Abstract
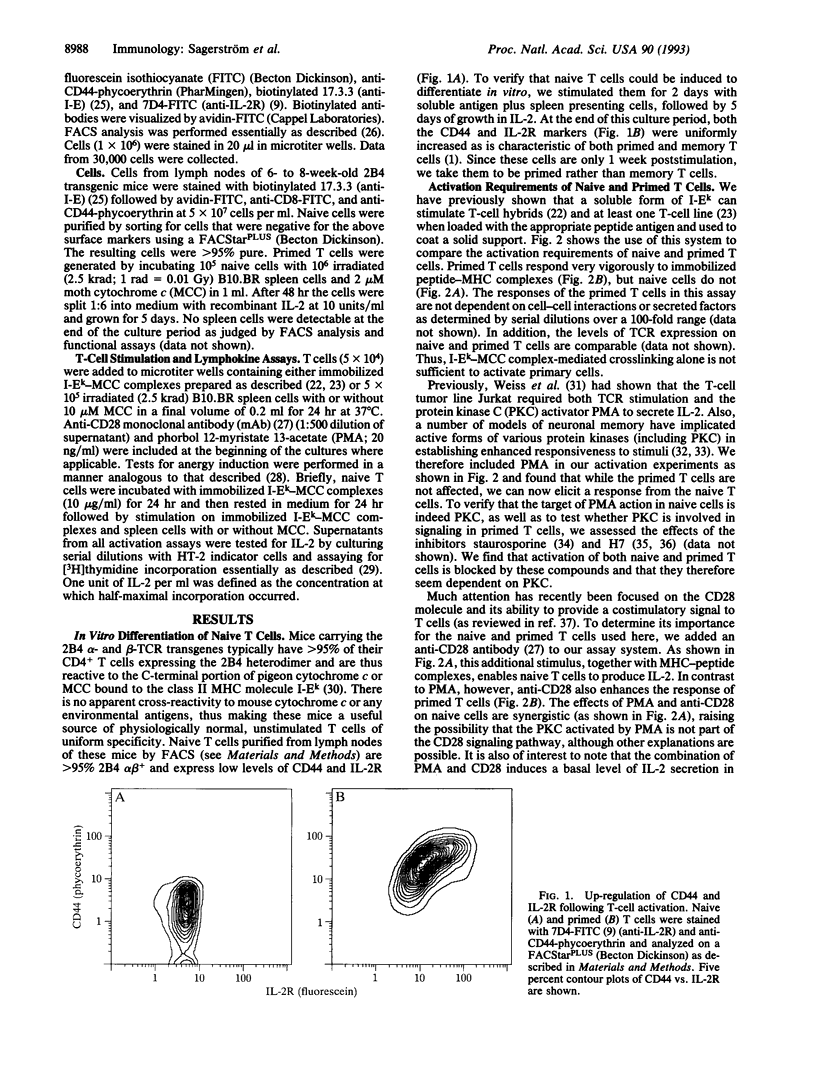
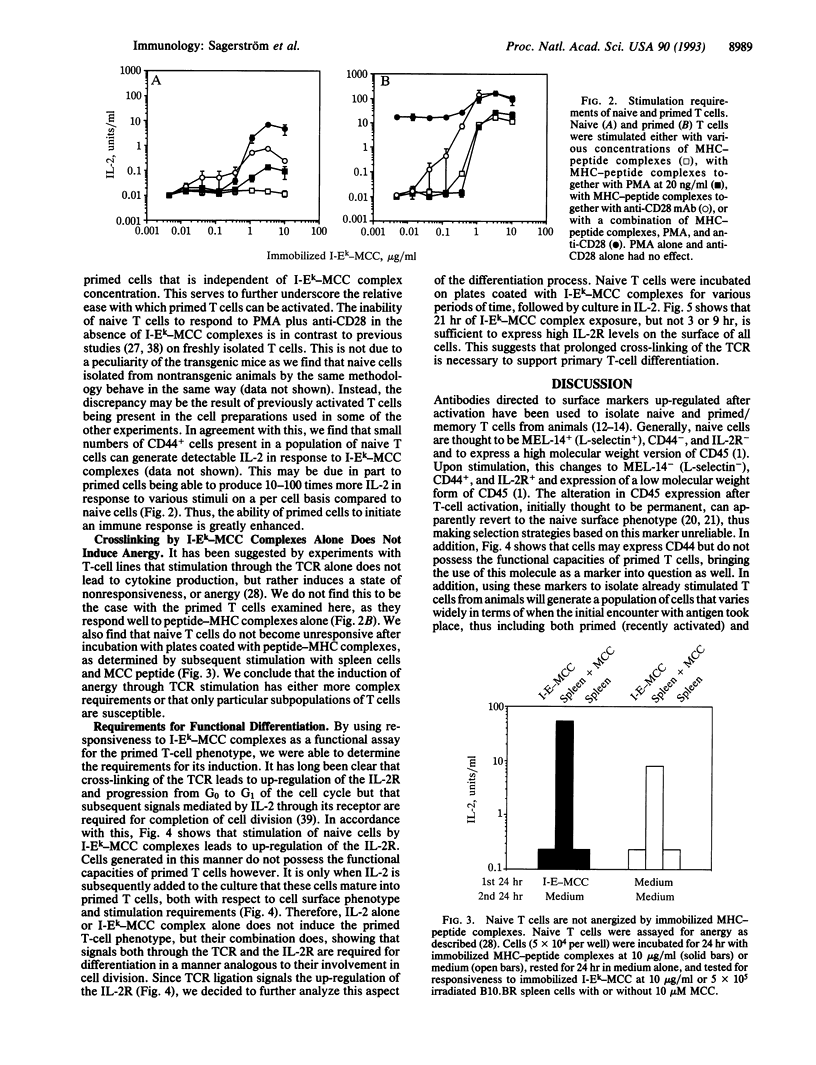
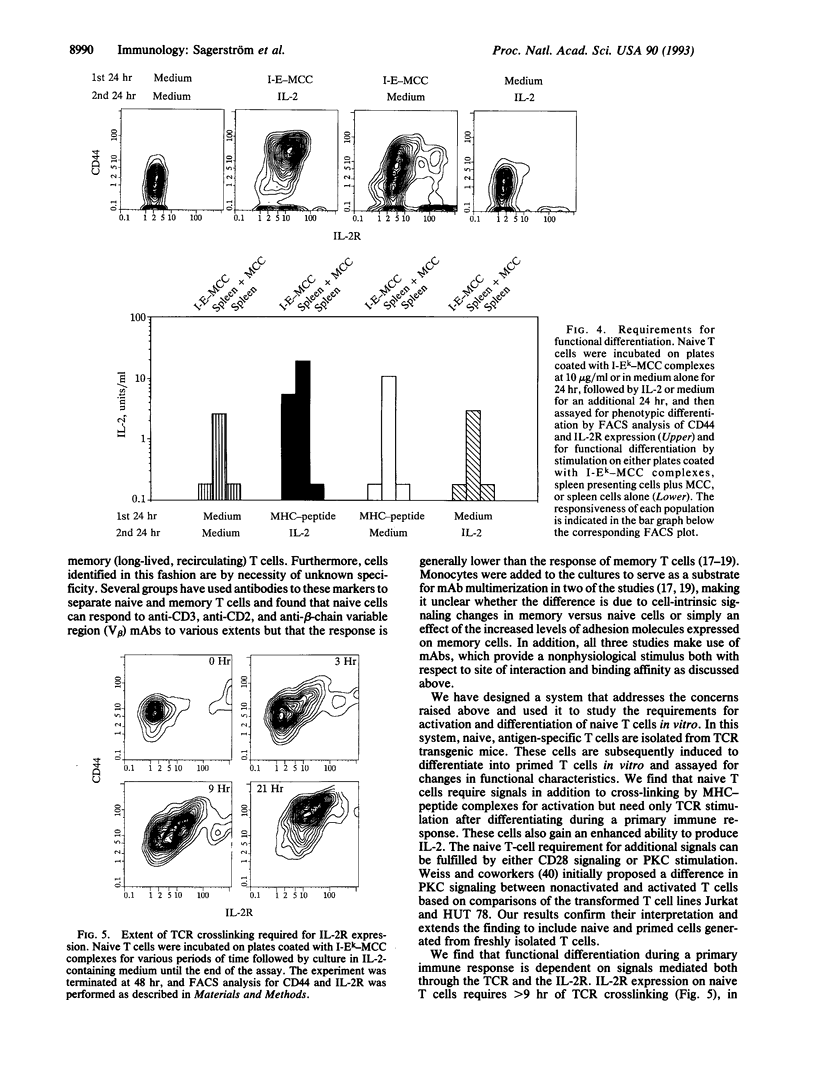
The progression of T cells from a quiescent or resting state to fully activated, proliferating cells is a crucial step in the initiation of an immune response. We have developed an in vitro system to study the requirements for triggering or hindering this pathway by using naive T cells derived from T-cell antigen receptor alpha beta transgenic animals and peptide-major histocompatibility (MHC) complexes coated on plates. Whereas previously stimulated T cells require only peptide-MHC complexes to produce interleukin 2 (IL-2), naive cells require at least one additional signal, which can be provided by either an anti-CD28 antibody or the protein kinase C stimulant phorbol 12-myristate 13-acetate. In contrast, the anti-CD28 antibody augments IL-2 production by primed T cells but is not required, and phorbol 12-myristate 13-acetate has no discernable effect. Thus we find that native T cells have significantly more stringent requirements for IL-2 production than primed cells and that this fits well with previous observations in other in vitro systems as well as in vivo models of autoimmunity. We also find that peptide-MHC complex stimulation of naive T cells, together with exogenous IL-2, is sufficient to convert these cells to primed T cells in vitro in 2 days, as assayed both by surface marker analysis and stimulation requirements. Taken together with the above results, this suggests that the activation of primary T cells requires at least two signals and that IL-2 produced by naive T cells in vivo may act in an autocrine fashion to allow them to proliferate and differentiate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell E. B. Function of CD4 T cell subsets in vivo: expression of CD45R isoforms. Semin Immunol. 1992 Feb;4(1):43–50. [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Frank G. D., Davis M. M. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990 Mar 23;60(6):1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., Horvath C., Bron C., Pedrazzini T., Howe R. C., MacDonald H. R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987 May 15;138(10):3120–3129. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Byrne J. A., Butler J. L., Cooper M. D. Differential activation requirements for virgin and memory T cells. J Immunol. 1988 Nov 15;141(10):3249–3257. [PubMed] [Google Scholar]
- Cerottini J. C., MacDonald H. R. The cellular basis of T-cell memory. Annu Rev Immunol. 1989;7:77–89. doi: 10.1146/annurev.iy.07.040189.000453. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Castellucci V. F., Bayley H., Schwartz J. H. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987 Sep 3;329(6134):62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- Gross J. A., Callas E., Allison J. P. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992 Jul 15;149(2):380–388. [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Scholz K. U., Duijvestijn A., Butcher E. C., Thiele H. G. Regulation of lymphocyte homing. I. Alterations in homing receptor expression and organ-specific high endothelial venule binding of lymphocytes upon activation. J Immunol. 1988 Feb 1;140(3):737–743. [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Jung T. M., Gallatin W. M., Weissman I. L., Dailey M. O. Down-regulation of homing receptors after T cell activation. J Immunol. 1988 Dec 15;141(12):4110–4117. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Krönke M., Peffer N. J., Depper J. M., Greene W. C. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6281–6285. doi: 10.1073/pnas.82.18.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman M., Bottomly K. Activation requirements for CD4+ T cells differing in CD45R expression. J Immunol. 1992 Oct 1;149(7):2300–2306. [PubMed] [Google Scholar]
- Mackay C. R. Migration pathways and immunologic memory among T lymphocytes. Semin Immunol. 1992 Feb;4(1):51–58. [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Manger B., Weiss A., Weyand C., Goronzy J., Stobo J. D. T cell activation: differences in the signals required for IL 2 production by nonactivated and activated T cells. J Immunol. 1985 Dec;135(6):3669–3673. [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., June C. H., Young H. A., Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol. 1989 May;19(5):803–808. doi: 10.1002/eji.1830190504. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M. Helper T cell memory: more questions than answers. Semin Immunol. 1992 Feb;4(1):59–68. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Wettstein D. A., Boniface J. J., Reay P. A., Schild H., Davis M. M. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J Exp Med. 1991 Jul 1;174(1):219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith D. C., McDevitt H. O., Steinman L., Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989 Jun 2;57(5):709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]



