Abstract
Background:
Posterior cortical atrophy (PCA) is a rare neurodegenerative syndrome characterized by early progressive visual dysfunction in the context of relative preservation of memory and a pattern of atrophy mainly involving the posterior cortex. The aim of the present study is to characterize the neuropsychiatric profile of PCA.
Methods:
The Neuropsychiatric Inventory was used to assess 12 neuropsychiatric symptoms (NPS) in 28 patients with PCA and 34 patients with typical Alzheimer disease (AD) matched by age, disease duration, and illness severity.
Results:
The most commonly reported NPS in both groups were depression, anxiety, apathy, and irritability. However, aside from a trend toward lower rates of apathy in patients with PCA, there were no differences in the percentage of NPS presented in each group. All those patients presenting visual hallucinations in the PCA group also met diagnostic criteria for dementia with Lewy bodies (DLB). Auditory hallucinations were only present in patients meeting diagnosis criteria for DLB.
Conclusion:
Prevalence of the 12 NPS examined was similar between patients with PCA and AD. Hallucinations in PCA may be helpful in the differential diagnosis between PCA-AD and PCA-DLB.
Keywords: neuropsychiatric symptoms, posterior cortical atrophy, neuropsychiatric inventory, visual hallucinations
Introduction
Posterior cortical atrophy (PCA) is a neurodegenerative syndrome characterized by progressive impairment of visual functions in the absence of visual acuity deficits and a pattern of atrophy involving parietal, occipital, and occipitotemporal cortex.1,2 Posterior cortical atrophy is considered the most common atypical presentation of Alzheimer disease (AD).3-5 Contrary to typical AD, episodic memory and insight are relatively preserved in the initial stages of the disease, while reading, object recognition, navigational orientation, and praxis are early affected. Other posterior symptoms, such as elements of Balint (simultanagnosia, optic ataxia, and ocular apraxia) and Gertsmann syndrome (agraphia, acalculia, finger agnosia, and right–left confusion), are also frequent. The syndrome is usually caused by AD pathology and less frequently by dementia with Lewy bodies (DLB), corticobasal degeneration, prion associated disease, and subcortical gliosis.3,6-8
A number of studies have focused on characterizing the cognitive and language profile, brain atrophy pattern, genetics, and possible therapeutic strategies in PCA6,9-17; however, little is known about its neuropsychiatric profile.18 Neuropsychiatric symptoms (NPS) are variably associated with the severity of cognitive decline in a number of neurodegenerative conditions19-22 and have an impact in patient’s quality of life, caregiver distress, and likelihood of institutionalization. By improving our understanding of NPS in PCA, we aim to contribute to (1) improving diagnosis and prognosis, (2) providing a more comprehensive picture of the evolution of clinical symptoms and associated problems across the course of the disease, and (3) planning and delivering effective support and psychoeducational training for caregivers.
The main goal of the present study is to characterize the neuropsychiatric profile of PCA. We aim to do so by (1) describing the differences in the NPS between PCA and AD and (2) investigating the relationship between NPS, age at onset, and cognitive impairment in PCA.
Methods
Participants were 28 patients with PCA and a convenience control sample of 34 patients with typical AD diagnosed at the Memory Disorders Unit of the Hospital Virgen del Rocio. Patients with PCA fulfilled current available clinical criteria6 comprising insidious onset and gradual progression, presentation of disabling visual complaints in the absence of significant primary ocular disease explaining the symptoms, and relative preservation of anterograde memory and insight early in the disorder. Patients with typical AD met diagnosis criteria for probable AD.23,24 Exclusion criteria included severe global cognitive impairment (defined by patient inability to undertake the cognitive assessment) and significant ischemic burden (scoring 1 or more on the Fazekas scale25). The local institutional review board approved this protocol.
In addition to standard demographic and clinical data, all patients and their relatives completed the 12-item Neuropsychiatric Inventory (NPI), a questionnaire that evaluates the presence, frequency, and severity of 10 different behavioral areas (delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, and abnormal motor behavior) and 2 neurovegetative areas (sleep and nighttime behavior disorders and appetite and eating disorders) over the past month.26 For the purpose of this study, only presence and not frequency or severity of NPS has been examined. Neuropsychological assessment consisted of tests of global cognitive function (Mini-Mental State Examination [MMSE]), executive function (phonemic and semantic verbal fluency27), memory (word list immediate recall and recognition28), language (Boston Naming Test29 and verbal/written comprehension27), and visuoperceptual and spatial function (Visual Object and Space Perception Battery).30
Three of the patients with PCA received a diagnosis of probable DLB31 at follow-up, more than 4 years after initial diagnosis and after having developed fluctuations, visual hallucinations (VH) or parkinsonism, and presenting low dopamine transporter uptake in basal ganglia demonstrated by DAT SPECT scan (123I-FP-CIT DATscan, GE Healthcare; patients present with 2 core features and a suggestive feature or one core feature and a suggestive feature). The NPI values of these individuals were collected within 6 months of the date of the SPECT. As the aim of the present study was to examine the neuropsychiatry of the PCA spectrum, these patients remained included in the set, avoiding the potential bias that would result from their exclusion in the overall analysis.
Chi-square or Fisher exact test was used for the analysis of dichotomous variables, while unpaired t test and Mann Whitney U were used for continuous variables depending whether or not they followed a normal distribution. A Spearman rank correlation coefficient was used to assess the relationship between cognitive performances and the occurrence of NPS.
Results
The demographic and clinical characteristics of the PCA and AD groups, as well as neuropsychological performances of the PCA group, are summarized in Table 1. Samples were matched for age, illness duration (from symptoms onset), and severity of cognitive decline (MMSE). There were no differences in the proportion of patients receiving related medication (cholinesterase inhibitors, antipsychotics, antidepressants, and benzodiazepines).
Table 1.
Demographic and Clinical Characteristics of the Study Groups.a
| PCA (n = 28) | AD (n = 34 ) | P Value | |
|---|---|---|---|
| Gender, female, n (%) | 15 (53) | 22 (64) | .37b |
| Age, y, mean ± SD | 64 ± 6.7 [54-79] | 66 ± 6.9 [53-79] | .16c |
| Disease duration, y, mean ± SD [range] | 4 ± 2.8 [1-13] | 4 ± 2.4 [1-10] | .47d |
| Age at onset, y, mean ± SD [range] | 59 ± 7.4 [42-75] | 62 ± 8.5 [43-78] | .14c |
| Percentage onset ≤ 65 years | 82% (23/28) | 61% (21/34) | .079b |
| Cholinesterase inhibitors, n (%) | 26 (92) | 31 (91) | .80b |
| Anti-psychotics, n (%) | 1 (3) | 0 | .45e |
| Antidepressants, n (%) | 6 (21) | 3 (8) | .27e |
| Benzodiazepines, n (%) | 1 (3) | 0 | .45e |
| MMSE (max. 30), mean ± SD | 13 ± 4.5 [5-22] | 14 ± 3.8 [6-22] | .57c |
| Neuropsychology data | |||
| Phonemic fluency | 8 ± 7.4 | – | |
| Semantic fluency | 3 ± 3.5 | – | |
| Word list immediate (WMS-R) | 6 ± 6.2 | – | |
| Word list recognition (WMS-R) | 10 ± 5.3 | – | |
| Naming (Brief BNT) | 8 ± 3.9 | – | |
| Comprehension: spoken sentences | 10 ± 5.9 | – | |
| Comprehension: written sentences | 2 ± 4.2 | – | |
| VOSP Incomplete letters | 2 ± 4.6 | – | |
| VOSP Dot counting | 3 ± 3.3 | – | |
Abbreviations: MMSE, Mini-Mental State Examination; WMS-R, Wechsler Memory Scale Revised (Wechsler28); BNT, Boston Naming Test (Goodglass et al29); VOSP, Visual Object and Space Perception Battery (Warrington & James30); PCA, posterior cortical atrophy; AD, Alzheimer disease; SD, standard deviation.
aValues expressed as mean ± SD or percentage as indicated.
bPearson χ2 test
cUnpaired t test.
dMann Whitney U test.
eFisher exact test.
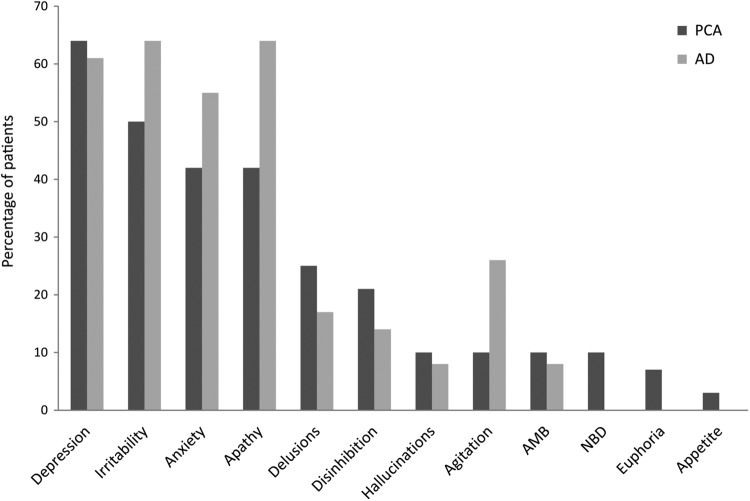
Chi-square tests revealed no differences between the PCA and the AD groups in the presence of each symptom on the NPI (see Figure 1). The most common symptoms were depression (64% PCA vs 61% AD, P = .83), irritability (50% PCA vs 64% AD, P = .24), anxiety (42% PCA vs 55% AD, P = .30), and apathy (42% PCA, vs 64% AD, P = .085). No differences were found in the presence of delusions or hallucinations between PCA and AD (delusions: 7 [25%] PCA vs 6 [17%] AD, P = .47; hallucinations: 3 [10%] PCA vs 3 [8%] AD, P = .80). Two (7%) patients in the PCA group but no one in the AD group presented with auditory hallucinations (AH), always accompanied by VH; these patients were 2 of the 3 individuals in the PCA group who were diagnosed with DLB.
Figure 1.
Prevalence of neuropsychiatric symptoms in patients with PCA and AD. AMB, indicates abnormal motor behavior; NBD, nightime behavioral disturbances; PCA, posterior cortical atrophy; AD, Alzheimer disease. Symptoms are ordered from more to less frequent in the PCA group.
To investigate the relationship between the age of onset and NPS, the PCA and AD sampled were split into early-onset (≤65 years) and late-onset (>65 years) subgroups (see Figure 2). In the PCA group, 47% (11 of 23) of the early-onset and 20% (1 of 5) of the late-onset patients presented anxiety, while the proportion of patients with anxiety in the AD group was 71% (15 of 21) in the early-onset and 30% (4 of 13) in the late-onset group. Levels of anxiety were significantly higher in the early-onset than late-onset AD subgroup (71% vs 30%, P = .02). Presence of anxiety did not differ between the PCA subgroups (47% vs 20%, P = .35) or between early-onset PCA and early-onset AD (47% vs 71%, P = .11). There were no other significant differences between early-onset and late-onset PCA or AD subgroups in the occurrence of any other NPS.
Figure 2.
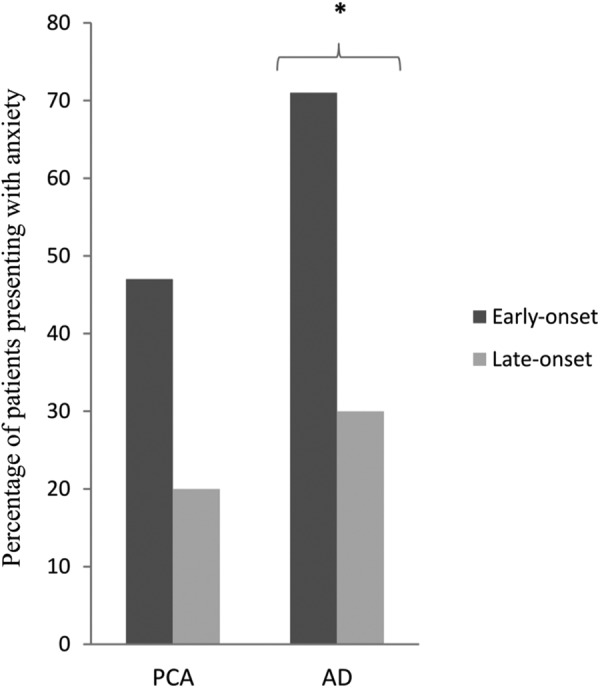
Relationship between age at onset and anxiety in PCA and AD. *Statistically significant difference, P = .020. PCA indicates posterior cortical atrophy; AD, Alzheimer disease.
Spearman correlation analysis yielded a significant inverse relationship between naming and the occurrence of delusions and agitation in PCA (delusions, r = −.48, P = .009; agitation, r = −.41, P = .029). There were no other significant correlations between NPS and neuropsychological test scores.
Discussion
This study compared the neuropsychiatric profile of PCA and typical AD. The overall results suggest a similar pattern of neuropsychiatric disturbance in PCA and AD, with a trend toward less apathy in the PCA group. There were no significant differences between both groups in the presence of NPS. The 4 most frequently occurring symptoms in our PCA sample were depression, irritability, apathy, and anxiety. These 4 features were also the 4 most common NPS in the AD control sample and are all in the top 5 most frequently occurring NPS averaging across 13 previous studies of neuropsychiatric features of AD (see Table 2).
Table 2.
Percentage of Participants Showing Individual NPS in the Current Study, the Previous Study of PCA, and 14 Previous Studies Using the NPI in AD.a
| Current study | Isella et al (2014) | Suarez- Gonzalez et al, (2014) | Van Vliet et al, (2012)33 | Toyota et al, (2007)34 | Zhang et al, (2012)35 | D’Onofrio et al, (2012)36 | Karttunen et al, (2011)37 | Yener et al, (2009)38 | Del Prete et al, (2009)39 | Hsieh et al, (2009)40 | Caputo et al, (2008)41 | Fuh et al, (2005)42 | Srikanth et al, (2005)43 | Senanarong et al, (2005)44 | Mean% showing NPS | Range% showing NPS | Rank order% showing NPS | Mean % (selected studiesb) | Range % (selected studiesb) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | PCA | AD | PCA | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | AD | ||||||||
| N | 28 | 34 | 20 | 20 | 85 | 79 | 68 | 46 | 261 | 46 | 166 | 240 | 81 | 159 | 78 | 94 | 45 | 53 | 25 | 690 | 188 | 107 | 25 | 44 | 73 | ||||||||
| MMSE | Mean | 13 | 14 | 24 | 25 | 16 | 19 | 19 | 17 | 19 | 23 | 14 | – | – | – | c- | d- | e- | 19 | 16 | 14 | – | – | – | 14 | 18 | |||||||
| SD | 5 | 4 | 3 | 2 | 6 | 7 | 4 | 8 | 6 | 2 | 5 | – | – | – | – | – | – | 4 | 6 | 6 | – | – | – | 5 | 6 | ||||||||
| CDR | – | – | - | – | – | – | – | – | – | – | – | – | 0.5 | (1-2) | – | – | – | – | – | – | 1 | 2 | 3 | – | – | ||||||||
| Age | Mean | 64 | 66 | 70 | 75 | 74 | 61 | 79 | 55 | 75 | 75 | 79 | 75 | 73 | 75 | 73 | 73 | 74 | 74 | 69 | 76 | 75 | 75 | 75 | 67 | 70 | |||||||
| SD | 7 | 7 | 8 | 4 | 7 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | 9 | 7 | 6 | 7 | 7 | 7 | 7 | 9 | 8 | ||||||||
| bSelected | b | b | b | b | b | b | b | ||||||||||||||||||||||||||
| Percentage participants showing NPMs | |||||||||||||||||||||||||||||||||
| Apathy | 42 | 64 | 60 | 55 | 61 | 38 | 39 | 56 | 64 | 87 | 58 | 47 | 49 | 47 | 59 | 54 | 66 | 77 | 88 | 51 | 43 | 55 | 64 | 93 | 45 | 59 | 38-93 | 1 | 56 | 43-87 | |||
| Depression | 64 | 61 | 45 | 40 | 70 | 16 | 45 | 43 | 39 | 30 | 58 | 37 | 32 | 39 | 42 | 42 | 33 | 79 | 48 | 40 | 46 | 44 | 54 | 75 | 30 | 44 | 16-79 | 2 | 39 | 30-46 | |||
| Irritability | 50 | 64 | 35 | 5 | 52 | 24 | 26 | 19 | 24 | 54 | 49 | 34 | 33 | 34 | 27 | 25 | 29 | 68 | 12 | 39 | 38 | 45 | 50 | 77 | 47 | 38 | 12-77 | 3 | 36 | 25-54 | |||
| Agitation | 10 | 26 | 25 | 15 | 23 | 21 | 25 | 28 | 44 | 34 | 44 | 29 | 23 | 32 | 28 | 29 | 33 | 62 | 44 | 41 | 28 | 49 | 64 | 68 | 35 | 37 | 21-68 | 4 | 31 | 23-49 | |||
| Anxiety | 42 | 55 | 55 | 15 | 44 | 16 | 26 | 28 | 38 | 32 | 56 | 25 | 28 | 24 | 39 | 39 | 36 | 75 | 28 | 41 | 32 | 43 | 44 | 6 | 42 | 35 | 6-75 | 5 | 33 | 24-43 | |||
| Sleep | 10 | 0 | 15 | 0 | 10 | 7 | 16 | – | – | 19 | 57 | – | – | – | – | – | – | 28 | 96 | 25 | 36 | 47 | 56 | 18 | 38 | 34 | 7-96 | 6 | 34 | 19-47 | |||
| AMB | 10 | 8 | 15 | 5 | 0 | 25 | 22 | 26 | 43 | 28 | 23 | 18 | 16 | 20 | 22 | 23 | 22 | 40 | 56 | 31 | – | – | 60 | 20 | 42 | 28 | 0-60 | 7 | 21 | 16-28 | |||
| Delusions | 25 | 17 | – | – | 32 | 7 | 23 | 13 | 50 | 23 | 17 | 22 | 17 | 25 | 27 | 28 | 27 | 11 | 28 | 22 | 19 | 43 | 58 | 9 | 27 | 25 | 7-58 | 8 | 26 | 17-43 | |||
| Apetite | 3 | 0 | 20 | 20 | 20 | 25 | – | – | 6 | 53 | – | – | – | – | – | – | 21 | 4 | 19 | 30 | 40 | 52 | 0 | 27 | 24 | 0-53 | 9 | 25 | 6-40 | ||||
| Hallucinations | 10 | 8 | – | – | 12 | 2 | 5 | 4 | 22 | 6 | 14 | 15 | 16 | 15 | 22 | 19 | 13 | 13 | 36 | 12 | 13 | 34 | 58 | 2 | 17 | 16 | 2-58 | 10 | 17 | 6-34 | |||
| Disinhibition | 21 | 14 | 10 | 0 | 12 | 11 | 5 | 4 | 16 | 13 | 0 | 14 | 18 | 12 | 15 | 11 | 9 | 17 | 0 | 9 | 13 | 32 | 32 | 45 | 30 | 15 | 0-45 | 11 | 16 | 11-32 | |||
| Euphoria | 7 | 0 | 5 | 0 | 3 | 2 | 2 | 8 | 7 | 2 | 4 | 5 | 4 | 6 | 11 | 15 | 7 | 9 | 48 | 4 | 5 | 9 | 16 | 6 | 6 | 8 | 2-48 | 12 | 7 | 2-15 | |||
Abbreviations: AMB, abnormal motor behavior; PCA, posterior cortical atrophy; AD, Alzheimer disease; SD, standard deviation; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; CDR, clinical dementia rating; NPS, neuropsychiatric symptoms.
aIn both PCA studies, the 4 most commonly observed NPS were apathy, depression, irritability, and anxiety (highlighted in gray). Across previous AD studies, apathy, depression, and irritability were also the 3 most commonly observed, with agitation marginally more common than anxiety. The percentage of anxiety in the Isella et al18 AD control group (highlighted in bold), who were significantly older than the corresponding patients with PCA, was unusually low and outside the range (also highlighted in bold) detected for previous studies involving patients with AD of comparable disease severity (estimated from MMSE/CDR score) and age (selected studies marked withb).
cMild AD, MMSE (21-26).
dModerate AD, MMSE (11-20).
eSevere AD, MMSE ≤ 10.
To our knowledge, there has only been 1 previous study evaluating NPS in PCA. Using the NPI, Isella et al18 compared 20 PCA and 20 patients with typical AD and reported higher rates of anxiety in PCA than in the AD group. The authors concluded that anxiety seems to be particularly common in PCA. As in Isella et al,18 in the present study, the 4 most common neuropsychiatric features in PCA are depression, irritability, apathy, and anxiety. However, unlike Isella et al18 who reported significantly higher rates of anxiety in their PCA (55%) than AD (15%) samples, we found no significant differences in anxiety (42% vs 55%, respectively) or any other NPS between our PCA and AD groups. One possible reason for this difference is that our PCA and AD groups were matched for age, unlike those in Isella et al study. Additionally, in Isella et al sample, the anxiety rate for patients with AD is unusually low in comparison to previous studies of NPS in AD, especially for those matched to the age and disease severity characteristics of the Isella et al group (see Table 2). The trend toward lower levels of apathy in the PCA group is consistent with findings described by Shakespeare et al,14 suggesting that patients with PCA may experience less apathy than individuals with AD. On the other hand, it has recently been claimed that individuals with early-onset AD exhibit significantly more anxiety symptoms than individuals with late-onset AD.32 Consistent with this claim, anxiety in the early-onset AD group was significantly more frequent than in the late-onset AD. Future studies using larger samples may determine whether this effect of age of onset upon anxiety symptoms holds true for patients with atypical AD phenotypes. Similarly, poor performance in naming tasks seems to be related to the appearance of higher rates of delusions and agitation. However, this finding needs to be replicated in larger longitudinal samples in order to ascertain the role of cognitive performance as potential predictors of psychiatric worsening.
Hallucinations and Delusions
Visual hallucinations have been reported in up to 25% of patients with PCA, although this proportion varies among studies. In the current sample, 3 (10%) patients presented with VH and met diagnostic criteria for DLB. These 3 cases presented features of Balint syndrome and occipital lobe atrophy on magnetic resonance imaging, and the presence of VH in these cases was not early but occurred at least 4 years after symptom onset. The presence of NPS was recorded within 6 months of the time of the SPECT. McMonagle et al10 reported 6 patients with VH, 5 of them were diagnosed with DLB. In the study of Tang-Wai et al,6 2 cases developed VH within 3 to 7 years, and coexisting Lewy body disease was found on autopsy assessment. In the series of Josephs et al,7 a quarter of the patients with PCA were found to have VH, and all of them met the clinical criteria for probable DLB. Moreover, AH have previously been reported as very specific of DLB and usually accompanying VH.21,22 Our results are mainly in line with these previous findings, suggesting that patients with PCA and VH, especially in the presence of AH, are likely to have DLB which might be of diagnostic relevance, as treatment approaches and prognosis for DLB differ from those for AD.
Strengths and Limitations
The main strengths of our study are the relatively large sample size and the availability of a routinely completed questionnaire to systematically evaluate NPS in both groups of patients. A further potential limitation may arise from the fact that only presence/absence of NPS has been considered in the present study. The frequency and severity with which these symptoms emerge might constitute a difference between patients with PCA which should be addressed in future studies. One difference between this and the previous study of NPS in PCA is the inclusion of individuals diagnosed with DLB. These individuals were retained in the current analysis, as our intention was to investigate the neuropsychiatric profile of the PCA syndrome not merely those individuals with PCA attributable to probable AD.
In summary, studies approaching NPS in PCA are scarce, and further efforts should be made in this direction. Future research looking at the frequency and severity of NPS and their course over time may contribute to shed light on the neuropsychiatric profile of PCA.
Acknowledgments
We thank patients and relatives involved in research at the Memory Disorders Unit of the University Hospital Virgen del Rocío and Emilie Brotherhood for her assistance with the manuscript’s proofreading.
Footnotes
Authors’ Note: Access to underlying research material: database can be at the disposal of editor if required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sources of financial support: This work was supported by an Alzheimer’s Research UK Senior Research Fellowship and ESRC/NIHR grant (ES/K006711/1) to S. Crutch. A. Suárez-González was supported by a Dunhill Medical Trust grant (R337/0214). This work was supported by the NIHR Queen Square Dementia Biomedical Research Unit.
References
- 1. Crutch SJ, Lehmann M, Schott JM, Ravinovici GD, Rossor MN, Fox N. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suárez-González A, Henley SM, Walton J, Crutch S. Posterior cortical atrophy, an atypical variant of Alzheimer disease. Psychiatr Clin N Am. 2015;38(2):211–220. doi:10.1016/j.psc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3. Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175–1180. [DOI] [PubMed] [Google Scholar]
- 4. Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(pt 10):2636–2645. [DOI] [PubMed] [Google Scholar]
- 5. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(8):614–29. [DOI] [PubMed] [Google Scholar]
- 6. Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. [DOI] [PubMed] [Google Scholar]
- 7. Josheps KA, Whitwell JL, Boeve BF, et al. Visual hallucinations in posterior cortical atrophy. Arch Neurol. 2006;63(10):1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Victoroff J, Ross GW, Benson DF, Verity MA, Vinters HV. Posterior cortical atrophy. Neuropathologic correlations. Arch Neurol. 1994;51(3):269–274. [DOI] [PubMed] [Google Scholar]
- 9. Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. [DOI] [PubMed] [Google Scholar]
- 10. McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66(3):331–338. [DOI] [PubMed] [Google Scholar]
- 11. Kas A, de Souza LC, Samri D, et al. Neural correlates of cognitive impairment in posterior cortical atrophy. Brain. 2011;134:1464–1478. [DOI] [PubMed] [Google Scholar]
- 12. Lehmann M, Barnes J, Ridgway GR, et al. Basic visual function and cortical thickness patterns in posterior cortical atrophy. Cereb Cortex. 2011;21(9):2122–2132. [DOI] [PubMed] [Google Scholar]
- 13. Seguin J, Formaglio M, Perret-Liaudet A. CSF biomarkers in posterior cortical atrophy. Neurology. 2011;76(21):1782–1788. [DOI] [PubMed] [Google Scholar]
- 14. Shakespeare TJ, Yong KX, Frost C, Kim LG, Warrington EK, Crutch SJ. Scene perception in posterior cortical atrophy: categorization, description and fixation patterns. Front Hum Neurosci. 2013;2(7):621 doi:10.3389/fnhum.2013.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84(4):460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weill Chounlamountry A, Poncet F, Crop S, et al. Physical medicine and rehabilitation multidisciplinary approach in a case of posterior cortical atrophy. Ann Phys Rehabil Med. 2012;55(6):430–439. [DOI] [PubMed] [Google Scholar]
- 17. Roca M, Gleichgerrcht E, Torralva T, Manes F. Cognitive rehabilitation in posterior cortical atrophy. Neuropsychol Rehabil. 2010;20(4):528–540. [DOI] [PubMed] [Google Scholar]
- 18. Isella V, Villa G, Mapelli C, Ferri F, Appollonio IM, Ferrarese C. The neuropsychiatric profile of posterior cortical atrophy. J Geriatr Psych Neurol. 2015;28(2):136–144. [DOI] [PubMed] [Google Scholar]
- 19. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. Mental and behavioural disturbances in dementia: findings form Cache County Study in Memory and Aging. Am J Psych. 2000;157(5):708–714. [DOI] [PubMed] [Google Scholar]
- 20. Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated caregiver stress. J Neurol Neurosurg Psychiatry. 2007;78(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ballard CG, Holmes C, McKeith I, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156(7):1039–1045. [DOI] [PubMed] [Google Scholar]
- 22. Suárez-González A, Serrano-Pozo A, Arroyo-Anlló E, et al. Utility of neuropsychiatric tools in the differential diagnosis of dementia with lewy bodies and Alzheimer disease: quantitative and qualitative findings. Int Psychogeriatrics. 2014;26(3):453–461. doi:10.1017/S1041610213002068 [DOI] [PubMed] [Google Scholar]
- 23. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–358. [DOI] [PubMed] [Google Scholar]
- 26. Cummings JL, Megan M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 27. Peña-Casanova J. Programa integrado de exploracion neuropsicologica. Manual. Barcelona: Masson; 1990. [Google Scholar]
- 28. Wechsler D. Wechsler Memory Scale – Revised (WMS-R). New York: Psychological Corporation; 1987. [Google Scholar]
- 29. Goodglass HK, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination (BDAE-3). Philadelphia: Lippincott, Williams and Wilkins; 2000. [Google Scholar]
- 30. Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Co; 1991. [Google Scholar]
- 31. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with lewy bodies. Neurology. 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 32. Kaiser NC, Liang LJ, Melrose R, Wilkins S, Sultzer DL, Mendez M. Differences in anxiety among patients with early- verus late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2014;26(1):73–80. [DOI] [PubMed] [Google Scholar]
- 33. Van Vliet D, de Vugt ME, Aalten P, et al. Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease-Part 1: findings of the two-year longitudinal NeedYD-Study. Dement Geriatr Cogn Disord. 2012;34:319-327. [DOI] [PubMed] [Google Scholar]
- 34. Toyota Y, Ikeda M, Shinagawa S, et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22:896–90110.1002/gps.1760. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Wang H, Li T, Yu X. Prevalence of Neuropsychiatric symptoms across the declining memory continuum: an observational study in a memory clinic setting. Dement Geriatr Cogn Disord Extra. 2012;2:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Onofrio G, Sancarlo D, Panza F, et al. Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr Alzheimer Res. 2012;9:759–771. [DOI] [PubMed] [Google Scholar]
- 37. Karttunen K, Karppi P, Hiltunen A, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Pscyhiatry. 2011;26:473–48210.1002/gps.2550. [DOI] [PubMed] [Google Scholar]
- 38. Yener G, Turkuaz Alzheimer Working Group The neuropsychiatric inventory scores change across the minimental state examination ranges in patients with Alzheimer’s disease: a multicentre study in Turkey. Cogn Behav Neurol. 2009;22(4):264–269. [DOI] [PubMed] [Google Scholar]
- 39. Del Prete, Spaccavento S, Craca A, Fiore P, Angelelli P. Neuropsychiatric symptoms and the APOE genotype in Alzheimer’s disease. Neurol Sci. 2009;30:367–373. [DOI] [PubMed] [Google Scholar]
- 40. Hsieh CJ, Chang CC, Lin CC. Neuropsychiatric profiles of patients with Alzheimer’s disease and vascular dementia in Taiwan. Int Geriatr Psychiatry. 2009;24:570-57710.1002/gps.2156. [DOI] [PubMed] [Google Scholar]
- 41. Caputo M, Monastero R, Mariani E, et al. Neuropsychiatric symptoms in 921 elderly subjects with dementia: a comparison between vascular and neurodegenerative types. Acta Psychiatr Scand. 2008;117:455–464. [DOI] [PubMed] [Google Scholar]
- 42. Fuh JL, Wang SJ, Cummings JL. Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. J Neurol Neurosurg Psychiatry. 2005;76:1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci. 2005;236(1-2):43–8. [DOI] [PubMed] [Google Scholar]
- 44. Senanarong V, Poungvarin N, Jamjumras P, et al. Neuropsychiatric symptoms, functional impairment and executive ability in Thai pateints with Alzheimer’s disease. Int Psychogeriatr. 2005;17:81–90. [DOI] [PubMed] [Google Scholar]