Abstract
Sheep scab, caused by infestation with the mite Psoroptes ovis, is highly contagious, causing intense pruritus and represents a major welfare and economic concern. Disease control strategies rely upon chemotherapy, however, sustainability is questionable due to issues of chemical residues, eco-toxicity and acaricide resistance. Control by vaccination is supported by demonstration of protective immunity in sheep previously infested with P. ovis. We identified vaccine candidates for P. ovis based on: (1) antigens selected by their interaction with host signalling pathways and the host immune-response; and (2) those shown to be either immunogenic or involved in mite feeding. This resulted in the development and validation, in repeated immunisation and challenge trials, of a seven recombinant protein sub-unit cocktail vaccine. Sheep were inoculated on three occasions, 2 weeks apart, along with QuilA adjuvant. Vaccination resulted in highly significant reductions in both lesion size (up to 63%) and mite numbers (up to 56%) following challenge. Mean lesion size in vaccinates was significantly smaller than controls from 1 week post infestation (wpi) until the end of the experiment at 6 wpi. All antigens elicited serum IgG responses following immunisation and prior to infestation, whereas controls did not produce antigen-specific IgG during the pre-infestation period. Vaccinated animals showed an amnestic response, with levels of antigen-specific IgG against muGST, Pso o 1 and Pso o 2 increasing following infestation. This vaccine represents the greatest reduction in lesion size to date with a sheep scab vaccine, providing encouragement for future production of a commercially-viable means of immunoprophylaxis.
Introduction
Psoroptic mange (sheep scab) caused by infestation with Psoroptes ovis, is highly contagious, causes intense pruritus and is a major welfare and economic concern [1, 2]. Currently, disease control relies on chemotherapy; however issues with chemical residues, eco-toxicity and acaricide resistance have raised concerns about the sustainability of this strategy and alternative means of control are desperately needed [3]. The concept of control by vaccination is supported by the demonstration of partial immunity in sheep following previous infestation with P. ovis [4–6]: During primary infestation an initial “lag phase”, with small numbers of mites and tight, focal lesions, is followed by a more rapid “growth phase”, with increasing mite numbers and expanding lesions. When this primary infestation is resolved (e.g. by treatment) and sheep are later re-infested, there is an extended lag phase, with lower mite numbers and reduced lesion sizes. Mite-specific IgG responses are similar in primary and secondary infestations but a more rapid induction of mite-specific IgE antibodies occurs in secondary infestations, suggesting that immediate hypersensitivity responses may contribute to immunity [4, 5, 7].
Attempts to vaccinate sheep against P. ovis using mite extracts have shown promise, with a 13-fold reduction in mite numbers and >65% reduction in lesion size in vaccinated sheep compared to controls [8]. Similarly, P. ovis extracts induce protection against mite challenge in cattle [9]. However, sub-fractionation of these complex extracts failed to identify the protective components involved. Furthermore, the practicality of a vaccine based on native P. ovis antigens is limited due to an inability to culture P. ovis in vitro, meaning that native antigen extracts would be prohibitively expensive to produce. We have adopted a “rational approach” to recombinant sub-unit vaccine design [10–14] in which host signalling pathways involved in the initial cutaneous pro-inflammatory response to P. ovis, upon which the mite relies to initiate its feeding and survival, were elucidated. This allowed identification of mite factors triggering these pathways, which could then be targeted by immunisation thus inhibiting mite survival. As the host:parasite interaction in sheep scab is complex, we hypothesised that incorporating multiple mite antigens into the vaccine, and thus targeting a number of host inflammatory pathways simultaneously, would be most likely to succeed. Similar multiple antigen approaches have been used in the development of effective vaccines against other parasites including the nematodes Necator americanus and Teladorsagia circumcincta [15, 16].
In this study we employed a recombinant sub-unit cocktail vaccine, using seven P. ovis proteins, four of which were identified through the approach described above (Pso o 1; Pso o 2; Pso o 3 and cyclophilin) with three additional antigens identified as either homologues of known allergens (Pso o 10); proteins upregulated during feeding (cathepsin L) or by immuno-screening of P. ovis cDNA libraries (mu class glutathione-S-transferase (muGST)) [10–14, 17–22]. Efficacy was tested across repeated trials in sheep using a P. ovis challenge model.
Materials and methods
Recombinant protein production
The vaccine was composed of seven recombinant proteins as described in Table 1 [14, 18–24]. Pso o 1, Pso o 10, cyclophilin and muGST were soluble in phosphate buffered saline (PBS), whilst Pso o 2, Pso o 3 and Cathepsin L were insoluble. Soluble and insoluble Escherichia coli-expressed proteins were induced and purified by nickel-affinity chromatography as described previously [24] and then dialysed against Dialysis Buffer (DB: 20 mM sodium phosphate, 0.5 M NaCl, pH 7.4) containing 2 M urea when purifying insoluble proteins. Protein concentrations were measured using a modified BCA protein assay (Pierce, UK) with BSA standards. Pso o 1 was expressed in Pichia pastoris, strain X-33 as described previously [21]. After purification, all antigens were stored at 4 °C, except for Pso o 1, which was stored at −20 °C.
Table 1.
Details of the recombinant P. ovis antigens used in the vaccine cocktail
| P. ovis antigen | Accession No. | Reference | Soluble in PBS a | Molecular weight (kDa) b | Expression system |
|---|---|---|---|---|---|
| Cathepsin L | BQ834906.1 | [23] | No | 25 | E. coli BL21-Codon Plus—pET-22b(+) |
| muGST | AM991140.1 | [14] | Yes | 25 | E. coli BL21-Codon Plus—pET-22b(+) |
| Pso o 1 | AM269885.1 | [21] | Yes | 25 | P. pastoris-X-33-pPICZαC |
| Pso o 2 | AF187083.1 | [24] | No | 14 | E. coli BL21-Codon Plus—pET-22b(+) |
| Pso o 3 | c | d, e | No | 25 | E. coli BL21-Codon Plus—pET-22b(+) |
| Pso o 10 | AM114276.1 | [20] | Yes | 37 | E. coli BL21-Codon Plus—pET-22b(+) |
| Cyclophilin | AAP03083.1 | d, f | Yes | 38 | E. coli BL21-Codon Plus—pET-SUMO |
aPso o 1, Pso o 10, cyclophilin and muGST were soluble in PBS, whilst Pso o 2, Pso o 3 and cathepsin L were formulated in Dialysis Buffer (DB).
bPredicted molecular weight in kilo Daltons.
cNot yet assigned.
dUnpublished data.
ePso o 3 identified as a homologue of the house dust mite allergen Der p 3 in an EST from a P. ovis cDNA library. The following primer sequences were used to amplify the coding region of Pso o 3, from cDNA derived from mixed stage P. ovis as described in [20], downstream of the predicted signal peptide sequence, and to allow subcloning into the expression vector (restriction sites underlined) :Pso o 3-For 5′ GATCCGAATTCGGCATATCGAATGTTTCCACTTCC3′, Pso o 3-Rev-5′ CCGCAAGCTTTACGATTCCGACAATCGTTTTAC3′.
f P. ovis cyclophilin identified as an EST from a P. ovis cDNA library. The following primer sequences were used to amplify full length P. ovis cyclophilin from cDNA derived from mixed stage P. ovis as described in [20] : Cyclophilin-For 5′ATGTCAACATGGACCCAAATTCAA′3, Cyclophilin-Rev 5′TTACATTTCACCACATTGTGATATGAT3′. Cyclophilin was subsequently expressed in E. coli, confirmed by matrix assisted laser desorption ionisation mass spectroscopy and its peptidyl prolyl cis–trans isomerase (PPIase) activity confirmed by a coupled enzyme assay as described in [41].
Immunisation and challenge protocols
Trial 1
Thirty sheep scab-naive (animals bred and reared on the MRI farm with no signs of scab observed prior to the start of the study) Texel crossbred lambs (~6 months old) were used in the study as this allowed us to ensure that they had no previous exposure to sheep scab. Lambs were randomly allocated into two equal-sized groups (“vaccine” and “control”) with each group of lambs housed in a separate pen. Lambs in the vaccine group were immunized on three occasions 2 weeks apart with 350 µg of recombinant protein cocktail (50 µg of each of the 7 P. ovis antigens) plus QuilA adjuvant (Brenntag Biosector). PBS-soluble proteins were administered together with 5 mg QuilA as a single sub-cutaneous injection into the lateral neck region, whilst insoluble proteins were administered via sub-cutaneous injection into the opposite side of the neck in DB with 5 mg QuilA. Lambs in the adjuvant-only control group were immunized at the same time and via the same routes with equivalent quantities of PBS, DB and QuilA. Two weeks after the final immunisation all lambs were infested, between the withers, with ~50 mixed-stage P. ovis mites. Lesion development was assessed weekly and blood samples were collected from all lambs immediately prior to each injection, 1 day pre-infestation and then weekly throughout a six-week infestation period, which is the maximum duration of infestation permitted to remain within the appropriate UK Home Office severity limits. At post mortem (pm), 6 weeks post infestation, 3 skin strips (approximately 5 cm × 1 cm) at the leading edge of the lesion were removed from each lamb for enumeration of mites.
Trial 2
Trial 2 was similar to Trial 1, with two exceptions: 10 lambs per group were used and 5 skin strips were taken at pm. Both trials were performed under the regulations of the UK Animal Procedures Act (1986) and a UK Home Office Project License. Experimental design and statistical power calculations were performed by Biomathematics and Statistics Scotland (BioSS) and were approved by the Moredun Research Institute Experiments and Ethics Committee (Approval Number: Trial 1 = E55/11; Trial 2 = E02/13).
Assessment of lesion size and mite numbers
The lesion area was measured weekly following infestation by multiplying the length and width of the lesion at the broadest point. Mite numbers were estimated by counting parasites on skin strips from the leading edge of each lesion at pm and expressed as mites per cm. An estimate of the total number of mites at the leading edge of the lesion was also determined by multiplying the mite count value by the total lesion perimeter [2 × lesion length (cm) + 2 × lesion width (cm)] for each animal.
Quantification of antigen-specific IgG
Recombinant antigen-specific IgG levels in serum across the pre- and post-infestation period were assessed for all vaccine antigens by ELISA as described previously [24] with the following exceptions: ELISA for Pso o 3 used a horse-radish peroxidase (HRP)-conjugate of polyclonal antibodies raised in pig against sheep IgG (Dako, UK). ELISA for Pso o 2 was as described in [24] but the antigen was diluted in ddH2O rather than carbonate buffer. The responses for each antigen were assessed for each sample in triplicate. OD450nm values were corrected against a reagent blank (no sample control), and all plates incorporated positive (pooled 6 wpi sera) and negative (pre-bleed from sheep scab naïve lambs) serum controls to account for inter- plate variation.
Statistical analyses
Estimated lesion sizes (cm2) were square root transformed and compared using a linear random coefficients model. The model incorporated fixed effects of trial, treatment group, linear and quadratic effects of time (in weeks as a covariate) and a treatment by time interaction, and random effects of intercept and time-specific slope for each lamb. Mite counts, recorded from skin strips from each lamb, were assumed to follow a Poisson distribution and modelled using a generalised linear mixed model (GLMM) with the logarithmic link function. The model included fixed effects of treatment group, trial and a treatment by trial interaction, an offset variable of the logarithm of skin strip length and a random effect of lamb with dispersion parameter estimated to account for the over-dispersion in mite count data. Estimates from this model were used to calculate the total number of mites by combining the estimated lesion perimeters. Mite numbers were not log transformed for statistical analysis, however, to present the data graphically in Figure 2, the log-scale was used to assist data presentation. The data for antibody levels were square root transformed and analysed by an additive linear mixed model, which included fixed effects of treatment, trial and a treatment by trial interaction. Separate smoothing curves were used for the non-linear relationship of the antibody response with time by treatment. The model also incorporated a first-order autoregressive correlation structure between observations at the 13 time points within the same animal and heterogeneity in variance for each trial. All statistical analyses were carried out using R software version 3.0.1 using relevant libraries (base, lme4, mgcv) [25].
Figure 2.
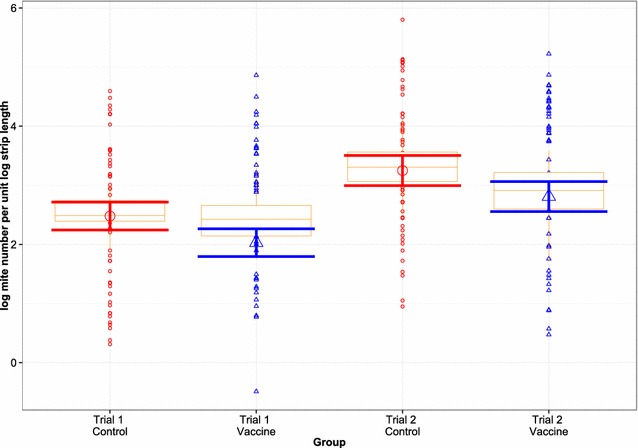
Mite numbers at the leading edge of the lesion, 6 weeks post-infestation with P. ovis. Data on mite number are presented as the logarithm of mite number per log strip length (cm). The plot shows observed mite number on lambs of vaccine (triangles) and control (circles) groups accompanied with boxplots presenting summary statistics of the observed data, and estimated mean mite number on the log scale (large triangle/circle) and corresponding 95% confidence interval (error bar) for vaccine and control groups during both trials.
Results
Lesion size
All lambs across both trials developed a single sheep scab lesion, originating from the site of challenge. Lesion measurements were based on these single and no additional lesions were detected during the infestation period. Figure 1 shows the estimated mean lesion size (transformed data, cm) for vaccine and control groups along with the observed lesion size for each animal at each wpi (across both trials). The mean lesion size, irrespective of treatment groups, did not differ between trials (p = 0.641) so the model excluding trial effect is reported. The increase in lesion size with time had a curvilinear relationship (p < 0.001). The mean lesion size increased over time for both vaccine and control groups though the rate of increase (cm/wpi) for the control group (8.08 cm ± 0.36) was significantly (p < 0.001) higher than in the vaccine group (5.20 cm ± 0.36). Mean lesion sizes (95% lower, upper confidence interval (CI)) in the vaccine and control groups were 52.68 (39.22, 68.13) and 106.46 (86.90, 128.00) cm2, respectively at 1 wpi, increasing to 1105.72 (900.77, 1331.64) and 2574.47 (2256.23, 2913.69) cm2 for the vaccine and control groups at 6 wpi, respectively. Based on these measurements, lambs in the vaccine group showed, on average, a >57% reduction in lesion size by 6 wpi compared with the control group, with a maximum reduction of 63% in lesion size at 3 wpi.
Figure 1.
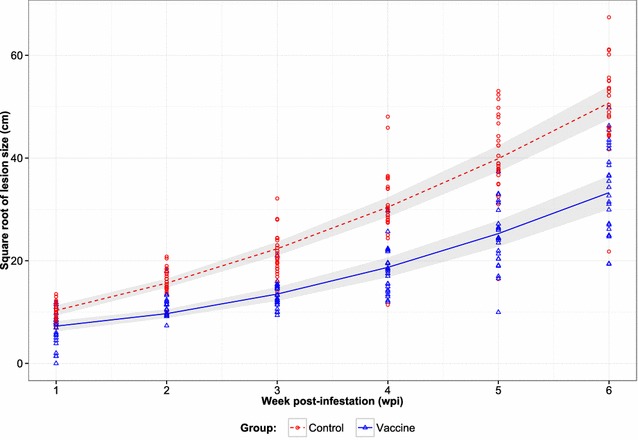
Lesion development over a 6 week period post-infestation with P. ovis across repeated vaccine trials. Lambs were infested with ~50 mites following immunisation with a seven recombinant protein cocktail vaccine with QuilA adjuvant (vaccine) or adjuvant only (control). Data on lesion size are presented on the transformed scale (cm, square root of lesion size). The plot shows observed lesion size of each lamb of vaccine (triangles) and control (circles) groups, estimated mean lesion size of vaccine (solid line) and control (dashed line) groups and corresponding 95% CIs envelop (shaded region).
Mite numbers
The mean mite counts in Trial 2 were significantly higher than in Trial 1 (p < 0.001) regardless of treatment group. However, in both trials, the vaccine group had significantly lower mean mite counts compared with the control group (Figure 2, p = 0.002). Estimated mean (95% lower, upper CI) mite counts per cm of skin for the vaccine group were 8 (6, 10) and 17 (13, 21) during Trial 1 and 2, respectively. In the control group, estimated mean mite counts were 12 (9, 15) and 26 (20, 34) in the two trials respectively. Accounting for the increased mean lesion perimeter at the leading edge of the lesion for the control group (202.80 cm) compared with the vaccine group (138.56 cm) across both trials, the estimated total mean (95% lower and upper CI) mite numbers for the vaccine and control groups for Trial 1 were 1055 (835, 1333) and 2414 (1915, 3048), respectively. Corresponding estimates for Trial 2 were 2292 (1767, 2976) and 5251 (4045, 6811), respectively. Across both trials the vaccinated lambs on average had a >56% reduction in total mite numbers at the leading edge of the lesion compared with the control group.
Serum IgG responses to recombinant P. ovis antigens
The antibody responses to all seven recombinant antigens for vaccine and control groups in Trial 2 are shown in Figure 3. All vaccinated animals generated an IgG antibody response to the seven antigens. The vaccine group had statistically significantly higher antibody levels to all antigens compared with the control group during both pre- and post-infestation periods. IgG levels for most vaccine antigens peaked at 7–14 days after the final immunisation and then declined. By 4 wpi, increased antigen-specific serum IgG levels were observed for the muGST, Pso o 1 and Pso o 2 indicating a potential amnestic response to these antigens. Serum from control animals had no measurable vaccine antigen-specific IgG prior to infestation, but did contain Pso o 2-specific IgG by 3 wpi and Pso o 1- and muGST-specific IgG by 6 wpi demonstrating that these antigens were recognised by the host immune response during exposure to P. ovis mites, following infestation.
Figure 3.
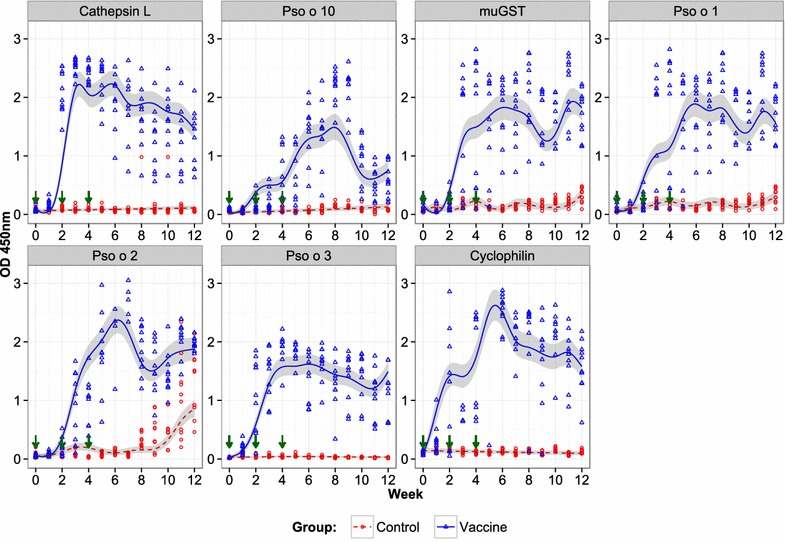
Antigen-specific antibody (IgG) levels in serum over a 6 week period post-infestation with P. ovis. Serum IgG responses specific for cathepsin L; Pso o 10; muGST; Pso o 1; Pso o 2, Pso o 3 and cyclophilin, respectively over a 6 week period of infestation with P. ovis during Trial 2 only (2013). Data on IgG levels are presented on the observed scale (OD450nm). The plot shows observed IgG levels of each lamb of the vaccine (triangles) and control (circles) groups, estimated mean IgG level of vaccine (solid line) and control (dashed line) groups and corresponding 95% confidence interval (CI) envelope (shaded region). Green arrows indicate timing of immunisations.
Discussion
The data presented here demonstrate the efficacy of a recombinant subunit sheep scab vaccine based on a cocktail of seven P. ovis antigens. When administered to lambs, the vaccine resulted in highly significant reductions in both lesion size (57%) and mite numbers (56%) following challenge in repeated protection trials. The lesions in the immunised lambs were significantly smaller than in matched control animals from 1 wpi until the end of the experiment at 6 wpi. In sheep scab, disease is transmitted via direct contact or fomites, so even modest decreases in lesion size and the concomitant reductions in mite numbers may limit disease spread [26–29]. All vaccine antigens elicited serum IgG responses following immunisation whereas animals in the control group did not possess antigen-specific IgG during the pre-infestation period. In addition, an amnestic response was observed in vaccinated animals, following mite challenge, with levels of antigen-specific IgG against muGST, Pso o 1 and Pso o 2 increasing following infestation with P. ovis. Serum IgG antibodies which bound recombinant Pso o 2 were demonstrated in the infested control animals, underpinning its use as a diagnostic antigen for the detection of sheep scab. In contrast no antigen-specific IgG response was observed for the mite antigens, Pso o 3 and Pso o 10. A previous study by our group demonstrated a similar lack of specific IgG reactivity to Pso o 10 in sheep undergoing a primary infestation with sheep scab and as such we would not expect to see an IgG response in these animals to Pso o 10 [20]. Detection of IgG to a defined antigen depends on many factors, including the amount of antigen that the host is exposed to and the relative immunogenicity of the antigen. It should also be noted that the Pso o 3 and Pso o 10 used for the ELISAs in this study were bacterial recombinant antigens, which may have structural differences to the native antigens and thus be poorly recognized by the mite-induced IgG response. The magnitude of the experimental challenge in the model described here is likely to be much greater than in a field outbreak, as the numbers of mites used (~50 mites per lamb) are likely to be substantially higher than those experienced during a natural infestation where only small numbers of mites, or even a single ovigerous individual, may be sufficient to establish a lesion [30–32]. Additionally, as a result of the potentially limited numbers of mites encountered in a natural challenge, field infestations may develop more slowly over a longer period of time encompassing several months rather than the 6 weeks described here [30]. Based on the estimates of slopes in conjunction with the line plots of lesion size for both vaccine and control groups (Figure 1) it may be inferred that the lesion size would increase progressively with time, beyond the period investigated in the current experiment, and hence, the difference in lesion size between control and vaccine groups could become more pronounced at later time points. Therefore, the ultimate efficacy of this vaccine may actually be greater than demonstrated here; however, further studies under field conditions are required to validate this hypothesis.
The subunit vaccine described here represents the greatest reduction in lesion size with a recombinant sheep scab vaccine to date, providing encouragement for future production of a commercially-viable means of immunoprophylaxis. Previous attempts have been made to produce an effective vaccine for sheep scab. For example, Nisbet et al. [14] produced a multi-protein recombinant vaccine based on P. ovis allergens, however the efficacy of this vaccine could not be determined due to the high degree of variability in the lesion size and mite numbers in the controls. Other efforts have focused on the use of native extracts of P. ovis to generate protective immunity: a vaccine based on P. ovis soluble proteins was previously tested in cattle, with 8/14 vaccinated calves being free of palpable lesions by 8 wpi compared to 3/14 in the controls [9]. Unfortunately, native extract based vaccines are unlikely to be commercially feasible due to the absence of in vitro culture systems for P. ovis to supply sufficient material for commercial production and also the lack of reproducibility with which these extracts can be produced. The use of a recombinant cocktail vaccine is, therefore, likely to be required for controlling complex eukaryotic parasites and may have advantages over single protein vaccines [33]. Whereas single point mutations in drug targets can lead to drug resistance, this is far less likely in a vaccine relying on multiple B cell epitopes present in a cocktail vaccine [28, 33].
Mathematical modelling has demonstrated that vaccines to control endoparasites may not need to achieve the same degree of efficacy as chemotherapeutics to achieve economic control of parasites, however, as these models were based on gastrointestinal nematode infections they may not be directly applicable to psoroptic mange [34–36]. Sterile immunity against many ectoparasites may not be achievable via vaccination and, unlike chemotherapeutics, an ectoparasite vaccine may not induce a rapid knockdown of parasite population nor necessarily protect individuals from being parasitized [29, 36]. Vaccination does have the potential to provide greater protection from re-infestation than achievable with chemotherapeutic control, which currently ranges from low levels of protection with a single dose of doramectin and up to 60 days for moxidectin in a long acting formulation. If used as part of an integrated control program, vaccines may reduce parasite populations over successive generations and, in the short term may mitigate the effects of parasitism by controlling population growth, limiting clinical pathology and alleviating the more extreme welfare symptoms [29]. Furthermore, vaccination may also reduce disease impact by blocking or reducing the spread of disease within and between flocks [29], although this is yet to be formally tested.
Given the likely efficacy achievable through vaccination, vaccines should not be considered as a single control measure for sheep scab but rather as an additional arm in a growing arsenal of tools available for coordinated control, including diagnostic tests, existing chemotherapeutics and effective biosecurity. However, to encourage producers to begin to switch from their current reliance on chemotherapeutics to a more coordinated approach involving anti-parasite vaccines, these products will have to demonstrate clear benefits, i.e. be efficacious, cost effective, environmentally friendly and sustainable [37]. One potential disadvantage of the cocktail approach is the additional costs involved in commercial production of a vaccine based on multiple antigens and, although not necessarily a barrier to commercial success, it is important to ensure that costs are reflective of the market. This may require further distillation of antigens required for protection, or formulation of protective antigens and/or epitopes within a single fusion protein, as recently demonstrated with an E. coli O157:H7 subunit vaccine [38] and also by co-expression of multiple copies of a rabies virus glycoprotein using a foot and mouth disease virus expression system incorporating the 2A peptide [39]. It is also critical at this stage to develop effective strategies to use this vaccine in the field. This will require identifying the optimal methods of integrating the vaccine with existing controls. For example this may involve combined use of diagnostic tests [24, 40] to identify and confirm outbreaks of disease, treatment with existing chemotherapeutic compounds and administration of vaccine to contiguous properties to limit further transmission.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed studies: STGB; JFH; TNM & AJN. Performed experiments: STGB; FN; DF; BW; EJM; JFH; TNM and AJN. Analysed data: STGB; MN; TNM and AJN. Prepared manuscript: STGB; MN; TNM and AJN.
Acknowledgements
This study was funded by the Department for Environment, Food and Rural Affairs (Defra), UK under project OD0555. We also gratefully acknowledge funding from The Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS).We would like to thank the Moredun Clinical Division for their continued help and expertise. The authors would also like to thank Yvonne Bartley for her assistance with the in vivo vaccine trials and Dr Sarah Brocklehurst (BioSS) for advice on an earlier draft of the manuscript.
Contributor Information
Stewart T. G. Burgess, Email: stewart.burgess@moredun.ac.uk
Francesca Nunn, Email: francesca.nunn@moredun.ac.uk.
Mintu Nath, Email: mintu.nath@bioss.ac.uk.
David Frew, Email: david.frew@moredun.ac.uk.
Beth Wells, Email: beth.wells@moredun.ac.uk.
Edward J. Marr, Email: edward.marr@moredun.ac.uk
John F. Huntley, Email: john.huntley@moredun.ac.uk
Tom N. McNeilly, Email: tom.mcneilly@moredun.ac.uk
Alasdair J. Nisbet, Email: alasdair.nisbet@moredun.ac.uk
References
- 1.Kirkwood AC. History, biology and control of sheep scab. Parasitol Today. 1986;2:302–307. doi: 10.1016/0169-4758(86)90124-9. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwhof GJ, Bishop SC. Costs of the major endemic diseases of sheep in Great Britain and the potential benefits of reduction in disease impact. Anim Sci. 2005;81:23–29. doi: 10.1079/ASC41010023. [DOI] [Google Scholar]
- 3.Nisbet AJ, Huntley JF. Progress and opportunities in the development of vaccines against mites, fleas and myiasis-causing flies of veterinary importance. Parasite Immunol. 2006;28:165–172. doi: 10.1111/j.1365-3024.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Bates P. Differences between primary and secondary infestations with the sheep scab mite, Psoroptes ovis. Vet Rec. 2000;146:528–529. doi: 10.1136/vr.146.18.528. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broek AH, Huntley JF, MacHell J, Taylor M, Bates P, Groves B, Miller HR. Cutaneous and systemic responses during primary and challenge infestations of sheep with the sheep scab mite, Psoroptes ovis. Parasite Immunol. 2000;22:407–414. doi: 10.1046/j.1365-3024.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith WD, Van den Broek A, Huntley J, Pettit D, Machell J, Miller HR, Bates P, Taylor M. Approaches to vaccines for Psoroptes ovis (sheep scab) Res Vet Sci. 2001;70:87–91. doi: 10.1053/rvsc.2000.0427. [DOI] [PubMed] [Google Scholar]
- 7.Van den Broek AH, Huntley JF, Machell J, Taylor MA, Miller HR. Temporal pattern of isotype-specific antibody responses in primary and challenge infestations of sheep with Psoroptes ovis–the sheep scab mite. Vet Parasitol. 2003;111:217–230. doi: 10.1016/S0304-4017(02)00379-5. [DOI] [PubMed] [Google Scholar]
- 8.Smith WD, Pettit DM. Immunization against sheep scab: preliminary identification of fractions of Psoroptes ovis which confer protective effects. Parasite Immunol. 2004;26:307–314. doi: 10.1111/j.0141-9838.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- 9.Pruett JH, Temeyer KB, Fisher WF, Beetham PK, Kunz SE. Evaluation of natural Psoroptes ovis (Acarina: Psoroptidae) soluble proteins as candidate vaccine immunogens. J Med Entomol. 1998;35:861–871. doi: 10.1093/jmedent/35.5.861. [DOI] [PubMed] [Google Scholar]
- 10.Burgess ST, Frew D, Nunn F, Watkins CA, McNeilly TN, Nisbet AJ, Huntley JF. Transcriptomic analysis of the temporal host response to skin infestation with the ectoparasitic mite Psoroptes ovis. BMC Genomics. 2010;11:624. doi: 10.1186/1471-2164-11-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess ST, McNeilly TN, Watkins CA, Nisbet AJ, Huntley JF. Host transcription factors in the immediate pro-inflammatory response to the parasitic mite Psoroptes ovis. PLoS One. 2011;6:e24402. doi: 10.1371/journal.pone.0024402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess ST, Nisbet AJ, Kenyon F, Huntley JF. Generation, analysis and functional annotation of expressed sequence tags from the ectoparasitic mite Psoroptes ovis. Parasit Vectors. 2011;4:145. doi: 10.1186/1756-3305-4-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess ST, Downing A, Watkins CA, Marr EJ, Nisbet AJ, Kenyon F, McNair C, Huntley JF. Development of a cDNA microarray for the measurement of gene expression in the sheep scab mite Psoroptes ovis. Parasit Vectors. 2012;5:30. doi: 10.1186/1756-3305-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nisbet AJ, Halliday AM, Parker L, Smith WD, Kenyon F, Knox DP, Huntley JF. Psoroptes ovis: identification of vaccine candidates by immunoscreening. Exp Parasitol. 2008;120:194–199. doi: 10.1016/j.exppara.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Hotez PJ, Diemert D, Bacon KM, Beaumier C, Bethony JM, Bottazzi ME, Brooker S, Couto AR, Freire Mda S, Homma A, Lee BY, Loukas A, Loblack M, Morel CM, Oliveira RC, Russell PK. The human hookworm vaccine. Vaccine. 2013;31(Suppl 2):B227–232. doi: 10.1016/j.vaccine.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nisbet AJ, McNeilly TN, Wildblood LA, Morrison AA, Bartley DJ, Bartley Y, Longhi C, McKendrick IJ, Palarea-Albaladejo J, Matthews JB. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine. 2013;31:4017–4023. doi: 10.1016/j.vaccine.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Stoeckli MR, McNeilly TN, Frew D, Marr EJ, Nisbet AJ, Van den Broek AH, Burgess ST. The effect of Psoroptes ovis infestation on ovine epidermal barrier function. Vet Res. 2013;44:11. doi: 10.1186/1297-9716-44-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AJ, Machell J, Van Den Broek AH, Nisbet AJ, Miller HR, Isaac RE, Huntley JF. Identification of an antigen from the sheep scab mite, Psoroptes ovis, homologous with house dust mite group I allergens. Parasite Immunol. 2002;24:413–422. doi: 10.1046/j.1365-3024.2002.00480.x. [DOI] [PubMed] [Google Scholar]
- 19.Huntley JF, Machell J, Nisbet AJ, Van den Broek A, Chua KY, Cheong N, Hales BJ, Thomas WR. Identification of tropomyosin, paramyosin and apolipophorin/vitellogenin as three major allergens of the sheep scab mite, Psoroptes ovis. Parasite Immunol. 2004;26:335–342. doi: 10.1111/j.0141-9838.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 20.Nisbet AJ, MacKellar A, Wright HW, Brennan GP, Chua KY, Cheong N, Thomas JE, Huntley JF. Molecular characterization, expression and localization of tropomyosin and paramyosin immunodominant allergens from sheep scab mites (Psoroptes ovis) Parasitology. 2006;133:515–523. doi: 10.1017/S0031182006000631. [DOI] [PubMed] [Google Scholar]
- 21.Nisbet AJ, MacKellar A, McLean K, Brennan GP, Huntley JF. Eukaryotic expression of recombinant Pso o 1, an allergen from Psoroptes ovis, and its localization in the mite. Parasitology. 2007;134:83–89. doi: 10.1017/S0031182006001235. [DOI] [PubMed] [Google Scholar]
- 22.McNair CM, Billingsley PF, Nisbet AJ, Knox DP. Feeding-associated gene expression in sheep scab mites (Psoroptes ovis) Vet Res. 2010;41:16. doi: 10.1051/vetres/2009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNair CM (2008) Identification and characterisation of novel antigens from the sheep scab mite Psoroptes ovis. PhD
- 24.Nunn FG, Burgess ST, Innocent G, Nisbet AJ, Bates P, Huntley JF. Development of a serodiagnostic test for sheep scab using recombinant protein Pso o 2. Mol Cell Probes. 2011;25:212–218. doi: 10.1016/j.mcp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Team RDC. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 26.Willadsen P. Vaccination against ectoparasites. Parasitology. 2006;133(Suppl):S9–S25. doi: 10.1017/S0031182006001788. [DOI] [PubMed] [Google Scholar]
- 27.Willadsen P. Novel vaccines for ectoparasites. Vet Parasitol. 1997;71:209–222. doi: 10.1016/S0304-4017(97)00028-9. [DOI] [PubMed] [Google Scholar]
- 28.Vercruysse J, Knox DP, Schetters TP, Willadsen P. Veterinary parasitic vaccines: pitfalls and future directions. Trends Parasitol. 2004;20:488–492. doi: 10.1016/j.pt.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Pruett JH. Immunological control of arthropod ectoparasites—a review. Int J Parasitol. 1999;29:25–32. doi: 10.1016/S0020-7519(98)00172-6. [DOI] [PubMed] [Google Scholar]
- 30.Van den Broek AH, Huntley JF. Sheep scab: the disease, pathogenesis and control. J Comp Pathol. 2003;128:79–91. doi: 10.1053/jcpa.2002.0627. [DOI] [PubMed] [Google Scholar]
- 31.Wall R, Smith KE, Berriatua E, French NP. Simulation analysis of the population dynamics of the mite, Psoroptes ovis, infesting sheep. Vet Parasitol. 1999;83:253–264. doi: 10.1016/S0304-4017(99)00062-X. [DOI] [PubMed] [Google Scholar]
- 32.Berriatua E, French NP, Wall R, Smith KE, Morgan KL. Within-flock transmission of sheep scab in naive sheep housed with single infested sheep. Vet Parasitol. 1999;83:277–289. doi: 10.1016/S0304-4017(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 33.Willadsen P. Antigen cocktails: valid hypothesis or unsubstantiated hope? Trends Parasitol. 2008;24:164–167. doi: 10.1016/j.pt.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Chan MS, Woolhouse ME, Bundy DA. Human schistosomiasis: potential long-term consequences of vaccination programmes. Vaccine. 1997;15:1545–1550. doi: 10.1016/S0264-410X(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 35.Donald AD. Parasites, animal production and sustainable development. Vet Parasitol. 1994;54:27–47. doi: 10.1016/0304-4017(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 36.Barnes EH, Dobson RJ, Barger IA. Worm control and anthelmintic resistance: adventures with a model. Parasitol Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 37.Dalton JP, Mulcahy G. Parasite vaccines—a reality? Vet Parasitol. 2001;98:149–167. doi: 10.1016/S0304-4017(01)00430-7. [DOI] [PubMed] [Google Scholar]
- 38.Gao X, Cai K, Li T, Wang Q, Hou X, Tian R, Liu H, Tu W, Xiao L, Fang L, Luo S, Liu Y, Wang H. Novel fusion protein protects against adherence and toxicity of enterohemorrhagic Escherichia coli O157:H7 in mice. Vaccine. 2011;29:6656–6663. doi: 10.1016/j.vaccine.2011.06.106. [DOI] [PubMed] [Google Scholar]
- 39.Tan Y, Liang H, Chen A, Guo X. Coexpression of double or triple copies of the rabies virus glycoprotein gene using a ‘self-cleaving’ 2A peptide-based replication-defective human adenovirus serotype 5 vector. Biologicals. 2010;38:586–593. doi: 10.1016/j.biologicals.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Burgess ST, Innocent G, Nunn F, Frew D, Kenyon F, Nisbet AJ, Huntley JF. The use of a Psoroptes ovis serodiagnostic test for the analysis of a natural outbreak of sheep scab. Parasit Vectors. 2012;5:7. doi: 10.1186/1756-3305-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kofron JL, Kuzmic P, Kishore V, Colon-Bonilla E, Rich DH. Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry. 1991;30:6127–6134. doi: 10.1021/bi00239a007. [DOI] [PubMed] [Google Scholar]


