variant contains the transmembrane and intracellular domains but is 5′ truncated and does not possess the WSXWS cytokine receptor activation motif. Like the 5′ truncated human variant it may bind the cytokines but not transduce the signal, possibly contributing to the low level of IL-12/IL-23 driven Th1/Th17 activity associated with multibacillary/lepromatous pathology.
Only a single sheep IL17RA transcript was identified. The predicted 5′ UTR of the sheep sequence shares 62.5% identity with the 5′ end of the human coding region (BC011624.2). Consequently the predicted sheep protein is only 249 amino acids rather than 866 amino acids of the full length human IL-17RA precursor (NP_055154.3). Two splice variants have been identified in humans, the full length transcript variant 1 (NM_014339.6) of 8607 bp and encoded by 13 exons and the soluble transcript variant 2 (NM_001289905.1) of 8506 bp without exon 11, which encodes the transmembrane domain [43]. However, no functional consequences have been associated with these variations. Sheep IL17RA includes exon 11 and expression is significantly higher in paucibacillary disease in comparison to both multibacillary and uninfected controls. IL-17RA is a component of the receptor (with IL-17RB) for both anti-inflammatory IL-25 and (with IL-17RC) the inflammatory Th17 cytokines IL-17A and IL-17F [44]; and high levels in paucibacillary animals could confirm a role of Th17 in paratuberculosis pathology [10].
The three splice variants of sheep IL17RB are all predicted to encode truncated proteins. IL17RBv2 and v3 encode identical short extracellular proteins that do not contain a transmembrane domain or intracellular region, it is therefore unlikely that they are cellular receptors but may bind and modulate IL-25 activity. The human truncated isoform 2 (Q9NRM) [45] has an unknown function and is produced at very low levels due to a premature stop codon in the mRNA, leading to nonsense-mediated mRNA decay. IL17RBv1 also encodes a truncated protein, with an amino-terminal truncated SEFIR (similar expression to fibroblast growth factor genes and IL-17R) domain and no TRAF6 binding motif. The SEFIR domain mediates protein interactions necessary for receptor signal transduction [46] and TRAF6 binding is essential for IL-25-mediated expression of IL-6 and TGFβ [47].
Only full length IL17RB was differentially expressed in the paratuberculosis diseased sheep, expression in the ICLN of the multibacillary sheep was significantly greater (2.49-fold, p = 0.04) than in uninfected controls. The levels in paucibacillary and control animals were not significantly different. This argues that IL-25 signalling is playing a role in multibacillary (lepromatous) pathology. IL-25 interaction with IL-17RA/IL-17RB induces the production of the pro-inflammatory cytokines IL-6 and IL-8 by endothelia [48], and IL-4 and IL-13 by T cells [49] leading to the development of Th2-associated pathology [50] especially at mucosal sites.
In conclusion, we have identified transcript variants in sheep of the four genes that make up the receptor complexes of IL-23 and IL-25; two cytokines important for the development of Th1/Th17 and Th2 T cells and in the pathogenesis of inflammatory disease at mucosal surfaces. RT-qPCR analysis of the different variants highlighted the role of the amino-terminal truncated IL12RB1v3 and full length IL17RB in multibacillary pathology; and full length IL17RA in paucibacillary disease. This study of the gene structure, sequence and expression of IL-23 and IL-25 has provided an initial insight into the role of differential T cell activation associated with the two pathological forms of sheep paratuberculosis.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JH conceived the study, is the principal investigator and collected the negative control tissues. LN performed most of the experiments including gene cloning, RT-qPCR and the data analysis, and was supervised by JH and RD. AG performed the IS900 PCR. CW performed the post mortems and collected tissues from the diseased animals. FC performed the histopathology and was responsible for disease diagnosis. JH drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the pathology and clinical staff at the Moredun Research Institute. This project was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant to The Roslin Institute. LN is a BBSRC postgraduate student funded by the Doctoral Training Grant BB/D526245/1.
Additional files
10.1186/s13567-016-0314-4 Primer sequences used for amplifying full length genes. The primer sequences and their Tm (°C) used to amplify the full length cytokine genes; and PCR product size.
10.1186/s13567-016-0314-4 Primer sequences used for RT-qPCR. The primer sequences and their Tm (°C) used to for relative RT-qPCR; and PCR product size.
10.1186/s13567-016-0314-4 Derived protein sequences of cytokine transcript variants. A. IL-23R derived protein sequences. Comparison of IL23R and four transcript variants. B. IL-12RB1 derived protein sequences. Comparison of IL12RB1 and IL12RB1v3. C. IL-17RB derived protein sequences. Comparison of IL17RB and three transcript variants.
Contributor Information
Louise Nicol, Email: louise_nicol89@hotmail.co.uk.
Anton Gossner, Email: anton.gossner@ed.ac.uk.
Craig Watkins, Email: craig.watkins@moredun.ac.uk.
Francesca Chianini, Email: francesca.chianini@moredun.ac.uk.
Robert Dalziel, Email: bob.dalziel@ed.ac.uk.
John Hopkins, Email: john.hopkins@ed.ac.uk.
References
- 1.Harris N, Barletta R. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin Microbiol Rev. 2001;14:489–512. doi: 10.1128/CMR.14.3.489-512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke C. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/S0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 3.Coussens P. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect Immun. 2004;72:3089–3096. doi: 10.1128/IAI.72.6.3089-3096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez V, Garcia Marin JF, Badiola JJ. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J Comp Pathol. 1996;114:107–122. doi: 10.1016/S0021-9975(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 5.Dennis MM, Reddacliff LA, Whittington RJ. Longitudinal study of clinicopathological features of Johne’s disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Vet Pathol. 2011;48:565–575. doi: 10.1177/0300985810375049. [DOI] [PubMed] [Google Scholar]
- 6.Casanova J-L, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 7.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 8.Smeed JA, Watkins CA, Rhind SM, Hopkins J. Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis. BMC Vet Res. 2007;3:18. doi: 10.1186/1746-6148-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrells C, Clarke CJ, Colston A, Kay JA, Porter J, Little D, Sharp JM. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne’s disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet Immunol Immunopathol. 1998;66:343–358. doi: 10.1016/S0165-2427(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 10.Allen AJ, Park KT, Barrington GM, Lahmers KK, Abdellrazeq GS, Rihan HM, Sreevatsan S, Davies C, Hamilton MJ, Davis WC. Experimental infection of a bovine model with human isolates of Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol. 2011;141:258–266. doi: 10.1016/j.vetimm.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens PM, Pudrith C, Skovgaard K, Ren X, Suchyta S, Stabel J, Heegaard P. Johne’s disease in cattle is associated with enhanced expression of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral blood mononuclear cells. Vet Immunol Immunopathol. 2005;105:221–234. doi: 10.1016/j.vetimm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Chacon O, Bermudez LE, Barletta RG. Johne’s disease, inflammatory bowel disease and Mycobacterium paratuberculosis. Annu Rev Microbiol. 2004;58:329–363. doi: 10.1146/annurev.micro.58.030603.123726. [DOI] [PubMed] [Google Scholar]
- 13.van de Vosse E, Hoeve MA, Ottenhoff THM. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 14.Saini C, Ramesh V, Nath I. CD4 + Th17 cells discriminate clinical types and constitute a third subset of non Th1, Non Th2 T cells in human leprosy. PLoS Negl Trop Dis. 2013;7:e2338. doi: 10.1371/journal.pntd.0002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 16.Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn’s disease. Annu Rev Genomics Hum Genet. 2009;10:89–116. doi: 10.1146/annurev-genom-082908-150013. [DOI] [PubMed] [Google Scholar]
- 17.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 18.Zurbrick BG, Czuprynski CJ. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect Immun. 1987;55:1588–1593. doi: 10.1128/iai.55.7.1588-1593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossner AG, Venturina VM, Peers A, Watkins CA, Hopkins J. Expression of sheep interleukin 23 (IL23A, alpha subunit p19) in two distinct gastrointestinal diseases. Vet Immunol Immunopathol. 2012;150:118–122. doi: 10.1016/j.vetimm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 23.Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 24.Morahan G, Kaur G, Singh M, Rapthap CC, Kumar N, Katoch K, Mehra NK, Huang D. Association of variants in the IL12B gene with leprosy and tuberculosis. Tissue Antigens. 2007;69(Suppl 1):234–236. doi: 10.1111/j.1399-0039.2006.773_3.x. [DOI] [PubMed] [Google Scholar]
- 25.Elloumi-Zghal H, Barbouche MR, Chemli J, Béjaoui M, Harbi A, Snoussi N, Abdelhak S, Dellagi K. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille calmette-guérin infection. J Infect Dis. 2002;185:1468–1475. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher G, Yuv R, Kan S, Mancini G (2009) Splice variants of human IL-23 receptor (IL-23R) mRNA and use of a Δ9 isoform in predicting inflammatory bowel diseases. In: USPTO. Patent number 8206925
- 27.Park JS, Park BL, Mo Kim, Heo JS, Jung JS, Bae DJ, Uh ST, Kim MK, Choi IS, Cho SH, Hong CS, Lee JY, Choi BW, Shin HD, Park CS. Association of single nucleotide polymorphisms on Interleukin 17 receptor A (IL17RA) gene with aspirin hypersensitivity in asthmatics. Hum Immunol. 2013;74:598–606. doi: 10.1016/j.humimm.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Jung JS, Park BL, Cheong HS, Bae JS, Kim JH, Chang HS, Rhim T, Park JS, Jang AS, Lee YM, Kim KU, Uh ST, Na JO, Kim YH, Park CS, Shin HD. Association of IL-17RB gene polymorphism with asthma. Chest. 2009;135:1173–1180. doi: 10.1378/chest.08-1595. [DOI] [PubMed] [Google Scholar]
- 29.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, Takizawa T, Koike M, Kudoh S, Costabel U, Guzman J, Rizzato G, Gambacorta M, du Bois R, Nicholson AG, Sharma OP, Ando M. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198–204. doi: 10.1128/JCM.40.1.198-204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauerfeind R, Benazzi S, Weiss R, Schliesser T, Willems H, Baljer G. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. J Clin Microbiol. 1996;34:1617–1621. doi: 10.1128/jcm.34.7.1617-1621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheep genome Assembly Oar v3.1 [online] http://www.livestockgenomics.csiro.au/sheep/oar3.1.php/
- 33.NCBI Primer BLAST [online] http://www.ncbi.nlm.nih.gov/tools/primer-blast/
- 34.Primer, N., Net Primer [online] http://www.premierbiosoft.com/netprimer/
- 35.The Ensembl Genome Browser [online] http://www.ensembl.org/
- 36.Kan SH, Mancini G, Gallagher G. Identification and characterization of multiple splice forms of the human interleukin-23 receptor a chain in mitogen-activated leukocytes. Genes Immun. 2008;9:631–639. doi: 10.1038/gene.2008.64. [DOI] [PubMed] [Google Scholar]
- 37.Dagil R, Knudsen MJ, Olsen JG, O’Shea C, Franzmann M, Goffin V, Teilum K, Breinholt J, Kragelund BB. The WSXWS motif in cytokine receptors is a molecular switch involved in receptor activation: insight from structures of the prolactin receptor. Structure. 2012;20:270–282. doi: 10.1016/j.str.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MW, O’Brien R, Mackintosh CG, Clark RG, Griffin JFT. Immunoregulatory cytokines are associated with protection from immunopathology following Mycobacterium avium subspecies paratuberculosis infection in red deer. Infect Immun. 2011;79:2089–2097. doi: 10.1128/IAI.00779-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, Tosi I, Capon F, Trembath RC, Peris K, Nestle FO. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One. 2011;6:e17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Vosse E, Lichtenauer-Kaligis EG, van Dissel JT, Ottenhoff TH. Genetic variations in the interleukin-12/interleukin-23 receptor (beta1) chain, and implications for IL-12 and IL-23 receptor structure and function. Immunogenetics. 2003;54:817–829. doi: 10.1007/s00251-002-0534-9. [DOI] [PubMed] [Google Scholar]
- 41.Robinson RT, Khader SA, Martino CA, Fountain JJ, Teixeira-Coelho M, Pearl JE, Smiley ST, Winslow GM, Woodland DL, Walter MJ, Conejo-Garcia JR, Gubler U, Cooper AM. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rβ1 isoform that enhances DC migration. J Exp Med. 2010;207:591–605. doi: 10.1084/jem.20091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, Bustamante J, Levy J, Candotti F, Casanova JL. A novel form of complete IL-12/IL-23 receptor b1 deficiency with cell surface-expressed nonfunctional receptors. Blood. 2004;104:2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- 43.Sohda M, Misumi Y, Tashiro K, Yamazaki M, Saku T, Oda K. Identification of a soluble isoform of human IL-17RA generated by alternative splicing. Cytokine. 2013;64:642–645. doi: 10.1016/j.cyto.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Uniprot [online] http://www.uniprot.org/
- 46.Novatchkova M, Leibbrandt A, Werzowa J, Neubüser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 47.Maezawa Y, Nakajima H, Suzuki K, Tamachi T, Ikeda K, Ji Inoue, Saito Y, Iwamoto I. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 48.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Brit J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 49.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]


