Abstract
Objective
Deficient retro-aortic rim has been identified as a risk factor for device erosion following trans-catheter closure of atrial septal defects (ASD). Transthoracic echocardiography (TTE) is the primary screening method for subjects for possible device closure of ASD, but its reliability in measuring retro-aortic rim size has not been assessed previously.
Design
A single institution cross-sectional analysis of children and adults referred for trans-catheter device closure of single ostium secundum ASD from January 1, 2005 to April 1, 2012 with reviewable TTE and trans-esophageal echocardiogram (TEE) images was performed. Inter-rater reliability of measurements was tested in a 24% sample. Accuracy of TTE measurement of retro-aortic rim was assessed using a Bland-Altman plot with TEE measurement as the gold standard. Test characteristics of TTE detection of deficient retro-aortic rim were calculated. Risk factors for misclassification of deficient retro-aortic rim were assessed using receiver operator characteristic curves (ROCC). Risk factors for measurement error were assessed through multivariate linear regression.
Results
In total, 163 subjects of median age 5 years (range: 0.3–46 years) were included. Trans-thoracic echocardiography had 90% sensitivity, 84% specificity, 90% positive predictive value, and 83% negative predictive value to detect deficient retro-aortic rim. Bland-Altman plot demonstrated no fixed bias (p=0.23), but errors in measurement increased on average as the aortic rim increased in size (p<0.001). Pre-specified patient level risk factors did not affect ROCC area under the curve, nor were any patient-level risk factors independently associated with increased measurement error on TTE.
Conclusions
TTE is a sensitive and specific screening test for deficient retro-aortic rim across a range of patient ages and sizes.
Keywords: Atrial septal defect, heart catheterization, pediatric cardiology, echocardiography
Introduction
Since King and colleagues first demonstrated the feasibility of device closure of atrial septal defects (ASD)1, accurate assessment of intra-cardiac anatomy has been an important challenge for interventional cardiologists. Early studies of pathological specimens and animal models defined the importance of circumferential tissue rims upon which to seat early devices2. The advent of two-dimensional echocardiography has enabled non-invasive measurement of intra-cardiac anatomy. Measurement of defect size on trans-thoracic (TTE) and trans-esophageal echocardiograms (TEE) has been validated against direct measurement in the operating room3–6.
Interest in measurement of the retro-aortic tissue rim is recent, fueled by concern about device erosion. Retrospective review of erosion cases identified deficient retro-aortic rim (rim less than 5 mm) as a potential risk factor for ersosion7,8. At the same time, case series have demonstrated that device closure in children with deficient retro-aortic rim can be accomplished with high rates of technical success and low rates of complication13–15. However, because of the potentially catastrophic results of device erosion, recommendations regarding use of the ASO device in the face of deficient retro-aortic rim continue to evolve9,10,16–18, and there is uncertainty regarding use of the ASO device for patients with deficient retro-aortic rim. TTE measurements of defect size, septal length, and tissue rims remain important in planning of ASD closure, determining 1) whether patients should be referred to the catheterization laboratory, 2) whether a strategy of “surgical back-up”, i.e. a plan to transition to operative closure if device closure is not feasible, should be employed to avoid risk and economic cost of multiple exposures to anesthesia and potentially admissions to hospital, and 3) to choose a closure devices to both optimize technical success and minimize risk of aortic erosion and other adverse events. To pursue this strategy, establishing the reliability of TTE to measure these features is vital. However, previous research about reliability and validity of measurements from transthoracic echocardiography has focused on defect size, and to our knowledge no studies have assessed the reliability of TTE measurement of retro-aortic rim.
The accuracy of screening TTE is, therefore, important not only in terms of its correlation with TEE but also 1) whether there is consistent over- or under-estimation of aortic rim in TTE measurements and 2) whether there are patient-level factors that affect the accuracy of TTE measurements. A single center cross-sectional study with retrospective data collection was undertaken to address these questions.
Methods
Study population
The study protocol was approved by the institutional review board of The Children’s Hospital of Philadelphia, and waiver of consent was granted. We conducted a cross-sectional study with retrospective data collection. All children and adults referred for possible device closure of ASD between January 1, 2005 and April 1, 2012 at The Children’s Hospital of Philadelphia were assessed for inclusion. Additional inclusion criteria included confirmation of single ostium secundum ASD and reviewable pre-procedural TTE and procedural TEE. No additional exclusion criteria were applied. Subjects were identified through our center’s cardiac catheterization laboratory and cardiac surgical databases.
Study procedures
Medical records were reviewed including pre-catheterization assessments and catheterization reports. Eligibility was confirmed during this review, and subject demographics (age, sex, and race), growth parameters (height, weight, body surface area, and body mass index), and clinical history (cardiac and non-cardiac medical and operative history) were extracted. During the study period, children and adults undergoing catheterization for possible device closure uniformly underwent pre-procedural TTE followed by a procedural TEE. Approach to catheterization, device selection, and follow-up was consistent across subjects in our institution over the study period. Pre-procedural TTE and procedural TEE images were reviewed by a single member of the study staff (MLO), blinded to the measurements made on the clinical study. Measurements from TTE and TEE in the same patient were not compared during data collection. On TTE, septal length and defect size in orthogonal views (subcostal frontal and sagittal) and tissue rims (right upper pulmonary vein, superior vena cava, inferior vena cava, mitral valve, and aorta) were measured. On procedural TEE, septal length, retro-aortic rim, maximal defect size, and defect diameter during balloon inflation using the stop-flow technique were measured. Across modalities, deficient retro-aortic rim was defined as a retro-aortic rim measuring less than 5 mm in any view as has been described previously8. Retro-aortic rim measurements were typically performed in the para-sternal short view on TTE and 45-degree view on TEE. With both modalities, a sweep was performed and the retro-aortic rim was measured in the frame in which it had the smallest length.
Analysis
Standard descriptive statistics were calculated. Continuous variables are expressed as mean ± standard deviation or median (range and interquartile range (IQR)) as appropriate. Categorical variables are described as proportions and counts.
To assess inter-rater reliability, 40 pre-procedural TTE studies were selected at random and measurements were performed by another member of the study staff (DJG), who was blinded to initial study measurements. This represented a 24% sample of the 166 digitally available TTE-TEE pairs. Intra-class correlation coefficients for continuous variables and simple kappa statistic for dichotomous variables were calculated.
The primary goal of the study was to assess the accuracy of measurement of retro-aortic rim on pre-procedural TTE, with TEE as the gold standard measurement. This was assessed through 1) univariate linear regression, a Bland-Altman plot, and receiver operator characteristic curves(ROCC). Univariate linear regression provided a measurement of correlation (r2) as well as the magnitude of error between TTE and TEE with the slope of the regression represented by the slope of the resultant regression equation. A Bland-Altman plot was produced to visually assess changes in inter-test agreement over the range of measurements11 and to assess for bias. The mean difference was calculated, including confidence interval. Fixed bias, bias across the range of rim sizes, was assessed through a one sample Student’s t-test of the difference of mean difference. Proportional bias, or bias that changed over the range of rim sizes, was assessed through linear regression of the difference between TTE and TEE and the mean of the two measurements. Because the focus of most studies to date has been to dichotomize retro-aortic rim between sufficient insufficient rims, we also assessed the ability of TTE to discriminate between the two, compared to TEE. To do this, test characteristics of TTE (sensitivity, specificity, positive predictive value, and negative predictive value) were calculated, and a ROCC was plotted (for detection of sufficient retro-aortic rim to simplify presentation). To assess whether patient characteristics affected the performance of TTE, ROCC areas under the curve (AUC) were compared using chi-squared test for a series of comparisons based on pre-identified patient level characteristics. These characteristics (age, sex, race, height, weight, body mass index (BMI), and defect size (measured in subcostal frontal) were identified prior to analysis as potential risk factors. Cut points were chosen based on visual inspection of scatterplots of the candidate covariate and the difference between TTE and TEE measurements of retro-aortic rim. If no obvious cut point was seen, the 25th, 50th, and 75th percentiles of the distribution of the covariates were used.
A second exploratory analysis was performed to assess the effect of covariates in magnitude of measurement error for retro-aortic rim size using multivariate linear regression. Difference was measured in two ways 1) the difference between TEE and TTE with sign of difference retained, i.e. with negative numbers for over-estimation of defect size by TTE and positive numbers for under-estimation of defect size and 2) the absolute difference between TEE and TTE measurements. The former models the difference in measurements as a continuum of over- and under-estimation, and assumes that there may be systematic over- or under-estimation of rim size by TTE. The latter assumes that error is random around a mean of zero. The same potential covariates considered in the ROCC analysis were identified prior to model building and were included based on clinical interest, rather than a bi-variable screening strategy12 with all factors retained in the final model.
Since the cohort size was fixed, no formal procedures were performed to calculate statistical power. No formal compensation for multiple comparisons was performed. The degree of uncertainty or dispersion in point estimates is represented in 95% confidence intervals (95% CI) where appropriate. Case based deletion was used to account for missing data. A threshold for statistical significance was set at p<0.05. All data analysis was performed using Stata SE v12 (Statacorp, College Station, TX).
Results
Study population
One hundred and sixty-six subjects met criteria for inclusion. Their demographic and clinical data are summarized in Table 1.
Table 1.
Subject characteristics
| N=166 | ||
|---|---|---|
| Age (years) | 5 (range: 0.3–46, IQR: 4–12) | |
| Female sex % (n) | 58% (96) | |
| Weight (kg) | 19.4 (range: 6.2–119.2, IQR: 25.2–47.3) | |
| Height (cm) | 110 (range: 66–186, IQR: 98–153) | |
| Body mass index | 16.5 (range: 12.9–40.1, IQR: 15.3–19.8) | |
| Race % (n) | ||
| White | 69% (114) | |
| African-American | 12% (19) | |
| Asian | 5% (8) | |
| Other | 15% (25) | |
| TTE Measurements | ||
| Defect size in frontal (mm) | 9.9 (range: 1.0–19.6, IQR: 7.5–11.9) | |
| Defect size in sagittal (mm) | 9.9 (range: 3.9–21.6, IQR: 7.8–12.1) | |
| Septal length in frontal (mm) | 39.3 (range: 11.2–65.5, IQR: 33.1–45.6) | |
| Septal length in sagittal (mm) | 37.7 (range: 18.4–63.3, IQR: 30.4–45.1) | |
| Retro-aortic rim (mm) | 4.0 (range: 0.8–15.3, IQR: 2.6–6.2) | |
| Deficient retro-aortic rim % (n) | 63% (104) | |
| TEE Measurements | ||
| Maximum defect size (mm) | 10.5 (range: 1.0–28 IQR: 8.2–13.9) | |
| Maximum septal length (mm) | 31.3 (range: 19.4–49.9, IQR: 27.3–35.8) | |
| Retro-aortic rim (mm) | 4.0 (range 0.04–19.0, IQR: 2.8–6.3) | |
| Deficient retro-aortic rim % (n) | 63% (105) | |
| Defect size on balloon inflation | 14 (range: 4–42.6, IQR: 11.7–21.8) |
Data are presented as median with (range and IQR) or percentage of total (count); Abbreviations: IQR: Inter-quartile range, TEE trans-esophageal echocardiogram, TTE transthoracic echocardiogram
Inter-rater Reliability
A sample of 40 subjects (24% of cohort) were used to assess inter-rater reliability of TTE measurements (Table 2). Inter-rater reliability of measurement of the retro-aortic rim was high (0.87). Inter-rater agreement for the diagnosis of deficient retro-aortic rim was 95%, with an unadjusted kappa statistic of 0.88, p<0.001.
Table 2.
Inter-rater reliability of TTE measurements
| Measurement | Intra-class coefficient |
|---|---|
| Septum in subcostal frontal | 0.62 |
| Defect in subcostal frontal | 0.53 |
| Septum in subcostal sagittal | 0.50 |
| Defect in subcostal sagittal | 0.62 |
| RUPV rim | 0.82 |
| Mitral valve rim | 0.74 |
| IVC rim | 0.73 |
| SVC rim | 0.59 |
| Retro-aortic rim | 0.87 |
Abbreviations: IVC inferior vena cava, RUPV right upper pulmonary vein, SVC super vena cava Agreement of diagnosis of deficient retro-aortic rim (<5mm) was 94.9%, unadjusted kappa=0.88, p<0.0001.
Comparison of retro-aortic rim measurements between TTE and TEE
Assessment of deficient retro-aortic rim on TTE demonstrated 90% sensitivity, 84% specificity, 90% positive predictive value, and 83% negative predictive value. The AUC of ROCC for TTE measurement of retro-aortic rim was 0.9 (Figure 1). Univariate linear regression (Table 3 and Figure 2) demonstrated moderate correlation between TTE and TEE measurements with TTE measurements overestimating retro-aortic rim (beta=0.93, 95% CI: 0.81–1.05, r-squared=0.6, p<0.001). A Bland-Altman plot was generated (Figure 3). On this, the limits of agreement were −4.0 to 3.7. Mean difference is 0.18 mm with a standard deviation of 1.9 mm (95% CI: −0.12 to 0.47). There was no significant evidence of fixed bias (p=0.23). There was evidence of proportional bias, and the difference between TTE and TEE measurements increased by 0.21 mm per mm increase in mean measurement of retro-aortic rim (p<0.001).
Figure 1. Receiver operator characteristic curve of TTE measurement of retro-aortic rim against TEE diagnosis of deficient retro-aortic rim.

Table 3.
Correlation between TTE and TEE measurements
| Measure | r2 | Beta | 95% CI | p | |
|---|---|---|---|---|---|
| Retro-aortic rim | 0.6 | 0.93 | 0.82 to 1.1 | <0.001 | |
| Defect size | |||||
| Subcostal frontal vs. maximum size on TEE | 0.22 | 0.54 | 0.38 to 0.70 | <0.001 | |
| Subcostal sagittal vs. maximum size on TEE | 0.28 | 0.67 | 0.50 to 0.83 | <0.001 | |
| Apical 4 chamber vs. maximum size on TEE | 0.32 | 0.58 | 0.44–0.71 | <0.001 | |
| Subcostal frontal vs. balloon size on TEE | 0.29 | 0.77 | 0.57 to 0.96 | <0.001 | |
| Subcostal sagittal vs. balloon size on TEE | 0.26 | 0.79 | 0.58 to 1.01 | <0.001 | |
| Apical 4 chamber vs. balloon size on TEE | 0.31 | 0.70 | 0.53 to 0.88 | <0.001 | |
| Septal length | |||||
| Subcostal frontal vs. maximum length on TEE | 0.19 | 0.31 | 0.21 to 0.40 | <0.001 | |
| Subcostal sagittal vs. maximum length on TEE | 0.22 | 0.32 | 0.22 to 0.42 | <0.001 | |
| Apical 4 chamber vs. maximum length on TEE | 0.28 | 0.40 | 0.29 to 0.50 | <0.001 | |
Abbreviations: CI confidence interval, TEE tran-esophageal echocardiogram, TTE trans-thoracic echocardiogram
Correlation between TTE and TEE measurements assessed by univariate linear regression.
Figure 2. TTE vs. TEE measurement of retro-aortic rim.
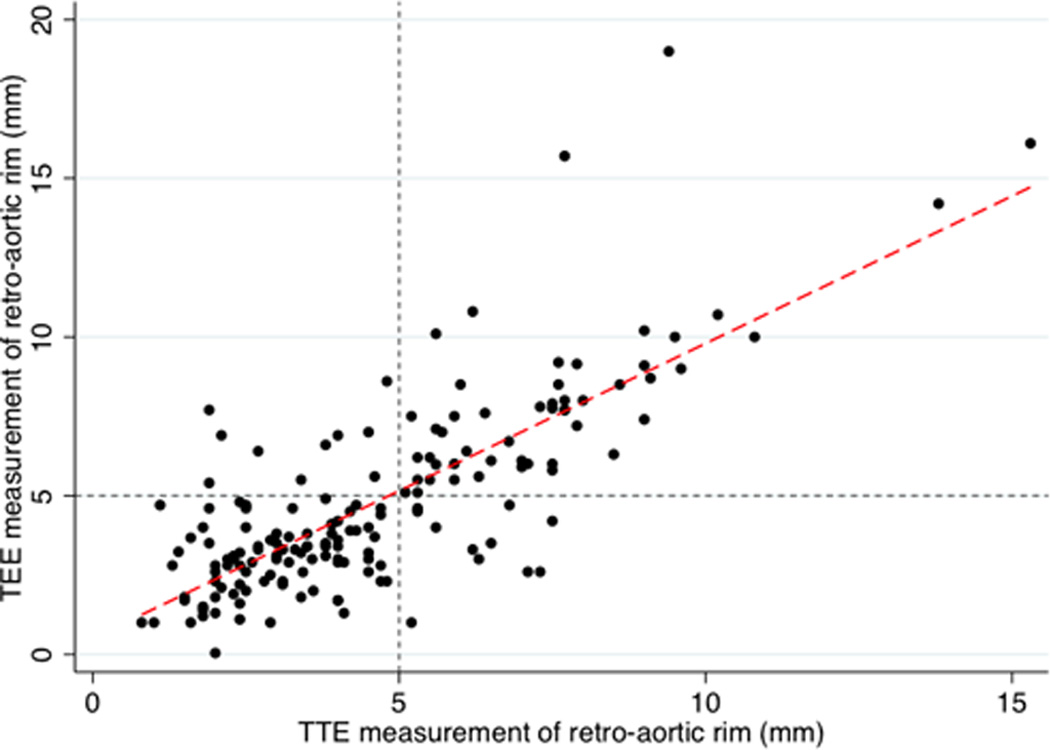
Individual level data for both measurements of retro-aortic rim are plotted (circles). Regression line is plotted (red dash) beta=0.9, r2=0.6, p<0.001.
Gray small dashes mark retro-aortic rim of 5 mm for TTE (vertical line) and TEE (horizontal line). These divide the grid into quadrants representing the relative distribution of accurate and inaccurate classification of aortic rim. Observations in lower left quadrant represent true positives for deficient retro-aortic rim, while those in the upper right quadrant are true negatives for rim deficiency (i.e. true positives for sufficient retro-aortic rim). Observations in the upper left quadrant (false positive) and lower right (false negative) represent inaccurate classification of retro-aortic rim by TTE measurement.
Figure 3. Bland-Altman plot of TTE vs. TEE measurement of retro-aortic rim.
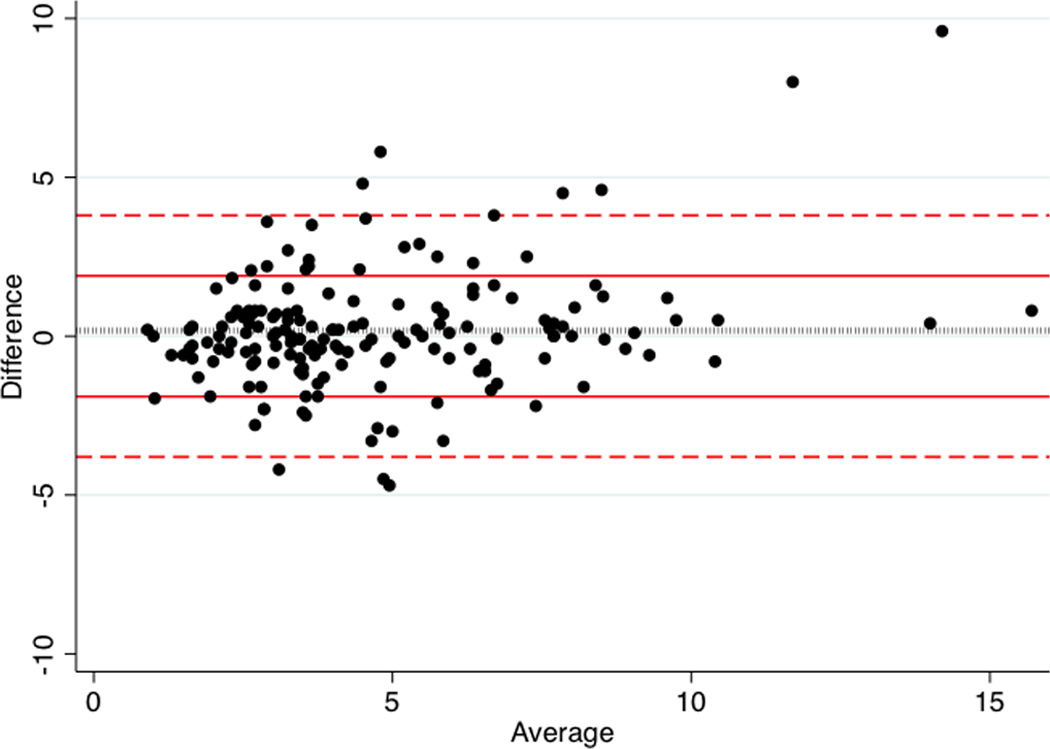
Average of TTE and TEE measurements (x-axis) and difference between TTE and TEE measurements (y-axis) are plotted for each subject (black circle). Bounds for one (red solid lines) and two standard deviations (red dashed lines) for difference are plotted, as is the mean difference between TTE and TEE (black dotted line). Limits of agreement are −4.0 to 3.7. Mean difference is −0.18±1.9mm (95% CI: 0.47–0.12).
Correlation of other measurements on TTE and TEE
Other TTE measurements were compared with those on TEE measurements. Measurements of defect size and septal length in three TTE projections (subcostal frontal, subcostal sagittal, and apical 4 chamber) all were correlated with TEE maximum dimension (Table 3).
Additional factors influencing association of TTE and TEE measurements of retro-aortic rim
Candidate risk factors for poor performance of TTE in measuring retro-aortic rim were assessed and comparisons were made between the resultant ROCC curves. Subdividing the cohort by pre-specified risk factors did not result in significant change in the ROCC AUC (Table 4).
Table 4.
Effect of patient level characteristics on ROCC AUC
| Factor | p |
|---|---|
| Sex | 0.46 |
| White vs. Non-white race | 0.59 |
| Age <3 years | 0.56 |
| Age <5 years | 0.22 |
| Age <12 years | 0.18 |
| Age <18 years | 0.52 |
| BMI >25 | 0.65 |
| BMI <15 | 0.38 |
| Height > 98 cm | 0.47 |
| Height >110 cm | 0.27 |
| Height >150 cm | 0.27 |
| Weight >20 kg | 0.25 |
| Weight >50 kg | 0.45 |
| Weight>75 kg | 0.67 |
| Defect size >7.5 mm | 0.25 |
| Defect size >9.9 mm | 0.95 |
| Defect size >11.9 mm | 0.98 |
Abbreviations: AUC: area under the curve, BMI body mass index
All p values are for chi-squared test of differences between the AUC for the two ROCC’s based on the cut point listed.
Next, multivariable analysis was performed to assess whether patient-level factors affected the difference between TEE and TTE measurements. No patient-level factors were independently associated with 1) the difference between TEE and TTE measurements of aortic rim size (Table 5a) or 2) the absolute difference between TEE and TTE measurements of rim size (Table 5b).
Table 5.
| a: Multivariate regression for magnitude of difference between TTE and TEE measurements of retro-aortic rim | |||
|---|---|---|---|
| Factor | Beta | 95% CI | p |
| Age (years) | −0.02 | −0.09 to 0.09 | 0.68 |
| Defect size (mm) in subcostal frontal* | 0.0 | −0.09 to 0.09 | 0.99 |
| Height (per 10 cm) | 0.13 | −0.13 to 0.38 | 0.33 |
| Male sex | −0.09 | −0.71 to 0.52 | 0.76 |
| Weight (per 5 kg) | −0.7 | −0.25 to 0.10 | 0.41 |
| White race versus non-White | 0.47 | −0.18 to 1.12 | 0.15 |
| b: Multivariate regression for magnitude of absolute difference between TTE and TEE measurements of retro-aortic rim | |||
|---|---|---|---|
| Factor | Beta | 95% CI | p |
| Age (years) | 0.02 | −0.04 to 0.08 | 0.48 |
| Defect size (mm) in subcostal frontal* | 0.03 | −0.04 to 0.09 | 0.41 |
| Height (per 10 cm) | 0.10 | −0.28 to 0.10 | 0.37 |
| Male sex | 0.19 | −0.27 to 0.66 | 0.41 |
| Weight (per 5 kg) | 0.07 | −0.11 to 0.16 | 0.48 |
| White race versus non-White race | 0.04 | −0.45 to 0.53 | 0.86 |
This is collinear with defect size measured in subcostal sagittal. Replacement with measurement from subcostal sagittal view was performed and there was no change (data not shown).
Discussion
This study demonstrates that TTE performs well as a screening test for deficient retro-aortic rim, demonstrating good inter-rater reliability, discrimination, sensitivity, specificity, and predictive value against a gold standard of TEE measurement. Though strongly correlated TTE measurements of retro-aortic rim had a standard deviation of 1.9 mm which suggests that the two tests are not truly interchangeable. There was no evidence for fixed bias. Proportional bias was seen with larger magnitude measurement errors for large retro-aortic rims, which may mitigate the observed errors in TTE and TEE measurements, since the focus on risk has been on small retro-aortic rims, in which the errors were smaller. These observations should be considered when interpreting TTE findings while planning for ASD closure.
To our knowledge no studies have assessed the reliability of TTE measurements of retro-aortic or other tissue rims relative to TEE. The current study demonstrates that TTE measurement of retro-aortic rim has excellent inter-observer reliability and test characteristics for screening for deficient retro-aortic rim. At this time, based on analyses of erosion cases7,8, a threshold of 5 mm for the retro-aortic rim has been established with rims <5 mm defined as deficient and as a relative contra-indication to use of the Amplatzer Septal Occluder device. It is not clear, at this time, if changes in rim size within deficient or sufficient retro-aortic rim categories further increase or decrease risk. In other words, it is not clear whether marginal decreases below 5 mm increase risk further or whether marginal increases above 5 mm decrease risk. Because of this, we assessed the accuracy of TTE measurements of retro-aortic rim with linear regression, a Bland-Altman plot, and ROCC techniques. The first two methods had conflicting results. Linear regression demonstrates moderate correlation between TTE and TEE measurements and that TTE over-estimates the true rim measurement by 7% on average. This conflicts with the analysis of fixed bias, which suggests that over the range of rim sizes, there was no over-estimation. In addition the Bland-Altman analysis, finds that the standard deviation of the mean difference between TTE and TEE measurements is almost 2 mm, which is a clinically relevant difference in a potential patient. This suggests that TTE and TEE cannot be considered interchangeable. However, testing for proportional bias demonstrates that measurement errors increased as rim size increased. Conversely, in cases with smaller rims, where these measurements are of greatest clinical importance, the errors were smaller. Additionally, in light of the focus on 5 mm as a threshold for deficient retro-aortic rim, we also used ROCC to assess the sensitivity, specificity, and discrimination of TTE for deficient retro-aortic rim over a range of patient characteristics. In these analyses, the test characteristics for detection of an insufficient retro-aortic rim were excellent. To further assist clinical decision making in interpreting screening TTE, attempts were made to identify whether patient-level factors (age, height, weight, BMI, sex, race, and defect size) affected TTE measurement of retro-aortic rim. Neither the odds of identifying deficient retro-aortic rim or the magnitude of error on TTE measurement were affected by patient level factors. Contrary to our expectations, TTE performed uniformly across the spectrum of patient sizes. The relationship between retro-aortic rim size and risk of adverse event remains uncertain. However, though TTE and TEE cannot be considered inter-changeable tests for measuring retro-aortic rim, TTE was an effective and accurate screening test for deficient retro-aortic rim. This supports the current practice of using TTE as a screening test, and reserving TEE as a confirmatory test for patients with persistent questions and as a method of providing procedural guidance.
Previous studies of echocardiographic measurements of intra-cardiac anatomy in ASD have focused on the reliability of TTE and TEE to measure defect size3–6. There are also studies assessing the relationship between TTE and TEE measurements and the stretched diameter of defects with various balloon sizing regimens19–26. The results of this study were consistent with these previous studies. Defect size on TTE, regardless of plane of interrogation, were significantly associated with TEE measurements. TTE measurements over-estimated defect size and were a closer approximation of defect size on during balloon inflation than they were of the un-stretched diameter, which should be considered when considering device choice from screening TTE. Septal length measurements on TTE were also significantly associated with those on TEE but had larger error rates than measurements of defect size. TTE measurements were consistently larger than on TEE. This is concerning because especially in small patients, septal length is a more important limiting factor on device closure than retro-aortic rim. Further study is necessary to explore this issue.
There are several limitations to this study. A single reader made measurements on TTE and TTE, in the hopes of reducing variability from multiple raters. To insure that TTE measurements were repeatable, repeat measurements were performed in a subsample, demonstrating excellent inter-rater reliability for retro-aortic rim measurement. To avoid bias, TTE and TEE were read separately and not compared during the data collection phase. Another issue is generalizability; the study addresses the test characteristics of TTE versus TEE for the cohort assessed at a single tertiary care pediatric institution. Quality of echocardiographic imaging (both TTE and TEE) may vary by center based on differences in equipment and personnel, which may limit the generalizability of this study. Further studies are necessary to clarify to address this concern. Additionally, it is the clinical practice of our institution to use TEE to guide device closure. Intra-cardiac echocardiography (ICE) is an alternative that has been shown to be safe, effective, and potentially more economical than TEE27–29. In our practice, ICE is used only in a minority of older and larger patients, so the study population was restricted to patients undergoing TEE, and therefore, our results may not be generalizable to measurements made with ICE. Additionally, the study population included subjects across a broad range of ages and sizes, but a minority were older (n=31 between 14–18 years and 5 subjects >18 years) and larger (10% of participants had BMI>25). This may limit generalizability. Finally, though this is a fairly large sample there is always the risk of type 2 error in retrospective studies without formal power calculations.
Accepting these limitations, TTE does appear to be a good screening test for identifying deficient retro-aortic rim in subjects referred for device closure of ASD. Measurement error is seen, which is greatest in larger rims that are of less clinical import. This supports the current practice at our institution of using TTE as a pre-procedure screening test, reserving TEE as a second-line confirmatory test and to guide device deployment. Patient characteristics do not appear to strongly influence either scalar measurement or identification of deficient retro-aortic rim. This study does not address how TTE should affect decisions regarding choice of interventional strategy. These decisions should be based on information regarding the accuracy of the measurements and the implication of those measurements to risk of adverse outcome. Though this study addresses the former, it cannot address the latter. Further studies are necessary to clarify how much of a risk factor deficient retro-aortic rim is for adverse outcome.
Supplementary Material
Acknowledgments
Funding sources: Dr. O’Byrne is supported by grants from the National Institute of Health (T32 HL007915) and is the recipient of the Entelligence Young Investigator Grant. Dr. Gillespie is supported by grants from the AHA and NIH, along with research support from Medtronic. Dr. Glatz is supported by grants from the AHA and Children’s Heart Foundation. Dr. Goldberg is supported by the National Institute of Health (NR002093 and U10HL068270). Dr. Dori receives research support from Siemens. The content is solely the responsibility of the authors and does not necessarily reflect the official view of the National Institutes of Health or other funding groups.
Footnotes
Disclosures: The authors have no other disclosures.
Author contributions:
All authors have met criteria for authorship, participating in design of the study, its conduct, and production of the ensuing manuscript. Dr. O’Byrne designed the study, performed primary data collection, data analysis, and wrote the manuscript. Dr. Gillespie acted as the primary investigator for the study overseeing design, data collection, data analysis, and the manuscript. Professor Shinohara provided consultation in regards to design and performance of statistical analysis. Doctors Glatz, Rome, Goldberg, and Dori provided commentary regarding study design, data analysis, and production of the manuscript. In addition, Dr. Goldberg performed data collection, making measurements in the subgroup of subjects in whom validation of TTE measurements was performed. All authors have reviewed the final draft of the manuscript and approved it for submission.
Reference
- 1.King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA: The Journal of the American Medical Association. 1976;235(23):2506–2509. [PubMed] [Google Scholar]
- 2.Lock JE, Rome JJ, Davis R, et al. Transcatheter closure of atrial septal defects. Experimental studies. Circulation. 1989;79(5):1091–1099. doi: 10.1161/01.cir.79.5.1091. [DOI] [PubMed] [Google Scholar]
- 3.Cheng TO, Xie M-X, Wang X-F, Wang Y, Lu Q. Real-time 3-dimensional echocardiography in assessing atrial and ventricular septal defects: An echocardiographic-surgical correlative study. American Heart Journal. 2004;148(6):1091–1095. doi: 10.1016/j.ahj.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Chau AK, Leung MP, Yung T, Chan K, Cheung Y, Chiu S. Surgical validation and implications for transcatheter closure of quantitative echocardiographic evaluation of atrial septal defect. AJC. 2000;85(9):1124–1130. doi: 10.1016/s0002-9149(00)00708-6. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto K, Matsuzaki M, Tohma Y, et al. Diagnosis and quantitative evaluation of secundum-type atrial septal defect by transesophageal Doppler echocardiography. 1990;66(1):85–91. doi: 10.1016/0002-9149(90)90741-i. [DOI] [PubMed] [Google Scholar]
- 6.Ishii M, Kato H, Inoue O, et al. Biplane transesophageal echo-Doppler studies of atrial septal defects: quantitative evaluation and monitoring for transcatheter closure. American Heart Journal. 1993;125(5 Pt 1):1363–1368. doi: 10.1016/0002-8703(93)91008-3. [DOI] [PubMed] [Google Scholar]
- 7.Amin Z. Echocardiographic predictors of cardiac erosion after amplatzer septal occluder placement. Catheter Cardiovasc Interv. 2013 doi: 10.1002/ccd.25175. [DOI] [PubMed] [Google Scholar]
- 8.Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Cathet Cardiovasc Intervent. 2004;63(4):496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 9.Amplatzer Septal Occluder and Delivery System: Instructions for Use. [Accessed November 25, 2013];professionalsjmcom. 2012 :1–16. [Google Scholar]
- 10.Mahapatra S, Graves AM. Letter to Physicians. [Accessed November 25, 2013];professionalsjmcom. 2013 :1–2. Available at: http://professional.sjm.com/resources/product-performance/amplatzer-septal-occluder-performance/ifu-updates. [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 12.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49(8):907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 13.Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF. Percutaneous Atrial Septal Defect Closure in Infants and Toddlers: Predictors of Success. Pediatr Cardiol. 2012;34(2):220–225. doi: 10.1007/s00246-012-0413-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Byrne ML, Glatz A, Sunderji S, et al. PREVALENCE OF DEFICIENT RETRO-AORTIC RIM AND ITS EFFECT ON OUTCOME IN DEVICE CLOSURE OF ATRIAL SEPTAL DEFECTS. Journal of the American College of Cardiology. 2014;63(12):A518. doi: 10.1007/s00246-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Byrne ML, Glatz AC, Sunderji S, et al. Prevalence of deficient retro-aortic rim and its effects on outcomes in device closure of atrial septal defects. Pediatr Cardiol. doi: 10.1007/s00246-014-0914-6. e-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallula K, Amin Z. Recent Changes in Instructions for Use for the Amplatzer Atrial Septal Defect Occluder: How to Incorporate These Changes While Using Transesophageal Echocardiography or Intracardiac Echocardiography? Pediatr Cardiol. 2012;33(7):995–1000. doi: 10.1007/s00246-012-0323-7. [DOI] [PubMed] [Google Scholar]
- 17.Rare Serious Erosion Events Associated with St. Jude Amplatzer Atrial Septal Occluder (ASO) United States Food and Drug Administration; 2013. [Accessed November 25, 2013]. Available at: http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm371145.htm. [Google Scholar]
- 18.Lange RA. Confidence in ASD occluder devices is eroding. Cathet Cardiovasc Intervent. 2012;80(2):175–176. doi: 10.1002/ccd.24533. [DOI] [PubMed] [Google Scholar]
- 19.van den Bosch AE, Harkel Ten D-J, McGhie JS, et al. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2006;19(6):815–821. doi: 10.1016/j.echo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Carlson KM, Justino H, O'Brien RE, et al. Transcatheter atrial septal defect closure: modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2005;66(3):390–396. doi: 10.1002/ccd.20443. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa MI, Balaguru D, McClure C, Kline CH, Radtke WAK, Shirali GS. Experience with use of multiplane transesophageal echocardiography to guide closure of atrial septal defects using the amplatzer device. Pediatr Cardiol. 2002;23(4):430–436. doi: 10.1007/s00246-002-1510-8. [DOI] [PubMed] [Google Scholar]
- 22.Forfar JC, Godman MJ. Functional and anatomical correlates in atrial septal defect. British Heart Journal. 1985;54:193–200. doi: 10.1136/hrt.54.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu X, Han YM, Berry J, Urness M, Amplatz K. A new technique for sizing of atrial septal defects. Catheter Cardiovasc Interv. 1999;46(1):51–57. doi: 10.1002/(SICI)1522-726X(199901)46:1<51::AID-CCD14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Rao PS, Langhough R, Beekman RH, Lloyd TR, Sideris EB. Echocardiographic estimation of balloon-stretched diameter of secundum atrial septal defect for transcatheter occlusion. American Heart Journal. 2003;124(1):172–175. doi: 10.1016/0002-8703(92)90937-q. [DOI] [PubMed] [Google Scholar]
- 25.Rao PS, Langhough R. Relationship of echocardiographic, shunt flow, and angiographic size to the stretched diameter of the atrial septal defect. American Heart Journal. 1991;122(2):505–508. doi: 10.1016/0002-8703(91)91008-b. [DOI] [PubMed] [Google Scholar]
- 26.Godart F, Rey C, Francart C, Jarrar M, Vaksmann G. Two-dimensional echocardiographic and color Doppler measurements of atrial septal defect, and comparison with the balloon-stretched diameter. AJC. 1993;72(14):1095–1097. doi: 10.1016/0002-9149(93)90873-b. [DOI] [PubMed] [Google Scholar]
- 27.Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. AJC. 2004;94(5):690–692. doi: 10.1016/j.amjcard.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Boccalandro F, Baptista E, Muench A, Carter C, Smalling RW. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. AJC. 2004;93(4):437–440. doi: 10.1016/j.amjcard.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Zanchetta M, Rigatelli G, Pedon L, et al. Transcatheter atrial septal defect closure assisted by intracardiac echocardiography: 3-year follow-up. Journal of Interventional Cardiology. 2004;17(2):95–98. doi: 10.1111/j.1540-8183.2004.09874.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


