Abstract
Sarcoidosis and tuberculosis are granulomatous disorders that mimic each other both clinically and radiologically. Both can present with fever and pulmonary nodules and often require the performance of transbronchial lung biopsy (TBLB) for diagnosis. In recent studies, the flexible cryoprobe for carrying out TBLB has been found to be useful in the diagnosis of disorders diffusely involving the lung parenchyma. Here, we present the case of a 29-year-old man who presented with fever and cough and was found to have multiple small nodules in both lungs. TBLB with a flexible cryoprobe helped in differentiating between sarcoidosis and tuberculosis.
KEY WORDS: Bronchoscopy, cryobiopsy, cryotherapy, lung biopsy, sarcoidosis, transbronchial lung biopsy, tuberculosis
INTRODUCTION
Diffusely distributed lung nodules are a common radiologic manifestation of several pulmonary diseases.[1] The differential diagnoses include infectious, inflammatory, and malignant disorders.[2] In high-prevalence areas, tuberculosis is an important consideration for a patient presenting with fever and lung nodules.[1] However, sarcoidosis can closely mimic tuberculosis, as both diseases may present with similar respiratory symptoms and diffuse lung nodules.[3,4,5] Perilymphatic nodules occur more commonly with sarcoidosis, while random or centrilobular nodules are seen more often with tuberculosis, although there is considerable overlap.[3] It is often difficult to differentiate between the two disorders solely on clinicoradiological grounds, and therefore pathological confirmation is essential.[4,6,7,8] On histopathological examination, both diseases are characterized by the presence of granulomatous inflammation.[9] While the presence of acid-fast bacilli virtually confirms the diagnosis of tuberculosis, the presence of necrosis and giant cells strongly favors the diagnosis of tuberculosis over sarcoidosis.[10]
Recently, the use of the flexible cryoprobe has been described for obtaining transbronchial lung biopsy (TBLB) in patients with diseases diffusely involving the lung parenchyma.[11] TBLB with a cryoprobe [cryobiopsy (CB) or cryo-TBLB] has been found useful in patients with suspected interstitial lung disease (ILD), patients with diffuse lung disease, immunocompromised patients with pulmonary infiltrates, and lung transplant recipients.[12,13,14,15] The major advantages of cryo-TBLB over TBLB with conventional flexible biopsy forceps (forceps biopsy or FB) are a higher yield, mainly due to the larger size of the sample, and an acceptable rate of complications.[15]
Here, we report the case of a patient who presented with fever and multiple lung nodules, and where a cryo-TBLB helped in confirming the diagnosis.
CASE REPORT
A 29-year-old man presented with complaints of fever and cough of 1 month's duration. There was no breathlessness, chest pain, hemoptysis, anorexia, or weight loss. There was no history of exposure to farm dust, metallic dust, fumes, or animal dander. The patient did not keep any pets or birds at home, and denied having any addictions. On examination, the pulse rate was 90 beats per min, respiratory rate 18 breaths per min, blood pressure 110/80 mmHg, and temperature 100°F. Pulse oximetric saturation was 98% while breathing room air. Auscultation of the chest revealed a few inspiratory crackles in both the lung bases. The rest of the physical examination was unremarkable.
The complete blood count and renal function tests were normal [Table 1]. There was elevation of the liver enzymes, with normal bilirubin and alkaline phosphatase (bilirubin 0.7 mg/dL, aspartate transaminase 66 U/L, alanine transaminase 88 U/L, alkaline phosphatase 126 U/L). The coagulation profile was normal. Urine analysis was normal, and blood and urine cultures were sterile. The tuberculin skin test was positive with an induration of 13 mm. The chest radiograph showed diffusely distributed nodules in bilateral lung fields. Computed tomography of the chest showed randomly distributed tiny nodules (1-2 mm in size) in both lungs [Figure 1], with mild hepatomegaly and a few subcentimetric lymph nodes at the porta and retroperitoneum. Two possibilities were considered: Disseminated tuberculosis and sarcoidosis. Flexible bronchoscopy and TBLB, both with conventional forceps and cryoprobe, were planned. The procedure was performed in the bronchoscopy suite with local anesthesia and conscious sedation.[16] The heart rate, oxygen saturation, blood pressure, and electrocardiogram (ECG) were monitored throughout the procedure. Continuous oxygen supplementation was provided through a face mask. The flexible bronchoscope (BF-1T180, Olympus, Tokyo, Japan) was introduced through the nose; the airway examination was normal, and subsequently, bronchoalveolar lavage was performed. TBLB was then performed using standard biopsy forceps (FB-19C, Olympus, Japan) from the basal segments of the right lower lobe. Six tissue specimens were obtained. Subsequently, cryo-TBLB was performed. The bronchoscope was introduced orally and maneuvered into the posterior segment of the right lower lobe. The flexible cryoprobe (1.9-mm outer diameter, 900-mm length, ERBE Elektromedizin, Tubingen, Germany) was introduced through the working channel of the bronchoscope into the selected segment and subsequently confirmed under fluoroscopic guidance. The probe was stationed 2 cm away from the chest wall. It was then cooled with nitrous oxide (this reduces the temperature of the probe's tip to -89°C) for 4 s. The entire bronchoscope was then retracted with the frozen lung tissue attached to the probe's tip. As soon as the bronchoscope was withdrawn from the patient, it was handed over to the technical assistant. The tip of the cryoprobe along with the first bronchoscope was immersed in normal saline at room temperature. Thawing resulted in the separation of the specimen from the tip. The specimen was then transferred into formalin. Meanwhile, the operator introduced another bronchoscope (FB-19TV, Pentax, Tokyo, Japan) immediately through the nose. Mild bleeding was visualized. The bronchoscope was immediately wedged into the posterior segment of the right lower lobe for 3 min and then withdrawn. There was no ongoing bleeding. The bronchoscope was subsequently withdrawn from the patient.
Table 1.
Hematological, biochemical, and other parameters of the patient
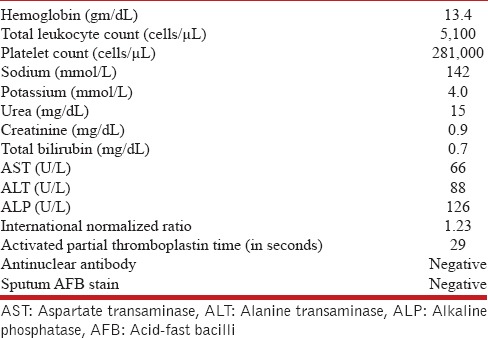
Figure 1.
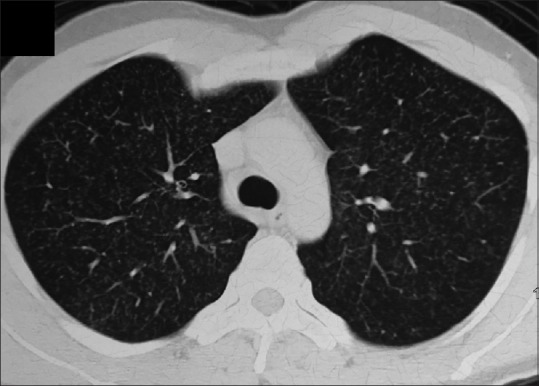
F Computed tomography of the chest showing multiple random nodules diffusely distributed in both the lung fields
Histopathological examination was done by an experienced pulmonary histopathologist. The CB was 0.5 cm in length, while the largest conventional FB specimen was 0.1 cm [Figure 2]. The surface areas of the CB and the FB were 14.18 mm2 and 1.65 mm2, respectively as measured with digital morphometry (Leica QWin version 3, Leica Microsystems Imaging Solutions Ltd, Cambridge, UK). Microscopic examination revealed 24 alveoli in FB and 212 alveoli in CB. Both the specimens revealed the presence of epithelioid cell granulomas with multiple Langhans type of giant cells and surrounding lymphocytes [Figure 3]. While there were nine granulomas in the CB, there was only one granuloma found in FB. The granuloma in the FB was a loose collection of epithelioid cells and did not have any central necrosis. The granulomas found in CB were well formed with Langhans type of giant cells and showed the presence of central necrosis with nuclear debris pointing toward a diagnosis of tuberculosis rather than sarcoidosis. There was no angiitis. Ziehl-Neelsen staining did not reveal acid-fast bacilli in either specimen. There was no artifact in the CB, while the FB showed minor crush artifacts. A diagnosis of pulmonary tuberculosis was made. The patient was started on antituberculosis treatment with oral rifampicin (600 mg), isoniazid (300 mg), pyrazinamide (1500 mg), ethambutol (1000 mg), and pyridoxine (10 mg). Fever abated in 2 weeks and cough disappeared. Chest radiograph showed reduction in the nodular opacities. Currently, at the time of writing, the patient is asymptomatic and under follow-up.
Figure 2.

Gross appearance of the flexible FB (left) and CB tissue (right). The difference in the sizes can be easily appreciated
Figure 3.
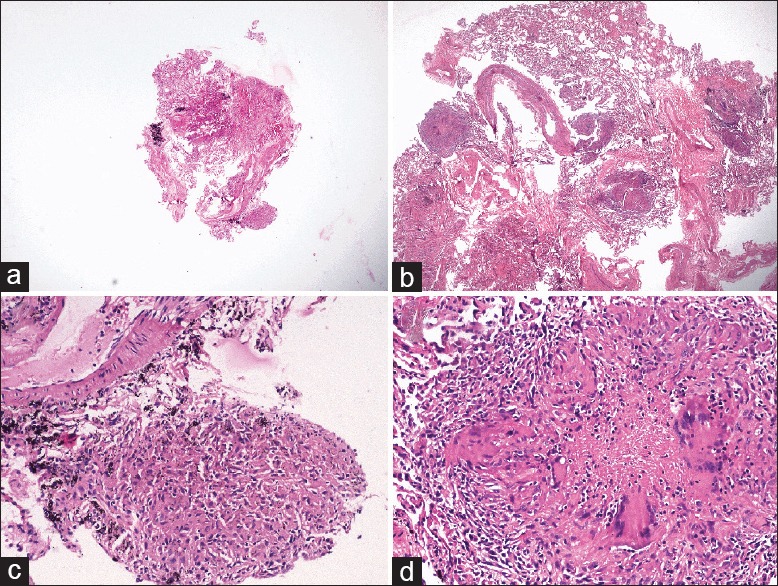
(a and b) Photomicrographs showing comparison of sizes of the flexible FB and CB under scanner view [hematoxylin and eosin (H and E), ×40], (c) FB showing single focus of loose collection of epithelioid cells forming granuloma (H and E, ×200), (d) CB showing well-formed epithelioid cell granuloma with Langhans giant cells and central necrosis with nuclear debris (H and E, ×200)
DISCUSSION
This case emphasizes the utility of cryo-TBLB in the diagnosis of diffuse lung disease. The use of a cryoprobe yields a larger specimen, which may prove to be diagnostically more useful than the small biopsies obtained with the conventional flexible forceps, as illustrated in this case. This is the first report of the use of cryo-TBLB in differentiating between pulmonary tuberculosis and sarcoidosis. To our knowledge, this is also the first description of the performance of cryo-TBLB from the Indian subcontinent, although the use of a cryoprobe for other purposes, such as removal of a tracheobronchial blood clot, has been described previously.[17]
The two major advantages of CB over FB are the larger size of the specimen and the absence of significant artifacts.[15] The larger size of the specimen not only provides a larger alveolated area and larger number of alveoli, but it also increases the probability of sampling the pathological tissue.[18] In disorders such as usual interstitial pneumonia, where pathological patterns such as temporal and spatial heterogeneity cannot be identified on small tissue specimens obtained with FB, the larger tissue obtained with CB offers a superior alternative and has been observed to increase the diagnostic yield from around 30% to over 90%.[19,20] In our patient, the larger size of the tissue not only yielded a larger number of granulomas (nine with CB versus one with FB), it also allowed identification of the necrosis inside the granulomas that was absent in the granuloma sampled with FB. This clinched the diagnosis of tuberculosis, as necrotizing granulomas are a rare occurrence in sarcoidosis.[21] Of several pathological characteristics, the presence of necrosis is the only morphologic finding that, in the absence of acid-fast bacilli, is specific enough to suggest a tubercular etiology of the granulomatous inflammation over sarcoidosis.[10] The absence of angiitis on a large lung biopsy also helped in ruling out the rare possibility of necrotizing sarcoid granulomatosis.[22]
In the current case, we performed only one biopsy with the cryoprobe, in contrast to six biopsies with the flexible forceps. Studies describing CB have reported anywhere from one to five biopsies being performed.[14,18] In our opinion, the number of biopsies obtained can be decided by the operator depending on the size of the biopsy obtained and the requirement of sampling from multiple bronchopulmonary segments of the same or different lobes, as in patients with ILD. In our case, the large tissue specimen obtained prompted us to limit the procedure to one CB attempt.
Several studies have shown that CB as compared to FB is remarkably free of artifacts.[14,15,23] This might be another factor contributing to an increase in the yield of CB. Only one previous study has described cold-related artifacts, a finding specific to CB.[19] We did not encounter any cold-related artifacts in the CB tissue, as the tissue was thawed properly before being transferred to formalin; there was the presence of minor crush artifacts in the FB tissue.
Due to the large size of the lung tissue obtained with CB, there may be the concern of a higher chance of bleeding and pneumothorax.[24] The incidence of pneumothorax described in previous studies is acceptable and only slightly higher than that encountered with FB (barring one study in which cryo-TBLB was performed in fibrotic diffuse lung disease, resulting in 28% incidence of pneumothorax).[19,25] The risk of pneumothorax can be minimized by the universal use of fluoroscopy during this procedure. The probe should be placed at least 20 mm away from the rib cage to avoid rupturing the visceral pleura.[13] We adhered meticulously to this protocol and were able to avoid the occurrence of pneumothorax.
Most studies show that the bleeding that accompanies the procedure is usually mild to moderate and gets easily controlled with routine measures, although severe bleeding has been described.[26] In the present case, too, the bleeding was only mild and was easily controlled by wedging the bronchoscope into the concerned segment. It is noteworthy that several previous studies have described the performance of CB in patients after placement of an artificial airway, namely the endotracheal tube, rigid bronchoscope, or laryngeal mask airway.[14,15] The artificial airway allows for easy suctioning and can prevent spillage of blood into the nonbiopsied side. Recently, Fruchter et al. have also described the safe performance of cryo-TBLB under conscious sedation without the use of artificial airway.[27] We have described a novel procedure with the use of two bronchoscopes wherein the second bronchoscope is immediately introduced into the airways after withdrawal of the first to control the postbiopsy bleeding by wedging the sampled bronchopulmonary segment and employing local measures such as instillation of ice-cold saline or adrenaline. The risk of bleeding is still present with our method due to the time required for placement of the second bronchoscope. Thus, our technique can be considered as betwixt and between avoiding an artificial airway (requiring operating room/anesthetist) with negligible risk of bleeding and an increased risk of bleeding while using a single bronchoscope.
CONCLUSION
In conclusion, TBLB with a cryoprobe is a novel technique that provides larger lung tissue specimens, which may be useful in the diagnosis of conditions requiring larger tissue volume, such as in differentiating between pulmonary tuberculosis and pulmonary sarcoidosis, chronic fibrosing interstitial pneumonias, and others. The procedure can be safely performed under conscious sedation in a spontaneously breathing patient without the use of an artificial airway.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gotway MB, Reddy GP, Webb WR, Elicker BM, Leung JW. High-resolution CT of the lung: Patterns of disease and differential diagnoses. Radiol Clin North Am. 2005;43:513–42. doi: 10.1016/j.rcl.2005.01.010. viii. [DOI] [PubMed] [Google Scholar]
- 2.Lad D, Malhotra P, Maskey D, Santhosh S, Mittal BR, Das A, et al. Pyrexia, lung nodules, granulomas: Pulmonary lymphomatoid granulomatosis. Indian J Hematol Blood Transfus. 2014;30(Suppl 1):418–21. doi: 10.1007/s12288-014-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchiori E, Zanetti G, Barreto MM, de Andrade FT, Rodrigues RS. Atypical distribution of small nodules on high resolution CT studies: Patterns and differentials. Respir Med. 2011;105:1263–7. doi: 10.1016/j.rmed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Gupta D. Tuberculosis and sarcoidosis: The continuing enigma. Lung India. 2009;26:1–2. doi: 10.4103/0970-2113.45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and tuberculosis: The same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18:506–16. doi: 10.1097/MCP.0b013e3283560809. [DOI] [PubMed] [Google Scholar]
- 6.Heinle R, Chang C. Diagnostic criteria for sarcoidosis. Autoimmun Rev. 2014;13:383–7. doi: 10.1016/j.autrev.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 9.El-Zammar OA, Katzenstein AL. Pathological diagnosis of granulomatous lung disease: A review. Histopathology. 2007;50:289–310. doi: 10.1111/j.1365-2559.2006.02546.x. [DOI] [PubMed] [Google Scholar]
- 10.Cancellieri A, Leslie KO, Tinelli C, Patelli M, Trisolini R. Sarcoidal granulomas in cytological specimens from intrathoracic adenopathy: Morphologic characteristics and radiographic correlations. Respiration. 2013;85:244–51. doi: 10.1159/000345386. [DOI] [PubMed] [Google Scholar]
- 11.Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration. 2009;78:203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 12.Fruchter O, Fridel L, Rosengarten D, Rahman NA, Kramer MR. Transbronchial cryobiopsy in immunocompromised patients with pulmonary infiltrates: A pilot study. Lung. 2013;191:619–24. doi: 10.1007/s00408-013-9507-z. [DOI] [PubMed] [Google Scholar]
- 13.Kropski JA, Pritchett JM, Mason WR, Sivarajan L, Gleaves LA, Johnson JE, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013;8:e78674. doi: 10.1371/journal.pone.0078674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarmus L, Akulian J, Gilbert C, Illei P, Shah P, Merlo C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: A pilot safety study. Chest. 2013;143:621–6. doi: 10.1378/chest.12-2290. [DOI] [PubMed] [Google Scholar]
- 15.Pajares V, Puzo C, Castillo D, Lerma E, Montero MA, Ramos-Barbon D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: A randomized trial. Respirology. 2014;19:900–6. doi: 10.1111/resp.12322. [DOI] [PubMed] [Google Scholar]
- 16.Kaur H, Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. A randomized trial of 1% vs.2% lignocaine by the spray-as-you-go technique for topical anesthesia during flexible bronchoscopy. Chest. 2015 doi: 10.1378/chest.15-0022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal IS, Dhooria S, Agarwal R, Behera D. Use of a flexible cryoprobe for removal of tracheobronchial blood clots. Respir Care. 2015;60:e128–31. doi: 10.4187/respcare.03861. [DOI] [PubMed] [Google Scholar]
- 18.Pourabdollah M, Shamaei M, Karimi S, Karimi M, Kiani A, Jabbari HR. Transbronchial lung biopsy: The pathologist's point of view. Clin Respir J. 2015 doi: 10.1111/crj.12207. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomassetti S, Cavazza A, Colby TV, Ryu JH, Nanni O, Scarpi E, et al. Transbronchial biopsy is useful in predicting UIP pattern. Respir Res. 2012;13:96. doi: 10.1186/1465-9921-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binesh F, Halvani H, Navabii H. Systemic sarcoidosis with caseating granuloma. BMJ Case Rep. 2012:2012. doi: 10.1136/bcr.05.2011.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quaden C, Tillie-Leblond I, Delobbe A, Delaunois L, Verstraeten A, Demedts M, et al. Necrotising sarcoid granulomatosis: Clinical, functional, endoscopical and radiographical evaluations. Eur Respir J. 2005;26:778–85. doi: 10.1183/09031936.05.00024205. [DOI] [PubMed] [Google Scholar]
- 23.Pajares V, Torrego A, Puzo C, Lerma E, Gil De Bernabe MA, Franquet T. Transbronchial lung biopsy using cryoprobes. Arch Bronconeumol. 2010;46:111–5. doi: 10.1016/j.arbres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Poletti V, Casoni GL, Gurioli C, Ryu JH, Tomassetti S. Lung cryobiopsies: A paradigm shift in diagnostic bronchoscopy? Respirology. 2014;19:645–54. doi: 10.1111/resp.12309. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Role of ultrasonography in the diagnosis and management of pneumothorax following transbronchial lung biopsy. J Bronchology Interv Pulmonol. 2015;22:14–9. doi: 10.1097/LBR.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 26.Hagmeyer L, Theegarten D, Wohlschlager J, Treml M, Matthes S, Priegnitz C, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2015 doi: 10.1111/crj.12261. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Fruchter O, Fridel L, Rosengarten D, Raviv Y, Rosanov V, Kramer MR. Transbronchial cryo-biopsy in lung transplantation patients: First report. Respirology. 2013;18:669–73. doi: 10.1111/resp.12037. [DOI] [PubMed] [Google Scholar]


