Abstract
AIMS:
We hypothesized that if we control for lifestyle changes during Ramadan, Ramadan Islamic intermittent fasting (IF) reduces oxidative stress. This study was conducted to examine the effect of Islamic IF during and outside of Ramadan on the circadian changes in lipid peroxidation marker malondialdehyde (MDA) during and outside while controlling for potential confounders.
METHODS:
Serum MDA concentration was measured in eight healthy male volunteers at baseline (BL), after fasting for 1 week before Ramadan (BL fasting), and during Ramadan. Blood samples were drawn at 22:00, 02:00, 04:00, 06:00, and 11:00. The participants were admitted to the sleep laboratory and monitored for 24 h on the day of the measurements. In the laboratory, each participant received meals of fixed compositions and caloric contents based on their ideal body weights. Light exposure, physical activity, and total sleep duration were uniformly maintained during the three study periods.
RESULTS:
The participants had a mean age of 26.6 ± 4.9 years and a mean body mass index of 23.7 ± 3.5 kg/m2. No significant changes were observed in MDA levels and blood glucose during BL, BL fasting, or Ramadan.
CONCLUSION:
In this pilot study, under conditions of fixed sleep-wake schedules and caloric intake, Ramadan IF does not alter serum MDA levels in healthy subjects. Larger studies are needed to confirm these findings.
Key words: Caloric restriction, circadian, intermittent fasting, malondialdehyde, oxidative stress, Ramadan, sleep
Lipid peroxidation reflects to the oxidative degradation of lipids, where free radicals take electrons from the lipids, which results in cell damage. Moreover, it is involved in the underlying pathophysiological mechanisms of several disorders and diseases such as cardiovascular diseases and aging.[1] Lipid peroxidation forms reactive aldehydes such as malondialdehyde (MDA). Assessing the end products of lipid peroxidation is considered as one of the best assays for oxidative damage.[1] In animal models, caloric restriction (CR) and experimental fasting have been shown to increase life span and benefit cardiovascular and cerebrovascular systems.[2] Although the exact mechanisms underlying these fasting-conferred health benefits are unknown, reduced oxidative stress has been proposed as a potential mechanism.[2] The few studies that have assessed correlations between oxidative stress and CR or experimental fasting have reported inconsistent results.[3,4] Ramadan fasting is a distinctive model for long-term intermittent fasting (IF) that is accompanied by several lifestyle changes, including alterations in meal times and frequency, caloric content and composition, sleep/wake patterns, circadian rhythm patterns, and physical activity.[5] Hence, results from experimental fasting cannot be generalized to Ramadan IF.[5] Studies that have assessed oxidative stress during Ramadan are limited and reported conflicting results.[6,7,8,9] Sleep duration, exercise, and meal composition have been reported to influence reactive oxygen species.[10,11,12] Few studies have addressed the effect of prolonged IF during Ramadan on oxidative stress. While some studies reported no change in lipid peroxidation,[7,8,9] others reported a significant reduction in lipid peroxidation.[6,13] However, previous studies during Ramadan did not control for the above confounders and did not measure sleep duration objectively. In addition, all previous studies collected a single blood sample to assess oxidative stress. Because oxidant concentration levels can be affected by the time of day that the samples are taken and by the relationship between these times and meal times[14,15] and because meal times change during Ramadan, the timing of the sample collection may have influenced oxidant measurements in previous studies. We hypothesize that if we control for lifestyle changes during Ramadan, IF reduces oxidative stress. Therefore, we conducted this study to examine the effects of intermittent Islamic IF on circadian alternations in the lipid peroxidation marker MDA during and outside Ramadan while controlling for several confounders. Fasting was conducted outside Ramadan to simulate Ramadan Islamic IF in the absence of the previously mentioned lifestyle modifications that occur during Ramadan.
Methods
Subjects
The College of Medicine Institutional Review Board at our institute approved the study protocol, and each participant signed informed consent. Eight healthy, nonsmoking Muslim male volunteers between 20 and 35 years of age participated in the study. Participants were recruited at the university using bulletin boards advertisements seeking research project volunteers. The following inclusion criteria were applied: Males who were not taking any prescription or over-the-counter medications or supplements before or during the study, do not drink alcohol, and did not perform shift work and were not on vacation during the study period or for 2 weeks before their enrollment. None of the participants had sleep disorders or was on a special dietary regimen for medical or weight loss purposes. Only males were included in this study because females break the fast during menstruation, as per religion regulations. In addition, gender-specific differences have been reported in oxidative stress development,[16,17] and greater oxidative stress values have been reported in males.[16,17] The exclusion criteria included a history of diabetes mellitus, hypertension, known sleep disorders or disturbances, body mass index (BMI) >25 kg/m2, chronic diseases, including lung, cardiovascular or neurological disease, and a previous history of supplement use, including antioxidant vitamins. All participants used a wrist actigraph device to assess the sleep/wake pattern and schedules prior to starting the study, (Philips/Respironics, Inc., Murrysville, PA, USA) for 1 week while living their normal routine. The working hours of the participants were from 07:30 to 16:30 before Ramadan and from 10:00 to 15:00 during Ramadan.
Study protocol
The study protocol has been previously described.[18] This study was conducted during the last week of the month of Rajab (month 7, Hijri), the first and last weeks of the month of Shaban (month 8, Hijri), and the 2nd week of the month of Ramadan (month 9, Hijri) during the Hijri calendar year 1432, which corresponded to the period between June 25 and August 15 2011 on the Gregorian calendar.
Participants visited the sleep laboratory on four occasions. During each visit, the subjects spent approximately 1 day and night in the sleep laboratory [Figure 1].
Figure 1.
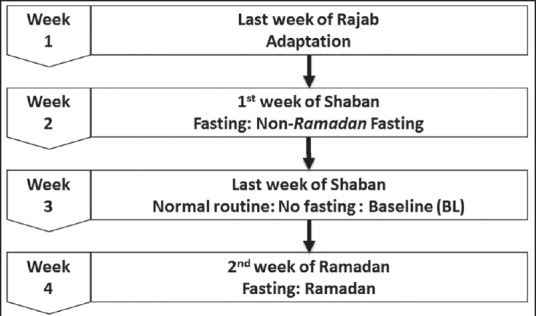
Study protocol
Adaptation night (during the last week of Rajab)
The subjects were adapted to the laboratory and sleeping setting to avoid the “ first night effect,”[19] which may result in altering the sleep patterns observed on the first night of sleep evaluation in the laboratory. During the adaptation visit, a medical checkup and basic blood tests (i.e., complete blood count, fasting blood sugar, kidney and liver function, and urine analysis) were performed to rule out comorbidities. BMI and demographic data were also obtained. Participants were instructed to maintain the same level of exercise and physical activity during the study period. Physical activity was assessed objectively using SenseWear Pro Armband™ (BodyMedia, Pittsburgh, PA, USA) as described below. To objectively assess sleep/wake schedules at home, each participant was asked to wear an actigraph monitor on his nondominant wrist at home.[20] A regular sleep/wake schedule was defined as a daily variability in bedtime and wake-up time of <1 h.[18]
Baseline fasting (during the 1st week of the month of Shaban [the month preceding Ramadan])
The participants were asked to perform the Islamic IF from dawn to sunset for 1 week only. We used this protocol to mimic Ramadan Islamic IF out of the month of Ramadan to control for the lifestyle, meal composition changes, and eating habits that occur during Ramadan and that could influence oxidant measurements. The participants presented to the study site on the last day of the fasting week for blood sample collection and sleep monitoring using polysomnography (PSG).
Baseline (nonfasting) (baseline) (during the last week of the month of Shaban)
The participants followed their normal living routines (at baseline [BL], without fasting). They reported to the study site on the last day of the week for PSG evaluation and blood sample collection.
During Ramadan
The participants reported to the laboratory during the last day of the 2nd week of Ramadan for PSG and blood samples collection.
The participants reported to the laboratory at approximately 18:00. The details of the study protocol have been previously described.[18,21] In the sleep laboratory, the participants were given meals of uniform composition (to control for the anti-oxidant properties of food sources), with fixed caloric intakes and fixed proportions of carbohydrates, fats, and proteins based on their ideal body weights. During BL, 3 meals were served; breakfast at 07:15, lunch at 12:00 (mid-day), and dinner at 20:00.[18] Three meals were served during BL fasting and Ramadan; Suhur was served before dawn (between 03:00 and 03:15), breakfast at sunset, (between 18:30 and 18:55) (to accommodate the changes in the time of dawn and sunset), and dinner at 21:00.
Fasting protocol
During BL fasting, the participants were asked to abstain from food and drinks for the 1st week of Shaban (7 days) from dawn to sunset. During the following 3 weeks of Shaban, the participants followed their normal activities and eating routines. The participants performed fasting during the whole month of Ramadan. During Ramadan, sunset (the end of fasting) and dawn (the beginning of fasting) occurred at approximately 18:34 and 04:00, respectively (fasting duration 14 h and 34 min). During the BL fasting period, dawn occurred at approximately 03:37, and sunset occurred at approximately 18:47 (fasting duration 14 h and 54 min). Blood glucose was measured at 15:30 during BL, BL fasting, and Ramadan.
Sleep monitoring in the laboratory
The PSG results are described in previous studies.[21] The subjects were directed to avoid taking naps during the sleep study day (monitored by actigraphy). Figure 2 demonstrates sleep schedules in the sleep laboratory during the three study visits. During BL, bedtime was at 23:00 and wake-up time was at 07:00. During BL fasting and Ramadan, bedtime was at 23:00. During Ramadan, the participants got up at 03:00 for Suhur, and sleep was allowed between 03:45 and 07:45. During BL fasting, the participants got up at 03:15 for Suhur (to account for the shift in dawn prayer time), and sleep was allowed between 04:00 and 07:45.
Figure 2.
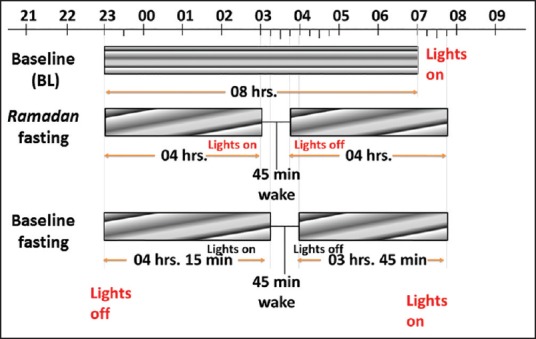
Sleep schedules during the three study visits: BL, BL fasting and Ramadan. BL: Baseline
During the study, a standard polysomnographic montage was used; four electroencephalography channels (C1-A4, C2-A3, O1-A4, and O2-A3), chin electromyography, and electrooculography (eye movement) were assessed. Arousal index, a reflection of sleep fragmentation, was defined as the number of arousals per 1 h of sleep. “Stage shift” was defined as total number of shifts in the sleep state from lights out to lights on.
Light exposure assessment
To control for the effect of circadian rhythm changes on oxidative stress, light intensity in the sleep laboratory was carefully controlled. The light level was kept at 50 lux from 18:00 until bedtime and during Suhur. During the specified sleep period (PSG recording), all lights were turned off, and the light level was maintained at <1 lux. Light intensity was measured using a Spectral Star Light Meter LX-1 (Japan).
Physical activity assessment
Energy expenditure was measured to reflect physical activity during the study period using the SenseWear Pro Armband™ (Body Media, Pittsburgh, PA, USA). Technical specifications of the Armband have been described previously.[22,23] The armband is a small, portable, convenient sensing device measuring 8.8 cm × 5.6 cm × 2.1 cm in size and 82 g in weight and is fixed around the triceps of the right arm. The armband measures several signals including skin temperature, galvanic skin response, heat flux from the body, and movement, which allows an advanced built-in algorithm to calculate the total energy expenditure.[23] This algorithm has been validated in several studies and compared with findings obtained using metabolic carts and doubly-labeled water.[22]
Lipid peroxide assay
Lipid peroxides were measured by analyzing thiobarbituric acid (TBA) reactive substances, which reflect MDA concentrations in human fluids, as previously described.[24] MDA is one of the final products of polyunsaturated fatty acid peroxidation in cells. An increase in free radicals stimulates MDA production. MDA reacts with TBA in the presence of acid and heat to form a red pigment that has a peak absorbance (l) of 530 nm. Blood samples were collected via an intravenous cannula in the antecubital vein at 22:00, 02:00, 04:00, 06:00, and 11:00. Samples were immediately centrifuged at 4°C, and stored at −70°C. Reagents were acquired from Sigma Chemical Company (St. Louis, MO, USA). Tetramethoxypropane was used to produce MDA to get a standard curve in the range of 0.1 µM to 5.0 μM. Blood serum (200 μl) was mixed with 5 mM butylated hydroxytoluene (25 μl), 0.2 M phosphoric acid (200 μl, H3PO4) and 0.11 M TBA (25 μl). The mixture was kept at 90°C for 45 min and then briefly put in ice to stop the reaction. MDA extraction was done by adding n-butanol (500 μl). Absorption was then measured in a 96-well plate reader (Spectra Microplate Autoreader, Tecan, Research Triangle Park, NC, USA) at 530 nm and 570 nm. The range of the standard curve was 0.1 µM to 5 μM/ml of blood serum.
Statistical analysis
Data are presented in the text and figures as the mean ± standard deviation. To assess the metabolic equivalents (METs), data were analyzed, and means were calculated to get the overall hourly average for each day. Comparisons between the BL, BL fasting, and Ramadan fasting groups were performed using one-way analysis of variance (ANOVA). Friedman's ANOVA by the rank test was used if the normality test failed. Results with a P value of <0.05 were considered to be statistically significant. Standard statistical software (Sigma Stat, version 3; SPSS, Chicago, IL, USA) was used for the analyses.
Results
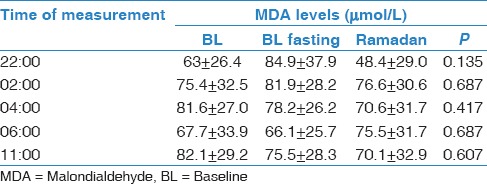
The mean age of the participants was 26.6 ± 4.9 years, and the mean BMI was 23.7 ± 3.5 kg/m2. No significant body weight changes were observed between the three periods (69.1 ± 8.4 kg, 67.5 ± 7.8 kg, and 66.3 ± 12.3 kg during BL, BL fasting, and Ramadan, respectively). Blood glucose measurements was performed at 15:30 and showed no significant differences between the three periods (6.1 ± 0.5, 5.7 ± 0.4, and 5.8 ±0.5 mmol/L, respectively). Table 1 demonstrates the sleep patterns generated at home using actigraphy. There was a shift delay in bedtime and wake-up time during Ramadan. However, there was no change in total sleep time (TST) between the three periods. Table 2 demonstrates the sleep parameters measured in the sleep laboratory using PSG monitoring. There were no significant changes in TST, sleep efficiency, sleep onset latency, REM onset latency, stage shifts, and arousal index between the BL, BL fasting, and Ramadan periods. Table 3 presents energy expenditure as a reflection of physical activity during the three periods. Data are expressed as kilocalorie per minute and METs during the entire day (24 h), during the daytime (from dawn to sunset), or at night (from sunset to dawn). There were no significant changes in energy expenditure during the three monitoring periods. Table 4 shows the concentrations of MDA during BL, BL fasting, and Ramadan. No significant changes in MDA concentration were observed at any of the five monitoring times (22:00, 02:00, 04:00, 06:00, and 11:00) during the three monitoring periods.
Table 1.
At-home sleep patterns observed using actigraphy
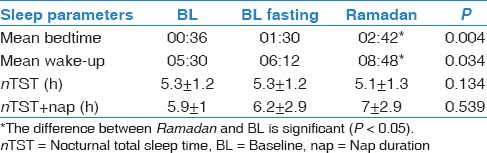
Table 2.
Sleep parameters in the sleep laboratory monitored using polysomnography (PSG)
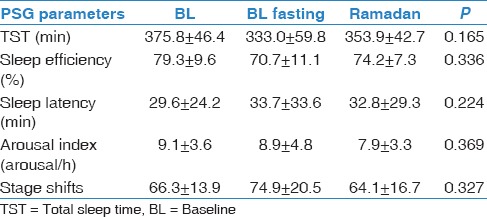
Table 3.
Energy expenditures during the three study periods measured using a SenseWear Pro Armband™
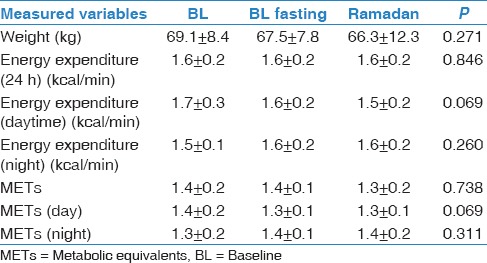
Table 4.
Measured MDA values during BL, BL fasting and Ramadan
Discussion
In the current study, the results do not support our hypothesis. We found no significant changes in the MDA serum levels measured at different times of the day during the three periods (BL, BL fasting and Ramadan). We measured MDA after 1 week of fasting during BL fasting and after 2 weeks of fasting during Ramadan. Although the fasting period during Ramadan was longer, there were no significant changes in the MDA levels.
Previous studies that aimed to assess oxidative stress during Ramadan fasting reported conflicting results. Moreover, those studies did not control for confounders that may affect oxidative stress such as sleep duration, physical activity, and meal composition. In a sample of healthy volunteers during Ramadan fasting, Faris et al. assessed oxidative stress using urinary 15-F12-Isoprostane.[8] This study showed that increased body weight during Ramadan was associated with increased lipid peroxidation and oxidative stress.[8] Interestingly, the study also showed that volunteers who lost weight had reduced levels of 15-F12-Isoprostane.[8] The above findings stress the importance of controlling for weight changes in oxidative stress measurements during IF. In another study, Ibrahim et al. observed no changes in serum MDA levels during Ramadan fasting.[7] However, they observed a reduction in MDA concentration in red blood cells (RBCs).[7] The reason for this discrepancy between serum MDA and RBCs MDA is unclear.[7] The influence of the dietary fat changes on the fatty acid composition of plasma lipids and RBCs fatty acids is well documented.[25] However, the fatty acid composition of plasma may not reflect the long-term nutritional status of dietary fats because of fast turnover and directional flux of various lipoproteins.[26] Therefore, RBCs fatty acid composition might be a better indicator of long-term dietary fatty acid intake than plasma lipid fatty acid composition, as RBC fatty acids do not change rapidly after dietary manipulations.[27,28] This may explain the differences between lipid peroxidation between plasma and RCBs lipids. In a third study, Ozturk et al. assessed oxidative stress among pregnant women and demonstrated that Ramadan fasting has no effect on oxidative stress.[9] The above studies contradict Al-Shafei, who reported a significant reduction in serum MDA among healthy volunteers and diabetic and hypertensive patients during Ramadan.[6,13] However, Al-Shafie did not report the participants’ weights before and during Ramadan. Weight reduction during fasting has been shown to reduce oxidative stress.[8] In addition, the investigators collected only a single early morning blood sample;[8] the timing of blood collection may have confounded the reported results. Comparing an early morning, overnight-fasted pre-Ramadan sample to blood collected after Suhur meal during Ramadan may have impacted the MDA measurements. During fasting, there is a greater tendency to utilize fat rather than glucose as a source of energy,[8] which results in higher fat oxidation.
Failure to control for potential confounders that may affect MDA levels, including meal composition, physical activity, sleep duration, fasting duration, smoking status, and medication use, may account for the inconsistencies between the different studies. Further research will be necessary to control for all potential confounders.
The current study has some limitations. The number of recruited volunteers is relatively low. Note that this limitation is inherent to experimental studies that use objective assessments in the laboratory under controlled conditions within a short specified period.[18,29] In addition, this type of studies is costly and a pilot study such as this is needed to convince funding agencies to fund a larger study. Moreover, the reported results cannot be applied to women, as gender-specific differences in the formation of oxidative stress have been reported.[16,17] Similarly, the current results cannot be applied to elderly as decreased antioxidant capacity and altered signaling mechanisms have been reported in elderly subjects.[30]
Conclusion
Our investigation of lipid peroxidation in eight fasting young men under controlled conditions revealed that Islamic IF does not alter serum MDA levels in healthy subjects during or before Ramadan. Larger studies are needed to assess oxidative stress during intermittent diurnal fasting while controlling for potential confounders.
Financial support and sponsorship
This study was supported by a grant from the College of Medicine Research Centre, Deanship of Scientific Research, King Saud University, Saudi Arabia.
Conflicts of interest
There are no conflicts of interest
References
- 1.Yoshida Y, Umeno A, Shichiri M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J Clin Biochem Nutr. 2013;52:9–16. doi: 10.3164/jcbn.12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Bartsch H, Nair J, Yoo DH, Hong YC, Cho SH, et al. Effect of short-term fasting on urinary excretion of primary lipid peroxidation products and on markers of oxidative DNA damage in healthy women. Carcinogenesis. 2006;27:1398–403. doi: 10.1093/carcin/bgi337. [DOI] [PubMed] [Google Scholar]
- 4.Thompson HJ, Heimendinger J, Haegele A, Sedlacek SM, Gillette C, O’Neill C, et al. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis. 1999;20:2261–6. doi: 10.1093/carcin/20.12.2261. [DOI] [PubMed] [Google Scholar]
- 5.Bahammam A. Does Ramadan fasting affect sleep? Int J Clin Pract. 2006;60:1631–7. doi: 10.1111/j.1742-1241.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shafei AI. Ramadan fasting ameliorates oxidative stress and improves glycemic control and lipid profile in diabetic patients. Eur J Nutr. 2014;53:1475–81. doi: 10.1007/s00394-014-0650-y. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim WH, Habib HM, Jarrar AH, Al Baz SA. Effect of Ramadan fasting on markers of oxidative stress and serum biochemical markers of cellular damage in healthy subjects. Ann Nutr Metab. 2008;53:175–81. doi: 10.1159/000172979. [DOI] [PubMed] [Google Scholar]
- 8.Faris MA, Hussein RN, Al-Kurd RA, Al-Fararjeh MA, Bustanji YK, Mohammad MK. Impact of ramadan intermittent fasting on oxidative stress measured by urinary 15-f(2t)-isoprostane. J Nutr Metab 2012. 2012 doi: 10.1155/2012/802924. 802924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozturk E, Balat O, Ugur MG, Yazicioglu C, Pence S, Erel Ö, et al. Effect of Ramadan fasting on maternal oxidative stress during the second trimester: A preliminary study. J Obstet Gynaecol Res. 2011;37:729–33. doi: 10.1111/j.1447-0756.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 10.Noguti J, Andersen ML, Cirelli C, Ribeiro DA. Oxidative stress, cancer, and sleep deprivation: Is there a logical link in this association? Sleep Breath. 2013;17:905–10. doi: 10.1007/s11325-012-0797-9. [DOI] [PubMed] [Google Scholar]
- 11.Camiletti-Moirón D, Aparicio VA, Aranda P, Radak Z. Does exercise reduce brain oxidative stress? A systematic review. Scand J Med Sci Sports. 2013;23:e202–12. doi: 10.1111/sms.12065. [DOI] [PubMed] [Google Scholar]
- 12.Bloomer RJ, Trepanowski JF, Kabir MM, Alleman RJ, Jr, Dessoulavy ME. Impact of short-term dietary modification on postprandial oxidative stress. Nutr J. 2012;11:16. doi: 10.1186/1475-2891-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Shafei AI. Ramadan fasting ameliorates arterial pulse pressure and lipid profile, and alleviates oxidative stress in hypertensive patients. Blood Press. 2014;23:160–7. doi: 10.3109/08037051.2013.836808. [DOI] [PubMed] [Google Scholar]
- 14.Hammouda O, Chahed H, Chtourou H, Ferchichi S, Miled A, Souissi N. Morning to evening difference of biomarkers of muscle injury and antioxidant status in young trained soccer players. Biol Rhythm Res. 2012;43:431–8. [Google Scholar]
- 15.Chtourou H, Hammouda O, Aloui A, Souissi N. Effect of time-of-day on muscle fatigue: A review. J Nov Physiother. 2013;3:160. [Google Scholar]
- 16.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22:438–42. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 17.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, et al. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 18.Alzoghaibi MA, Pandi-Perumal SR, Sharif MM, BaHammam AS. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS One. 2014;9:e92214. doi: 10.1371/journal.pone.0092214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamaki M, Nittono H, Hayashi M, Hori T. Spectral analysis of the first-night effect on the sleep-onset period. Sleep Biol Rhythms. 2005;3:122–9. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.BaHammam AS, Almushailhi K, Pandi-Perumal SR, Sharif MM. Intermittent fasting during Ramadan: Does it affect sleep? J Sleep Res. 2014;23:35–43. doi: 10.1111/jsr.12076. [DOI] [PubMed] [Google Scholar]
- 22.Dorminy CA, Choi L, Akohoue SA, Chen KY, Buchowski MS. Validity of a multisensor armband in estimating 24-h energy expenditure in children. Med Sci Sports Exerc. 2008;40:699–706. doi: 10.1249/MSS.0b013e318161ea8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BaHammam A, Alrajeh M, Albabtain M, Bahammam S, Sharif M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite. 2010;54:426–9. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Alzoghaibi MA, Bahammam AS. The effect of one night of continuous positive airway pressure therapy on oxidative stress and antioxidant defense in hypertensive patients with severe obstructive sleep apnea. Sleep Breath. 2012;16:499–504. doi: 10.1007/s11325-011-0531-z. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: A study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr. 1987;45:443–55. doi: 10.1093/ajcn/45.2.443. [DOI] [PubMed] [Google Scholar]
- 26.Phillips GB, Dodge JT. Composition of phospholipids and of phospholipid fatty acids of human plasma. J Lipid Res. 1967;8:676–81. [PubMed] [Google Scholar]
- 27.Hill JG, Kuksis A, Beveridge JM. The effect of diet on the phospholipid composition of the red blood cells of man. J Am Oil Chem Soc. 1965;42:137–41. doi: 10.1007/BF02545050. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar JW, Ahrens EH., Jr Effects of dietary fats on human erythrocyte fatty acid patterns. J Clin Invest. 1963;42:675–85. doi: 10.1172/JCI104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BaHammam A. Effect of fasting during Ramadan on sleep architecture, daytime sleepiness and sleep pattern. Sleep Biol Rhythm. 2004;2:135–43. [Google Scholar]
- 30.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]


