Abstract
Background:
Emergence of drug resistance has complicated the treatment of tuberculosis (TB). WHO reports India to be one among 27 “high burden” multidrug-resistant (MDR) TB countries.
Objective:
To diagnose TB and detect drug resistance of mycobacterial isolates in acid-fast bacilli (AFB) smear negative HIV reactive patients (Group A) and compare them with HIV seropositive AFB smear positive (Group B) and HIV-seronegative AFB positive cases (Group C).
Materials and Methods:
Clinical specimens collected in all groups were processed as per the standard protocol except blood, which was processed by lysis centrifugation technique. They were then inoculated with Lowenstein-Jensen media and the isolates obtained were subjected to drug susceptibility test (DST) by proportion method and genotype MTBDR plus assay.
Results:
In Group A, 162 patients were included. Of the 443 clinical samples collected, 76 mycobacterial strains were obtained from 67 (41%) patients. Of these, 50 (65.8%) were sensitive to all drugs and 26 (34.2%) resistant to one or more anti-tubercular drugs. Antibiogram of Group A when compared with Group B and C showed that the MDR rate 6.6%, 6.7% and 8% respectively) did not differ much; but resistance to at least single drug was (26 [34.2%], 3 [10%], and 8 [16%]), respectively.
Conclusion:
Our study suggests that HIV has no influence on the anti-tubercular resistance pattern, but increased MDR rate along with HIV in high TB burden setting stresses the need for early diagnosis and DST in providing proper regimens and improve prognosis.
KEY WORDS: Drug-resistant tuberculosis, genotype MTBDR, HIV, proportion method
INTRODUCTION
Tuberculosis (TB) is the first infectious disease declared by WHO as a global health emergency in 1993. It is one of the India's oldest and most neglected public health challenges due to mismanagement of TB patients in both public and private sectors.[1] HIV and TB convergence have worsened and complicated the situation. One-third of the TB-infected patients are among 34 million people living with HIV.[2] Global control of TB is hampered due to delayed TB diagnosis and drug resistance. This reduces patient's life expectancy, and if infected with drug-resistant strains, TB can easily be transmitted among HIV-infected patients and the general community. Resistance of M. tuberculosis to antitubercular drugs is the result of a spontaneous genetic event and “manmade amplification of the natural phenomenon.”[3] There are two ways by which people get drug-resistant TB; first, when TB treatment is inadequate, i.e., when patients fail to adhere to proper treatment regimes, wrong drugs are prescribed, when the supply of drugs is not continuous; or substandard drugs are used for the treatment, and second, when there is a direct transmission of drug-resistant TB from one person to another.
WHO has reported an alarming rise in multidrug-resistant (MDR) TB and extremely drug-resistant (XDR) TB globally. In 2011, WHO estimated 630,000 cases of MDR-TB among world's 12 million prevalent cases of active TB. Almost 60% of MDR-TB cases are in India, China, and the Russian Federation. It is estimated that about 9% of MDR-TB cases have (XDR)-TB.[2] India stands, one among 27 “high burden” MDR countries and has over 2 million new TB cases every year and TB kills nearly 1000 people every day. WHO currently estimates that India has about 100,000 people with MDR-TB.[4] A team from Mumbai in January 2012 reported twelve cases of the strain as totally drug-resistant TB and suggested that it cannot be cured because of resistance to all TB drugs tested.[5]
Epidemiological studies are important to assess the local prevalence rate and detect drug resistance pattern to optimize drug therapy and to prevent the dissemination of resistant strains in the community.[6] With this background, the study was carried out to diagnose TB, as well as to detect the drug resistance pattern of the mycobacterial isolates in smear negative HIV-positive patients.
MATERIALS AND METHODS
Study settings
This study was undertaken from August 2010 to December 2012 at a tertiary care hospital located in the city center of Mysore, Karnataka, India, in Microbiology Department in consultation with the Medicine Department. Three groups were included for the study to compare the drug resistance of mycobacterial isolates:
Group A – HIV-positive acid-fast bacilli (AFB) smear negative
Group B – HIV-positive AFB sputum smear positive
Group C – HIV-negative AFB sputum smear positive.
Inclusion criteria
All newly detected HIV reactive patients during the study period with one or more of the following features were included in the study: History of prolonged fever, weight loss, cough for more than 2 weeks (sputum smear negative), radiological evidence suggestive of TB, pleural effusion, diarrhoea persisting for more than a month, pain abdomen/ascites/lymphadenopathy, or any other features suggestive of TB.
Of the 416 HIV, seropositive patients, 162 patients with suggestive features of TB but smear negative were included in Group A. For Group B and C, 30 and 50 patients were included, respectively. Ethical approval was obtained from the Institutional Ethics Committee and accordingly informed written consent was obtained from all patients.
Sample processing
Early morning sputum, stool, blood, and other clinical samples depending on the site of pathology were collected from Group A patients. From the Group B and C only sputum samples were included for the study. Sputum samples collected were processed by Petroff's method. The stool was suspended in Middlebrook 7H9 broth, and an equal amount of 4% NaOH was added as a part of decontamination. Both sputum and stool were incubated at 37°C for 20 min. Sputum, stool, and body fluids except blood were centrifuged at 3000 g for 30 min. The stool was neutralized with N/10 HCL and re-centrifuged for 10 min at 3000 g. The centrifuged deposits of all samples were then inoculated onto Lowenstein-Jensen (LJ) media.
About 5 ml of the blood collected from each patient, with sodium citrate as an anticoagulant, was centrifuged for 10 min. The buffy coat was transferred into Wintrobe tube by lumbar puncture needle and centrifuged for 30 min at 3000 g. The concentrated buffy coat was then transferred to eppendorf tube containing 0.1% saponin and coarse glass beads. The above mixture was vortexed for 10–12 min to release the intracellular mycobacteria and then inoculated onto LJ medium. All the slants were incubated at 37°C for 6–8 weeks.
The isolates recovered on LJ media were confirmed to be AFB by Ziehl-Neelsen staining of the culture smear. Later they were inoculated to LJ media with paranitrobenzoic acid and other biochemical tests to identify the species of mycobacteria. Mycobacterial culture was then tested for drug susceptibility by both proportion method using LJ media and genotype MTBDR plus assay. Standard drug susceptible strain H37Rv was used as control.
Drug susceptibility test
Conventional-proportion method
The antitubercular drugs tested were isoniazid (INH) – 0.2 mcg/ml, rifampicin (RIF) – 40 mcg/ml, ethambutol (EMB) – 2 mcg/ml, streptomycin (SM) –4 mcg/ml, and pyrazinamide (PZA) – 200 mcg/ml (Hi-Media). A loop full of fresh mycobacterial culture on LJ slant including H37Rv strain was suspended in 1 ml sterile distilled water. This was homogenized by vortexing with glass beads for 10 min. Opacity of suspension was adjusted to match Mc Farland 0.5 standard with saline giving approximately 1.5 × 108 CFU/ml. The suspension was diluted in 1:10000 and 100 µl of this was seeded on 5 different drug containing media and control media. All slants were then incubated at 35–37°C for 6–8 weeks and were examined for growth twice a week.
The isolate was termed either resistant/sensitive depending upon the duration of growth compared to the standard strain and expressed in percentage using the formula, i.e., number of colonies on the drug medium/number of colonies on the control medium ×100. The results were interpreted as resistant if the percentage was >1 and sensitive if <1.
Molecular method-genotype MTBDR plus assay
The assay was performed to detect MTB and drug susceptibility as per manufacturer's instructions (Hain Lifescience GmbH). It includes 3 steps: DNA extraction, polymerase chain reaction amplification, and reverse hybridization. Barnard reports that the assay is of limited use when used on smear negative samples (Barnard 2008 art 41).[7] Therefore, cultures were used to detect the drug resistance. The Hain strip contains TUB, rpoB,katG, and inhA wild-type and mutated probes for RIF, high and low-level of INH, respectively. The band in TUB zone indicates that the tested mycobacteria belong to MTB complex, and the absence indicates atypical mycobacteria. The absence of a band in at least one of the wild type probes and/or the presence of a band in mutation probe regions of the locus control zones of rpoB, katG, and inhA indicates drug resistance.
RESULTS
Among 416 HIV-positive patients, 162 (40%) patients were with features suggestive of TB but smear negative. Out of 443 clinical samples collected from 162 patients (Group A), 76 mycobacterial isolates were obtained from 67 (41%) patients. Of 76 isolates, 69 (91%) were MTB complex and 7 (9%) were Mycobacterium avium complex (MAC).[8]
Drug susceptibility results
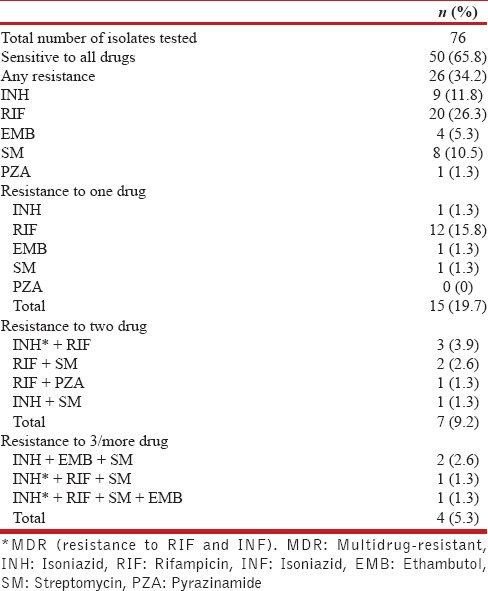
Group A
Of the 76 strains, 50 (65.8%) were sensitive to all drugs tested, and 26 (34.2%) were resistant to one or more drugs [Table 1]. Among 7 MAC isolates, only one showed resistance to RIF. Among 76 isolates, resistance to the single drug was observed in 15 (19.7%), two drugs in 7 (9.2%), 3 drugs in 3 (3.9%), and 4 drugs in 1 (1.3%) isolates. Resistance to INH, RIF, EMB, SM, and PZA was observed in 9 (11.8%), 20 (26.3%), 4 (5.3%), 8 (10.5%), and 1 (1.3%) isolate, respectively. MDR (resistance to both RIF and INH) was observed in 5 (6.6%) isolates.
Table 1.
Resistance pattern of mycobacterial isolates of Group A: HIV-positive AFB smear negative
Group B
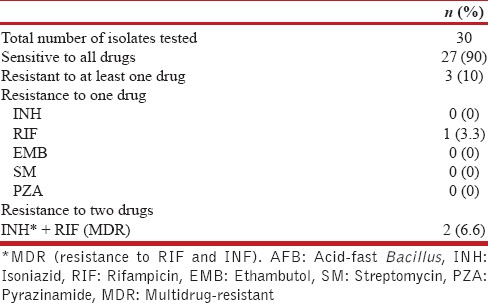
Of the 30 isolates, 27 were sensitive to all drugs and 3 were resistant to at least one drug. MDR was observed in 2 isolates, and only one showed resistance to RIF [Table 2].
Table 2.
Resistance pattern of mycobacterial isolates of Group B: HIV-positive AFB smear positive
Group C
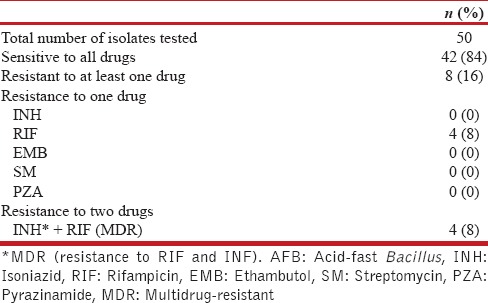
Of the 50 isolates, 42 were sensitive to all drugs and 8 were resistant; 4 were MDR and other 4 isolates showed resistance only to RIF [Table 3].
Table 3.
Resistance pattern of mycobacterial isolates of Group C: HIV-negative AFB smear positive
Drug resistance to at least one drug of the three groups were 26 (34.2%), 3 (10%), and 8 (16%), respectively. MDR of the three groups were 5 (6.6%), 2 (6.7%), and 4 (8%), respectively [Table 4].[8] The MDR rate detected in these groups were statistically (Chi-square test) found to be insignificant.
Table 4.
Multidrug resistance pattern of mycobacterial isolates of three groups

All the results of the conventional proportion method concurred with the molecular genotype MTBDR plus assay except one isolate which showed resistance to RIF solely by the latter method.[8]
DISCUSSION
In this study, mycobacterial culture-based TB detection provided added diagnostic sensitivity in detecting 41% of TB cases and 6.6% MDR-TB in smear negative HIV patients. The prevalence of primary drug resistance observed in different studies from India was found to be about 18.8% (7.9–27.1%).[9] The study conducted by National Tuberculosis Institute in the districts of Mysore (2001), Hoogly, Mayurghanj, Naogan, and in Bangalore city in 2003 showed MDR-TB level as 1.2, 3.0, 0.7, and 2.2%, respectively, in new cases.[10,11,12] Our findings show 6.6% MDR in newly detected TB cases (only in smear negative HIV patients) indicating a rise in MDR in the last decade.
Our observation of 34.2% drug resistance to one or more drug concurs with Bammann et al. studies in Brazil.[13] MDR-TB prevalence was 43% in HIV-positive and 3.9% in HIV-negative patients in Peru (2003).[14] Two separate studies from South Africa (2010, 2011) report 20% and 18% MDR-TB.[15,16] Studies from Peru (2006) and Pune (2004) reports 43% and 4.4% MDR-TB in HIV patients, respectively; the latter showed 7.3% resistance to more than one drug.[17,18] Another study from Pune (2005) reported 10% drug resistance to INH, 6.6% to EMB, 6.6% to SM, and 10% MDR in HIV patients.[19] This study noted maximum resistance to RIF (26.3%), followed by INH (11.8%), SM (10.5%), EMB (5.3%), and PZA (1.3%) which concurred with the 5 years (2005–2009) study of Mumbai, which also showed maximum resistance to RIF (74.4%), followed by SM (70%), INH (53.2%), and EMB (21.7%) and 47.54% strains were MDR.[9]
A high incidence of 22.11%-MDR were reported in TB patients from Dhaka (2009)[20] with 53.84% resistant to at least one drug. Our findings of 8% MDR and 16% resistance to at least one drug of Group C are in agreement with the results of Northern Kerala (2006), i.e., 8.8% and 17.7% resistance to more than one drug[21] from Pune (2006) which shows 2.9% MDR.[22] The drug susceptibility test (DST) undertaken by TRC, Chennai in North Arcot, Raichur, Jabalpur, and Wardha districts during 2000–2002 reports MDR-TB in 2.8%, 2.5%, 1.1%, and 0.5%, respectively.[4,23,24]
A single isolate which exhibited discordant result may confer mutation at low-level but could be clinically resistance which remained undetected by the phenotypic method.[25,26]
Culture-based TB detection and DST methods are accurate and inexpensive. However, the only disadvantage is that it is time-consuming. Comparison of drug resistance among three groups showed that MDR did not differ significantly, and RIF showed maximum resistance in all three groups. Implementation of genotype MDR-TB plus assay for DST in smear negative, culture positive samples can diagnose drug resistance in a short period of time. Rapid methods are not a replacement for culture, and many of them are not reliable when used on smear negative specimens.[27]
The cost of drugs alone to treat MDR-TB patient is 50–200 times higher than that used for drug-susceptible TB patient and the overall costs of care is found to be 10 times higher or more. According to the stop, TB Partnership's Global Plan to Stop TB, 2006–2015, 1.3 million MDR-TB cases will need to be treated in the 27 high MDR-TB burden countries between 2010 and 2015 at an estimated cost of US$16.2 billion.[4]
DST is not performed routinely in India except in a few laboratories. A team from Mumbai, who reported drug-resistant TB totally, stress that newly diagnosed patients should be tested for resistance if they continue to test positive for TB after 2 months of the regular treatment, or if they have been treated before for TB and are sick again.[5]
There are several limitations in our study. The study was limited exclusively to inpatients of one hospital and not the whole community. We have not included patients attending government/other health care services. All patients who fall under Group A and B, who attended hospital during the study period, were included in the study, but for Group C the number of patients was limited to 50. Screening of all HIV-seropositive patients can provide TB prevalence rate and MDR-TB status in HIV patients.
CONCLUSION
The incidence of MDR-TB in high TB burden setting stresses the need for DST to be done for every patient who is culture positive for M. tuberculosis, especially in HIV patients who carry a high mortality risk. Moreover, early diagnosis may prevent the therapy with inappropriate regimens and improve prognosis from causing MDR-TB strains to develop additional resistance, reduce transmission, and manage drug-resistant TB. The molecular diagnostic strategies could be used to identify patients with or without MDR/XDR TB strains, but efforts to reduce their cost and simplify for resource-limited settings are needed. Though mycobacterial culture-based studies detect TB and drug resistance early detection by rapid techniques may prevent the spread and patients could be treated earlier. TB control program can be effective if they function well with HIV detection and management. However, if private practitioners diagnose TB at an early stage, test for DST, and join hands with RNTCP and ICTC services in providing a proper regimen.
Financial support and sponsorship
ICMR.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Our sincere thanks to the Indian Council of Medical Research (ICMR-IRIS No. 5/8/5/8/2008-ECD-I), New Delhi for financial assistance in carrying out this study. We also thank Mr. B. Mahadevaswamy, Dr. K. Jayashree, Dr. K. Anurada, Dr. B. Sumangala, and Dr. Chitra Chandrashekar for their valuable inputs in the study.
REFERENCES
- 1.Prasad R. India's Tuberculosis Challenge. The Hindu. [Last cited on 2012 Dec 13]. Available from: http://www.thehindu.com/health/indias-tuberculosis-challenge/article4017246.ece .
- 2.WHO Fact Sheet No. 104. [Last update on 2012 Oct 23; Last cited on 2012 Dec 31]. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/
- 3.Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79:3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Multidrug and Extensively Drug-resistant TB (M/XDR-TB) 2010 Global Report on Surveillance and Response. WHO Global Report. 2010. [Last cited on 2012 Dec 31]. Available from: http://www.apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf .
- 5.Loewenberg S. India reports cases of totally drug-resistant tuberculosis. Lancet. 2012;379:205. doi: 10.1016/s0140-6736(12)60085-3. [DOI] [PubMed] [Google Scholar]
- 6.Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug-resistant pulmonary tuberculosis in Western Turkey: Prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005;25:313–8. doi: 10.5144/0256-4947.2005.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 8.Umamaheshwari S, Neelambike SM, Anuradha K, Sumangala B. Diagnosis of mycobacterial drug resistance in HIV reactive patients by phenotypic and genotypic assay – A comparative study. BMC Infect Dis. 2014;14:P58. [Google Scholar]
- 9.Menon S, Dharmshale S, Chande C, Gohil A, Lilani S, Mohammad S, et al. Drug resistance profiles of Mycobacterium tuberculosis isolates to first line anti-tuberculous drugs: A five years study. Lung India. 2012;29:227–31. doi: 10.4103/0970-2113.99104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahadev B, Jagota P, Srikantaramu N, Gnaneshwaran M. Surveillance of drug resistance in Mysore district, Karnataka. [Last cited on 2012 Oct 23];NTI Bull. 2003 39:5–10. Available from: http://www.popline.org/node/254514 . [Google Scholar]
- 11.Mahadev B, Kumar P, Agarwal SP, Chauhan LS, Srikantaramu N. Surveillance of drug resistance to anti-tuberculosis drugs in districts of Hoogli in West Bengal and Mayurbhanj in Orissa. [Last cited on 2012 Oct 23];Indian J Tuberc. 2005 52:5–10. Available from: http://www.imsear.li.mahidol.ac.th/handle/123456789/146942 . [Google Scholar]
- 12.Vijay S, Balasangameshwara VH, Jagannatha PS, Kumar P. Initial drug resistance among tuberculosis patients under DOTS programme in Bangalore city. [Last cited on 2012 Oct 18];Indian J Tuberc. 2004 51:17–21. Available from: http://www.medind.nic.in/ibr/t04/i1/ibrt04i1p17.pdf . [Google Scholar]
- 13.Bammann RH, Zamarioli LA, Pinto VS, Vázquez CM, Litvoc MN, Klautau GB, et al. High prevalence of drug-resistant tuberculosis and other mycobacteria among HIV-infected patients in Brazil: A systematic review. Mem Inst Oswaldo Cruz. 2010;105:838–41. doi: 10.1590/s0074-02762010000600019. [DOI] [PubMed] [Google Scholar]
- 14.Campos PE, Suarez PG, Sanchez J, Zavala D, Arevalo J, Ticona E, et al. Multidrug-resistant Mycobacterium tuberculosis in HIV-infected persons, Peru. Emerg Infect Dis. 2003;9:1571–8. doi: 10.3201/eid0912.020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heysell SK, Thomas TA, Gandhi NR, Moll AP, Eksteen FJ, Coovadia Y, et al. Blood cultures for the diagnosis of multidrug-resistant and extensively drug-resistant tuberculosis among HIV-infected patients from rural South Africa: A cross-sectional study. BMC Infect Dis. 2010;10:344. doi: 10.1186/1471-2334-10-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NS, Moodley P, Babaria P, Moodley S, Ramtahal M, Richardson J, et al. Rapid diagnosis of tuberculosis and multidrug resistance by the microscopic-observation drug-susceptibility assay. Am J Respir Crit Care Med. 2011;183:1427–33. doi: 10.1164/rccm.201009-1449OC. [DOI] [PubMed] [Google Scholar]
- 17.Kawai V, Soto G, Gilman RH, Bautista CT, Caviedes L, Huaroto L, et al. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg. 2006;75:1027–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Praharaj AK, Kalghatgi AT, Varghese SJ, Nagendra A. Incidence and drug susceptibility pattern of Mycobacterium tuberculosis in HIV infected patients. [Last cited on 2012 Nov 02];Med J Armed Forces India. 2004 60:134–6. doi: 10.1016/S0377-1237(04)80103-5. Available from: http://www.popline.org/node/254455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira M, Tripathy S, Inamdar V, Ramesh K, Bhavsar M, Date A, et al. Drug resistance pattern of Mycobacterium tuberculosis in seropositive and seronegative HIV-TB patients in Pune, India. Indian J Med Res. 2005;121:235–9. [PubMed] [Google Scholar]
- 20.Wadud AB, Rahman AS, Miah RA, Saleh AA. Drug resistance pattern of Mycobacterium tuberculosis isolated from patients attending a referral hospital. [Last cited on 2012 Oct 28];Banglad J Med Microbiol. 2010 3:13–7. Available from: http://www.banglajol.info/index.php/BJMM/article/view/5321 . [Google Scholar]
- 21.Ravindran C, James PT, Jyothi E. Prevalence of initial drug resistance of Mycobacterium tuberculosis in northern Kerala. [Last cited on 2012 Oct 28];Lung India. 2006 23:106–8. Available from: http://www.lungindia.com/textasp?2006/23/3/106/44401 . [Google Scholar]
- 22.Chand K, Khandelwal R, Vardhan V. Resistance to antituberculosis drugs in pulmonary tuberculosis. [Last cited on 2012 Oct 23];Med J Armed Forces India. 2006 62:325–7. doi: 10.1016/S0377-1237(06)80097-3. Available from: http://www.mjafi.net/article/S0377-1237(06) 80097-3/abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paramasivan CN, Bhaskaran K, Venkataraman P, Chandrasekaran V, Narayanan PR. Surveillance of drug resistance in tuberculosis in the state of Tamil Nadu. Indian J Tuberc. 2000;47:27–33. [Google Scholar]
- 24.Paramasivan CN, Venkataraman P, Chandrasekaran V, Bhat S, Narayanan PR. Surveillance of drug resistance in tuberculosis in two districts of South India. Int J Tuberc Lung Dis. 2002;6:479–84. doi: 10.5588/09640569512977. [DOI] [PubMed] [Google Scholar]
- 25.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009;47:3501–6. doi: 10.1128/JCM.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacoma A, Garcia-Sierra N, Prat C, Ruiz-Manzano J, Haba L, Rosés S, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2008;46:3660–7. doi: 10.1128/JCM.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson ML. Recent advances in the laboratory detection of Mycobacterium tuberculosis complex and drug resistance. Clin Infect Dis. 2011;52:1350–5. doi: 10.1093/cid/cir146. [DOI] [PubMed] [Google Scholar]


