Abstract
OBJECTIVES:
To evaluate the bacterial colonization and associated risk factors in patients with bronchiectasis.
METHODS:
A total of 121 patients followed at the Bronchiectasis Unit, between 1996 and 2013 and diagnosed as having noncystic fibrosis bronchiectasis with high resolution computed tomography or multi-slice computed tomography were included in this retrospective study. The following definition of colonization was used for study purposes: Detection of at least two isolates of an organism separated by at least 3 months in a year.
RESULTS:
Of these 121 patients, 65 (54%) were female and 56 (46%) were male. Mean age was 50.6 ± 16.1 years. Mean duration of illness was 20.3 ± 15.5 years. 43 (35.5%) cases had colonization. The major pathogens responsible for colonization were Pseudomonas aeruginosa (n = 25; 20.6%) and Haemophilus influenzae (n = 14, 11.5%). The stepwise logistic regression analysis showed a significant association between colonization and a low percentage of forced vital capacity (FVC%) and the presence of cystic bronchiectasis (P < 0.05).
CONCLUSION:
The following factors have been found to be associated with colonization in patients with bronchiectasis: Low FVC% and the presence of cystic bronchiectasis.
Key words: Bacterial colonization, bronchiectasis, risk factors
The lower airways in patients with bronchiectasis are often colonized with pathogenic microorganisms, which are associated with pulmonary infections and secretion of inflammatory mediators that can lead to progressive tissue damage and airway obstruction.[1,2] The triad of chronic infection, bronchial inflammation, and progressive lung injury represents a “vicious cycle” justifying prompt evaluation of suspected infections.[3] Identification of the causative bacterial agents and administration of appropriate anti-microbial therapy may help break off this vicious cycle.[1,2,4] In this regard, sputum culture represents a simple, noninvasive, low-cost diagnostic tool to evaluate the pattern of bronchial colonization in clinically stable patients with bronchiectasis.[1] There are scarce studies about the bronchial colonization of patients with bronchiectasis. The aim of the present study was to evaluate the bacterial colonization and associated factors in patients with bronchiectasis.
Methods
A total of 121 patients followed at the Bronchiectasis Unit, Department of Pulmonology, Cerrahpasa Medical School between 1996 and 2013 and diagnosed as having noncystic fibrosis (CF) bronchiectasis with high resolution computed tomography (HRCT) or multi-slice computed tomography (CT) were included in this retrospective study. The study protocol was approved by the Ethics Board of Cerrahpasa Medical School, Istanbul University (Approval No: 83045809-33960).
Study design
This study setting was an outpatient clinic specializing in bronchiectasis where HRCT or multi-slice CT, in addition to sputum cultures, is routinely performed in patients with bronchiectasis. Patient data for a total of 121 participants was evaluated retrospectively. Colonization is defined as “at least two results of sputum culture separated by at least 3 months in a year.”[5] The results of sputum cultures were divided into two groups; colonized and noncolonized. Possible risk factors for colonization (age, gender, age at the etiologic factor onset and the symptoms onset, duration of the etiologic factors and symptoms, follow-up period, having symptoms, smoking history, etiology of bronchiectasis, type of bronchiectasis, localization of bronchiectasis, number of lobes with bronchiectasis, pulmonary functions) and the treatment including antibiotics, inhaled corticosteroids, inhaled long acting beta-2 agonists, inhaled anticholinergics, and oral N-acetyl cysteine were evaluated.
Clinical assessments
Age, gender, status and duration of the etiologic factor and symptoms were evaluated. In the presence of symptoms such as purulent sputum which are strongly suggestive for bronchiectasis, the date on which symptoms emerged was used as the disease onset; in the absence of symptoms, the date of the first radiologic detection of bronchiectasis was used as the disease onset. A patient was considered smoker if he or she was smoking daily at least for 1 year. Smoking was quantified in terms of pack-year. If a patient quit smoking at least 1 year before the study, he/she was accepted as an ex-smoker.
Radiological evaluation
All patients underwent an HRCT or CT imaging to determine the type bronchiectasis (cystic, varicose, or cylindrical) and the total number of lobes involved. HRCT or CT imaging of patients was accepted after interpretation of a faculty member of Radiology Department.
Spirometric evaluation
Spirometry was performed in all patients during the stable phase using a SensorMedics Vmax device (SensorMedics Series 22, USA) according to the criteria proposed by American Thoracic Society.[6]
Microbiological evaluation
Bronchial colonization was examined in all patients. All sputum samples with <10 epithelial cells under ×100 magnification were considered valid sputum samples qualifying for further analysis.[7] Sputum samples were plated onto 5% sheep-blood agar, chocolate agar, and Mc Conkey agar. Gram and Ehrlich Ziehl–Neelsen staining were routinely performed. Bacterial colonization was defined as the detection of at least two isolates of an organism separated by at least 3 months in a year. Patients with no pathogens isolated in sputum samples were considered to have no bacterial colonization.
Treatment
The previous and ongoing treatment and drug dose including antibiotics, inhaled corticosteroids, inhaled long acting beta-2 agonists, inhaled anticholinergics, and oral N-acetyl cysteine are investigated and recorded. Furthermore, the type and duration of antibiotic treatments before colonization were recorded in detail.
Statistical analyses
SPSS software version 21.0 (AIMS, Istanbul, Turkey) was used for statistical analyses. Continuous variables were expressed as mean ± standard deviation, and all categorical variables were expressed as the number and percent. Continuous variables were compared using Student's t-test or Mann-Whitney U-test depending on the distribution of data, and categorical variables were compared using Chi-square test. Variables considered statistically significant in the univariate analysis were entered into the stepwise logistic regression analysis in order to define the most relevant predictive variables, and their odds ratios (ORs) with 95% confidence interval (CI) are also shown.
Results
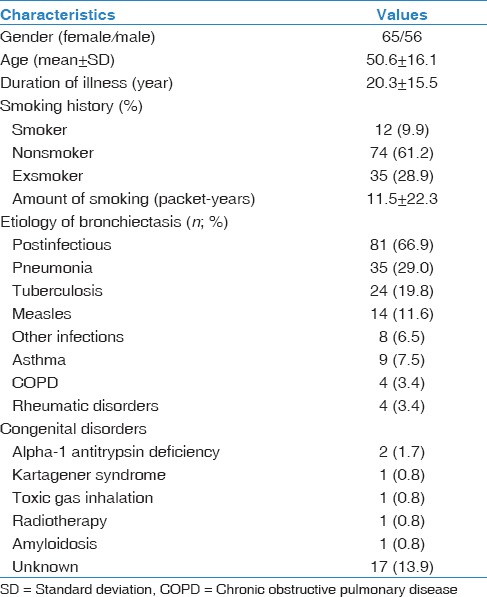
Of the 121 patients included in the study, 65 (54%) were female and 56 (46%) were male. Mean age was 50.6 ± 16.1 years. Mean duration of disease was 20.3 ± 15.5 years (median: 15 years). From an etiological viewpoint, postinfectious bronchiectasis represented the majority of the cases (n = 81; 66.9%). Patient characteristics are shown in Table 1.
Table 1.
Baseline characteristics of 121 patients
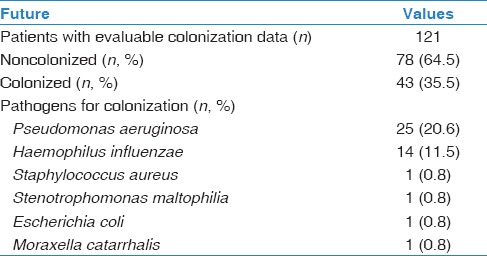
Colonization was present in 43 (35.5%) subjects, mostly caused by Pseudomonas aeruginosa (n = 25; 20.6%) and Haemophilus influenzae (n = 14; 11.5%) [Table 2].
Table 2.
The main pathogens for colonization
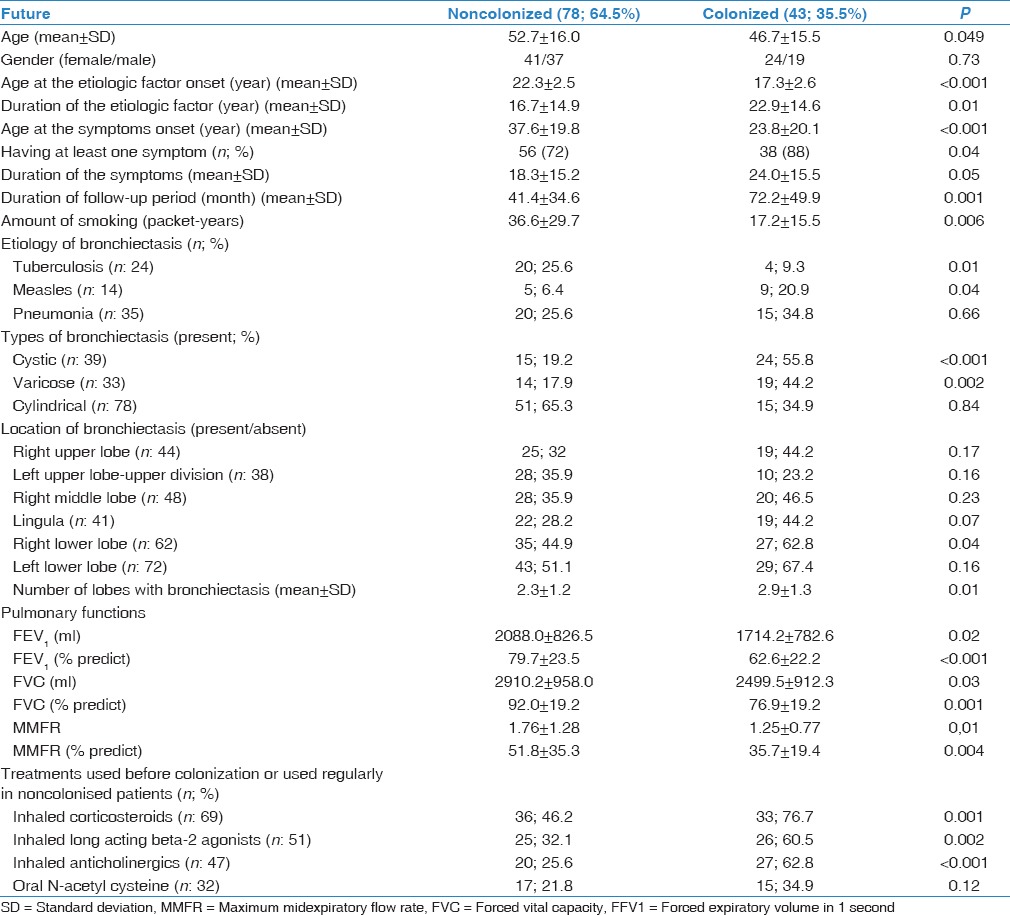
Young age, young age at onset of the etiology, young age at symptoms onset (the onset of the symptoms signifies the onset of the disease), having at least one symptom, long duration of symptoms, long follow-up period, low amount of smoking (packet-years), tuberculosis, and measles as etiologic factors, the presence of cystic or varicose bronchiectasis, location in right lower lobe, severity of radiological signs (total number of lobes with bronchiectasis), low pulmonary function tests including forced vital capacity (FVC), FVC%, forced expiratory volume in 1 s (FEV1), FEV1%, percentage of maximum midexpiratory flow rate values and using inhaled corticosteroids, inhaled long acting beta-2 agonists, and inhaled anticholinergics emerged as significant risk factors for colonization at univariate analyzed. (P < 0.05, for all). Comparison of subjects with or without colonization is shown in Table 3.
Table 3.
The comparison of the colonized and noncolonized patients
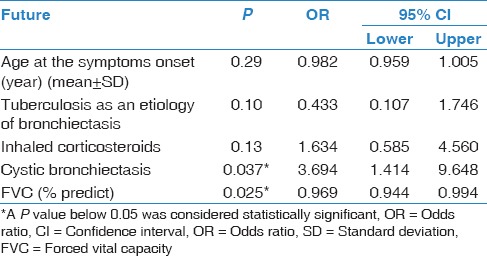
The stepwise logistic regression analysis showed a significant association between colonization and cystic bronchiectasis (P: 0.037; OR: 3.7; CI: 1.4-9.6) and low FVC% (P: 0.025; OR: 0.9; CI: 0.9-1.0) [Table 4].
Table 4.
The results of the stepwise logistic regression analysis
Discussion
Patients with bronchiectasis often suffer from chronic colonization or infection of the lower respiratory tract, which is sterile under normal conditions. Previous studies have used a variety of definitions for chronic colonization in patients with bronchiectasis.[8] These definitions have included at least three isolates of an organism over a period of at least 3 months, and at least two isolates 3 months apart over 1 year or three or more consecutive cultures separated by at least 1 month over a period of 6 months.[5,9,10,11,12] In case on the inability to reach microbiological test results, the information about the type of colonized bacteria and its antibiogram is quite important to decide about the effective antibiotics during the early phase of exacerbations. We used the definition of Pasteur et al.; “at least two results of sputum culture separated by at least 3 months in a year” was used.[5]
Main pathogens responsible for colonization were P. aeruginosa (20.6%) and H. influenzae (11.5%) in our bronchiectasis patients. This is similar to the report of Pasteur et al., where P. aeruginosa (24%) was the most frequent pathogen isolated, followed by H. influenzae (17%).[5] Similarly, pathogens isolated in some other studies were P. aeruginosa (5-75%), and H. influenzae (10-47%), Streptococcus pneumoniae (2-14%).[1,10,13,14,15,16,17,18,19,20,21,22,23] Angrill et al.,[1] Evans et al.,[10] Pasteur et al.,[5] and King et al.[17] have reported the results of sputum cultures in different times apart each other to determine colonization. In the literature, although most of the studies have published their results based on a single sputum culture, the results have been partially similar. In addition, new techniques have been developed and can provide the opportunity to examine in detail the bacterial profile of air way.[15,23] Rogers et al.[15] analyzed the induced sputum and bronchoalveolar lavage by 16S ribosomal RNA gene pyrosequencing and showed the significant correlation between the bacterial profile of the lower airway and disease severity in 41 patients with non-CF. In another study of Rogers et al.[23] that was complementary of previous study,[15] they used “Microbiota Stratification System” and reported that; “the stratification system is so effective and may predict the risk of future exacerbations in non-CF bronchiectasis by finding the dominant bacterial taxa that are not ordinarily identified by culture and have not previously been considered to be pathogenic.” However, further investigations are needed to verify the effectiveness of these new techniques.
The effects of treatments mostly corticosteroids and antibiotics on colonization in bronchiectasis are unknown research topics. In this study, the treatment including antibiotics, inhaled corticosteroids, inhaled long acting beta-2 agonists, inhaled anticholinergics, and oral N-acetyl cysteine were not found to be related with colonization.
Low FVC% and the presence of cystic bronchiectasis were more likely to be associated with airway colonization in this study (P <0.05). This is partially similar to the findings reported by Angrill et al.,[1] who demonstrated that the three following variables were associated with airway colonization:
Confirmation of diagnosis before the age of 14 years;
The presence of varicose-cystic bronchiectasis on HRCT; and
FEV1 lower than 80%.[1]
In contrast to the report published by Angrill et al., our results showed that lower values of FVC%, not lower FEV1, were associated with colonization in patients with bronchiectasis. Furthermore in studies by Evanset al.,[10] Miszkiel et al.,[24] and Ho et al.,[13] a correlation between the colonization (especially with P. aeruginosa) and lung function impairment was reported. Our results are supportive of these findings as well. We would like to emphasize that our results are similar to those of the previous studies from different European and Asian countries (Angrill et al. from Spain, Evans et al. from UK, Pasteur et al. from UK, King et al. from Australia, Ho et al. from Hong Kong, Rogers et al. from UK, Miszkiel et al. from UK, Tunney et al. from UK, Palwatwichai et al. from the Southeast Asia). We may conclude that microbiological profile of patients with bronchiectasis is similar in all around the Europe and the Asia. The ongoing multidisciplinary collaborative studies in non-CF bronchiectasis, such as “EMBARCH” Registry (European Multicentre Bronchiectasis Audit and Research Collaboration), are expected to find responses to lots of questions about the field of bronchiectasis in a near future.
This study has several strengths. It includes a fairly high number of bronchiectasis patients with a long follow-up time in a specified reference Bronchiectasis Unit established in 1996; which has the mission to accept the patients with bronchiectasis as a special group of patients, who should be followed up by a specified reference clinic. Furthermore, considering the lack of recent publications on this issue, we report a relatively new data to literature in the field of lower airway colonization in this group of patients.
However, its retrospective design with lack of the absence of data on compliance with respiratory physiotherapy to optimize bronchial hygiene is among its limitations.
Conclusion
Risk factors associated with bacterial colonization in patients with bronchiectasis are low FVC% and the presence of cystic bronchiectasis. Patients with at least one of these two risk factors require closer monitoring for colonization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Alef Bakır from Biostatistics Department of Istanbul University Cerrahpasa Medical Faculty.
References
- 1.Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, et al. Bacterial colonisation in patients with bronchiectasis: Microbiological pattern and risk factors. Thorax. 2002;57:15–9. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foweraker JE, Wat D. Microbiology of non-CF bronchiectasis. Eur Respir Soc Monogr. 2011;52:68–96. [Google Scholar]
- 3.Cole PJ. Inflammation: A two-edged sword - the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. [PubMed] [Google Scholar]
- 4.Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27–34. doi: 10.1016/j.molimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1277–84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 6.Standardization of spirometry, 1994 update, American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 7.James L, Hoppe-Baver JE. Processing and interpretation of lower respiratory tract specimens. In: Isenberg HD, editor. Clinical Microbiology Procedures Handbook. Washington, DC: ASM Press; 1992. pp. 1151–8. [Google Scholar]
- 8.Pasteur MC, Bilton D, Hill AT British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 9.Shah PL, Mawdsley S, Nash K, Cullinan P, Cole PJ, Wilson R. Determinants of chronic infection with Staphylococcus aureus in patients with bronchiectasis. Eur Respir J. 1999;14:1340–4. doi: 10.1183/09031936.99.14613409. [DOI] [PubMed] [Google Scholar]
- 10.Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: The influence of Pseudomonas aeruginosa. Eur Respir J. 1996;9:1601–4. doi: 10.1183/09031936.96.09081601. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell MJ, Ward C, Lordan JL, Rutherford RM. Non-cystic fibrosis bronchiectasis. QJM. 2013;106:709–15. doi: 10.1093/qjmed/hct109. [DOI] [PubMed] [Google Scholar]
- 12.Cantón R, Cobos N, de Gracia J, Baquero F, Honorato J, Gartner S, et al. Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect. 2005;11:690–703. doi: 10.1111/j.1469-0691.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 13.Ho PL, Chan KN, Ip MS, Lam WK, Ho CS, Yuen KY, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114:1594–8. doi: 10.1378/chest.114.6.1594. [DOI] [PubMed] [Google Scholar]
- 14.Chan TH, Ho SS, Lai CK, Cheung SW, Chan RC, Cheng AF, et al. Comparison of oral ciprofloxacin and amoxycillin in treating infective exacerbations of bronchiectasis in Hong Kong. Chemotherapy. 1996;42:150–6. doi: 10.1159/000239435. [DOI] [PubMed] [Google Scholar]
- 15.Rogers GB, van der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax. 2013;68:731–7. doi: 10.1136/thoraxjnl-2012-203105. [DOI] [PubMed] [Google Scholar]
- 16.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–61. doi: 10.1378/chest.108.4.955. [DOI] [PubMed] [Google Scholar]
- 17.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–8. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–26. doi: 10.1164/rccm.201210-1937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–56. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 20.Goeminne P, Dupont L. Non-cystic fibrosis bronchiectasis: Diagnosis and management in 21st century. Postgrad Med J. 2010;86:493–501. doi: 10.1136/pgmj.2009.091041. [DOI] [PubMed] [Google Scholar]
- 21.Kelly MG, Murphy S, Elborn JS. Bronchiectasis in secondary care: A comprehensive profile of a neglected disease. Eur J Intern Med. 2003;14:488–492. doi: 10.1016/j.ejim.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Palwatwichai A, Chaoprasong C, Vattanathum A, Wongsa A, Jatakanon A. Clinical, laboratory findings and microbiologic characterization of bronchiectasis in Thai patients. Respirology. 2002;7:63–6. doi: 10.1046/j.1440-1843.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogers GB, Zain NM, Bruce KD, Burr LD, Chen AC, Rivett DW, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc. 2014;11:496–503. doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 24.Miszkiel KA, Wells AU, Rubens MB, Cole PJ, Hansell DM. Effects of airway infection by Pseudomonas aeruginosa: A computed tomographic study. Thorax. 1997;52:260–4. doi: 10.1136/thx.52.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]


