Abstract
Inflammatory myofibroblastic tumors (IMT) are uncommon neoplasms of the lung in adults. They constitute less than 1% of all lung neoplasms and usually present as parenchymal masses. Diagnosis requires a high index of suspicion. They are characterized by spindle-shaped tumor cells (fibroblasts/myofibroblasts) in a background of lymphoplasmacytic infiltrate. About 50% of the tumors harbor an ALK gene rearrangement. They have to be differentiated from inflammatory pseudotumors (IPT), which show increased number of IgG4 plasma cells on immunostaining and are negative for anaplastic lymphoma kinase (ALK) protein. Herein, we present a case of a 28-year old female who presented with hemoptysis and was diagnosed with an IMT of lung in the first trimester of pregnancy. We have not only reviewed the occurrence of IMT during pregnancy but also discuss the management options for IMT during pregnancy.
KEY WORDS: Carcinoid, inflammatory myofibroblastic tumor, lung tumor, pregnancy, surgical resection
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT), the most common pulmonary neoplasm in children, is an uncommon neoplasm of the respiratory tract in adults.[1,2] It can occur at any site of the body, lung being one of the common sites of origin. Although a benign neoplasm on pathology, the clinical presentation of IMT is variable. In a majority of the patients, IMT presents as a benign, slow-growing tumor without local invasion or distant metastases. However, in upto 5% of the patients it can present as an aggressive locally invasive tumor or a metastatic disease.[3,4] The symptoms at clinical presentation and radiologic features are non-specific and diagnosis of these tumors requires a high index of suspicion. Most tumors present as lobulated masses with distinct margins in the peripheral parenchyma. They have a predilection for lower lobe involvement. Calcification, heterogeneous attenuation and contrast enhancement may be present.[5] An accurate diagnosis of these lesions can only be made after histopathology and immunohistochemical analysis. It is important to differentiate IMT from its closest histopathologic differential, the IgG4-related inflammatory pseudo tumor (IPT) as both share common histopathologic features of prominent fibroblastic/myofibroblastic proliferation in a background of inflammatory cells (plasma cells and lymphocytes).[6]
Complete surgical resection is considered to be the treatment of choice for IMT of the thorax.[7,8] Other modalities which have been tried include bronchoscopic resection (for endotracheal lesions),[9] steroids, ALK inhibitor crizotinib[10] and non-steroidal anti-inflammatory drugs (NSAIDS).[11] In this report, we describe a 28-year-old lady who was diagnosed with an IMT during the first trimester of her pregnancy and was managed with surgical resection. We also discuss the possible therapeutic options for management during pregnancy.
CASE REPORT
A 28-year-old lady presented with dry cough for 2 years and hemoptysis for 1 week. She had three episodes of hemoptysis (100 ml per episode). There was no history of breathlessness, fever, anorexia or weight loss. She was in the first trimester of her third pregnancy. Her chest examination was unremarkable. A chest radiograph showed a well-circumscribed lesion in the left hilar region. A non-contrast computed tomography scan revealed a left hilar mass lesion causing abrupt cut off of the left lower lobe bronchus [Figure 1a]. The fat planes with the adjacent mediastinal structures were well-preserved. The patient was subjected to flexible bronchoscopy, which showed a vascular growth occluding the left lower lobe bronchus. In view of the clinical presentation with massive hemoptysis and the presence of a vascular growth occluding the bronchus, a clinical suspicion of carcinoid tumor was considered. An endobronchial biopsy was obtained using an electrocoagulation-enabled biopsy forceps with the application of electrocoagulation current (40 W for 10 seconds in a monopolar mode). The histopathologic examination of the biopsy specimen showed a tumor composed of a mixture of spindle cells arranged in short and long fascicles [Figure 1b]. These cells were positive for smooth muscle actin (SMA) confirming their myofibroblastic nature. The tumor stroma showed an inflammatory infiltrate which was predominantly composed of plasma cells. There were no mitoses or necrosis seen. Immunostaining for anaplastic lymphoma kinase (ALK) antigen was positive [Figure 1c] in the tumor cells and the IgG4 stain did not highlight excess of IgG4-positive plasma cells, thus confirming the diagnosis of an inflammatory myofibroblastic tumor.
Figure 1.
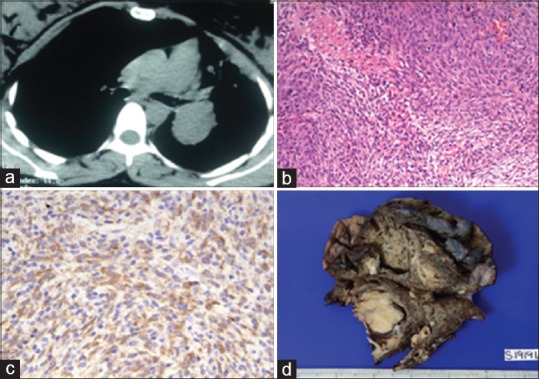
(a) Computed tomography image (axial view) showing a well-defined mass lesion (4.5 × 3.5 × 4.4 cm) occluding the left lower lobe bronchus and causing partial obstruction of the left upper lobe bronchus (b) Photomicrograph showing spindle-shaped tumor cells arranged in short and long fascicles admixed with few lymphoplasmacytic cells. (H and E, x200) (c) Photomicrograph showing granular cytoplasmic positivity for ALK (ALK immunostaining, x400) (d) Gross photograph of left lower lobectomy specimen, showing circumscribed greyish white tumor measuring 4 × 4 cm and occluding the main bronchus
She had ongoing hemoptysis despite being treated with cough suppressants and antifibrinolytic agents (tranexamic acid). The treatment options were discussed with the patient and she opted for surgical resection following a medical termination of pregnancy (MTP). The resected left lower lobectomy specimen showed a circumscribed greyish white tumor (4 × 4 × 3 cm) occluding the left lower lobe main bronchus [Figure 1d]. Histopathological examination showed a tumor with similar morphology and immunohistochemical profile as endobronchial biopsy. The bronchial resection margin and the lymph nodes were free of tumor. The patient is currently asymptomatic and on follow-up.
DISCUSSION
To the best of our knowledge, this is the second case report of IMT diagnosed during pregnancy. Amir et al. described the first case of a 21-year-old lady in her first trimester with an IMT of the trachea who was managed with endoscopic resection of the tumor.[12] The management of IMT of the lung during pregnancy is difficult as the therapeutic options are limited. Crizotinib is considered a category D drug for use during pregnancy. However, there have been reports of successful use of crizotinib for lung cancer in pregnancy.[13] Celecoxib is also considered a category C drug (Category D if used beyond 30 weeks of gestation) for use during pregnancy. Though steroids can be used safely in pregnancy, use in the first trimester has been associated with an increased risk of cleft lip. Also, the clinical response of IMT to steroids is inconsistent. Surgery during pregnancy is not without complications. Second trimester is considered the optimal time for non-obstetric surgery during pregnancy. Other options include endoscopic therapy (for endotracheal lesions) and close monitoring with definitive procedure performed after delivery (for asymptomatic or minimally symptomatic patients). As the index patient had ongoing life-threatening hemoptysis and a large mass with significant extraluminal component, it was decided to proceed for surgery at the earliest. The therapeutic decisions for the management of IMT in pregnancy should be individualized. The management of IMT during pregnancy depends on the patient's symptoms, the duration of pregnancy and the location of tumor.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oztuna F, Pehlivanlar M, Abul Y, Tekinbas C, Ozoran Y, Ozlu T. Adult inflammatory myofibroblastic tumor of the trachea: Case report and literature review. Respir Care. 2013;58:e72–6. doi: 10.4187/respcare.02198. [DOI] [PubMed] [Google Scholar]
- 2.Jindal A, Bal A, Agarwal R. Inflammatory myofibroblastic tumor of the trachea in the pediatric age group. Case report and systematic review of the literature. J Bronchology Interv Pulmonol. 2015;22:58–65. doi: 10.1097/LBR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 3.Carillo C, Anile M, De Giacomo T, Venuta F. Bilateral simultaneous inflammatory myofibroblastic tumor of the lung with distant metastatic spread. Interact Cardiovasc Thorac Surg. 2011;13:246–7. doi: 10.1510/icvts.2011.271932. [DOI] [PubMed] [Google Scholar]
- 4.van den Heuvel DA, Keijsers RG, van Es HW, Bootsma GP, de Bruin PC, Schramel FM, et al. Invasive inflammatory myofibroblastic tumor of the lung. J Thorac Oncol. 2009;4:923–6. doi: 10.1097/JTO.0b013e3181a76e28. [DOI] [PubMed] [Google Scholar]
- 5.Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719–29. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat P, Bal A, Das A, Singh N, Singh H. Pulmonary inflammatory myofibroblastic tumor and IgG4-related inflammatory pseudotumor: A diagnostic dilemma. Virchows Arch. 2013;463:743–7. doi: 10.1007/s00428-013-1493-2. [DOI] [PubMed] [Google Scholar]
- 7.Melloni G, Carretta A, Ciriaco P, Arrigoni G, Fieschi S, Rizzo N, et al. Inflammatory pseudotumor of the lung in adults. Ann Thorac Surg. 2005;79:426–32. doi: 10.1016/j.athoracsur.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Kim JS, Choi YS, Kim K, Shim YM, Han J, et al. Treatment of inflammatory myofibroblastic tumor of the chest: The extent of resection. Ann Thorac Surg. 2007;84:221–4. doi: 10.1016/j.athoracsur.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Ray A, Suri JC, Bhattacharya D, Gupta A. Bronchoscopic resection of endobronchial inflammatory myofibroblastic tumor: A case report and systematic review of the literature. Lung India. 2014;31:172–5. doi: 10.4103/0970-2113.129866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tothova Z, Wagner AJ. Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors. Curr Opin Oncol. 2012;24:409–13. doi: 10.1097/CCO.0b013e328354c155. [DOI] [PubMed] [Google Scholar]
- 11.Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: A case report and review of the literature. Oncol Lett. 2013;5:1672–6. doi: 10.3892/ol.2013.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amir R, Danahey D, Ferrer K, Maffee M. Inflammatory myofibroblastic tumor presenting with tracheal obstruction in a pregnant woman. Am J Otolaryngol. 2002;23:362–7. doi: 10.1053/ajot.2002.128041. [DOI] [PubMed] [Google Scholar]
- 13.Sariman N, Levent E, Yener NA, Orki A, Saygi A. Lung cancer and pregnancy. Lung Cancer. 2013;79:321–3. doi: 10.1016/j.lungcan.2012.11.014. [DOI] [PubMed] [Google Scholar]


