Abstract
During the metabolism of different arsenic-containing compounds in human, a variety of metabolites are produced with significantly varying toxicities. Currently available analytical methods can only detect a limited number of human metabolites in biological samples during one run due to their diverse characteristics. In addition, co-elution of species is often unnoticeable with most detection techniques leading to inaccurate metabolic profiles and assessment of toxicity. A high performance liquid chromatography inductively coupled mass spectrometry (HPLC-ICP-MS) method was developed that can identify thirteen common arsenic metabolites possibly present in human with special attention dedicated to thiolated or thiol conjugated arsenicals. The thirteen species included in this study are arsenite (AsIII), arsino-glutathione (As(GS)3), arsenate (AsV), monomethylarsonous acid (MMAIII), monomethylarsino-glutathione (MMAIII(GS)2), monomethylarsonic acid (MMAV), dimethylarsinous acid (DMAIII (from DMAIIII)), S-(dimethylarsinic)cysteine (DMAIII(Cys)), dimethylarsino-glutathione (DMAIII(GS)), dimethylarsinic acid (DMAV), dimethylmonothioarsinic acid (DMMTAV), dimethyldithioarsinic acid (DMDTAV), dimethylarsinothioyl glutathione (DMMTAV(GS)). The developed method was applied for the analysis of cancer cells that were incubated with Darinaparsin (DMAIII(GS)), a novel chemotherapeutic agent for refractory malignancies, and the arsenic metabolic profile obtained was compared to results using a previously developed method. This method provides a useful analytical tool which is much needed in unequivocally identifying the arsenicals formed during the metabolism of environmental arsenic exposure or therapeutic arsenic administration.
Keywords: High performance liquid chromatography inductively coupled mass spectrometry (HPLC-ICP-MS), arsenic speciation, arsenic metabolism in humans, dimethylarsinous glutathione
1. Introduction
Arsenic (As), through naturally occurring from the Earth's crust to groundwater and anthropogenic activities such as nonferrous metal mining and smelting, coal combustion, and pesticide application, has resulted in the contamination of water, soil, and food around the world [1-4]. The chronic toxicity of As exposure through drinking water and food (e.g., rice) poses serious health risks to millions of people [5-9]. Toxic effects of As include diabetes, anemia, diarrhea, and more importantly, the development of different types of cancers such as carcinomas of the skin, lung, bladder, kidney and liver [10-12].
Despite of its known toxicity and potential to cause cancer, As has been historically used for medicinal purposes to treat such diseases as ulcers, head lice, and plague [13-16]. Although the current use of medical As has been limited due to its high toxicity and carcinogenicity, some As compounds have been recently rediscovered for the treatment of certain cancers [13-14, 17]. For example, arsenic trioxide (As2O3, ATO, Trisenox) was revived for the treatment of both newly diagnosed and relapsed acute promyelotic leukemia (APL) [13, 18]. S-dimethylarsino-glutathione (Darinaparsin, DAR) has been in clinical trials for hematological cancers and refractory solid tumors [19-22].
Both toxicity and anticancer activity of As are determined by the metabolism of As in human body. Arsenic metabolism is rather complicated, as the metabolic profile depends on the As species introduced, the route of administration, and the type of cells involved in As elimination. A wide variety of As species, including methylated arsenicals such as DMAV, MMAV, DMAIII, and MMAIII and As-glutathione (GSH) conjugates, have been observed during As metabolism (see Table 1 for the names, structures, and abbreviations of all thirteen arsenicals included in this study). In particular, a new type of arsenicals, thiolated As compounds including DMMTAV, DMDTAV and DMMTAV(GS), has been recently detected in humans and/or mammals [23-28]. Although efforts have been made to reveal the pathways of As metabolism, much remains unclear about how these As species are formed and what role these arsenicals play in the toxicity and therapeutic efficacy of As [29-30].
Table 1. Names, abbreviations and structures of compounds of interest.
| Most common | Structure | Occurrence in humans |
pKa [47] |
|
|---|---|---|---|---|
| Names | Abbreviatio ns |
|||
| Arsenate, Arsenic acid | AsV |

|
Urine [48-52] Fingernail [50] Blood plasma [50, 53] | 2.3 6.7 11.6 |
| Dimethyldithioarsinic acid Dimethylarsinodithioic acid | DMDTAV |

|
In mammals [24, 26] | |
| Arsenite, Arsenious acid | AsIII |

|
Urine [48-52] Fingernail [50] Blood plasma [53] | 9.2 |
| Monomethylarsonic acid, Methylarsonic acid | MMAV MAsV |

|
Urine [48-52] Fingernail [50] Blood plasma [50, 53] Hepatocytes [54] | 3.6 8.2 |
| Monomethylarsonous acid, Methylarsonous acid, Methylarsonite | MMAIII MAsIII |

|
Urine [48-52, 55] Hepatocytes [54] | |
| Dimethylmonothioarsinic acid, Dimethylarsinothioic acid, Thio-dimethylarsinate | DMMTAV Thio-DMA DMASV |

|
Urine [23] Red blood cells [27] | |
| Dimethylarsinic acid, Dimethylarsinate | DMAV DMAsV |

|
Urine [48-52] Fingernail [50] Blood plasma [50, 53] Hepatocytes [54] | 6.3 |
| Arsino-glutathione | AsIII(GS)3 ATG |

|
In mammals [56-57] | |
| Dimethylarsinothioyl glutathione | DMMTAV-GS DMATG DMASV-GS DMMTAV(G S) |

|
[25] | |
| Monomethylarsino-glutathione, Monomethylarsonic diglutathione | MMAIII(GS)2 MADG |

|
In mammals [56-57] | |
| S-(Dimethylarsenic) Cysteine | DMAIIICys |

|
||
| Dimethylarsinous acid, Dimethylarsinite | DMAIII DMAsIII |

|
Urine [48-52, 55] Fingernail [50] Hepatocytes [54] | |
| Dimethylarsinous glutathione, Dimethylarsinic glutathione, Dimethylarsino-glutathione, Darinaparsin, ZIO-101, DAR | DMAIII(GS) DAR DMAG DMAsIIIGS |

|
[25] | |
In order to understand the metabolic pathways of As, the mode of action of As toxicity, and the mechanisms underlying As chemotherapy, we must have an analytical method that is capable of unequivocally identifying these arsenicals formed during the metabolism of environmental As exposure or therapeutic As administration. Speciation analysis of As metabolites is important, because each As species possesses varying toxicities. For example, MMAIII and DMAIII were shown to be more genotoxic than inorganic As (iAsIII) and more potent inhibitors of enzymes while DMMTAV is more cytotoxic than DMAV [23, 31-33]. Generally trivalent arsenicals are much more toxic than pentavalent ones, and are taken up more efficiently by cells than pentavalent ones [34-35].
Existing As speciation techniques usually employ high performance liquid chromatography (HPLC) separation on reverse phase or ion exchange column, coupled to an element specific detector such as inductively coupled plasma mass spectrometer (ICP-MS), atomic fluorescence spectrometer (AFS), or atomic absorption spectrometer (AAS) [36-40]. Unfortunately, currently available techniques have shortcomings and cannot meet the need of “full-spectrum” analysis of As metabolites. First, ion chromatography was found to decompose glutathione (GSH) complexes of As, failing to detect these As-GSH complexes [41-42]. Since As-GSH complexes are thought to be necessary intermediates for As methylation, their unequivocal identification is important to depict the pathways of As metabolism. Second, many current As speciation methods are unable to detect thiolated arsenicals such as DMMTAV, DMDTAV, and DMMTAV(GS), and in particular cannot simultaneously determine methylated species (trivalent and pentavalent) and sulfur-containing arsenicals. It is necessary to identify thiolated arsenicals as these As species may play an important role in As metabolism, toxicity, and anticancer activity. Finally, current analytical methods usually are able to separate a limited number of As species and co-elution of species occurs during analysis. For example, many of the methods employing anion exchange columns could not distinguish MMAIII from MMAV and DMAIII from DMAV, providing incomplete information on the profile of As metabolism.
The objective of this study was to develop an analytical method using HPLC-ICP-MS that is capable of performing a “full-spectrum” analysis of As metabolites. The method was aimed to simultaneously determine the newly discovered thiolated or thiol conjugated As metabolites (e.g., DMMTAV, DMDTAV, and DMMTAV(GS)) and highly toxic trivalent methylated As (DMAIII and MMAIII), in addition to the As species of traditional interest (e.g. inorganic AsV and AsIII and organic DMAV and MMAV). Thirteen possible As metabolites, representing most of the As species reported so far during As metabolism, were included in this study. As the method was intended for analysis of human As metabolites, thiolated arsenicals, trivalent methylated As species, and As-GSH complexes were the primary targets of separation and detection. A variety of chemical and instrumental parameters were optimized for the method, with a focus on the key parameters influencing separation and detection of these thiolated arsenicals. The method was applied to the analysis of human cancer cells treated with Darinaparsin to demonstrate its application in determining human metabolites of this anticancer drug.
2. Materials and Methods
2.1. Reagents
Argon (Ar) purged double deionized water (DDIW) (18.2 MΩ·cm, Barnstead Nanopure Diamond) was used throughout the experiments. Acetonitrile (ACN) and methanol were purchased from Thermo Fisher Scientific, USA. Ammonium hydroxide (NH4OH), sodium hydroxide (NaOH), potassium hydroxide (KOH), phosphoric acid (H3PO4), hydrochloric acid (HCl), and nitric acid (HNO3) used for pH adjustments were also purchased from Thermo Fisher Scientific. Isopropyl alcohol was obtained from Fluka. Glycine, L-glutathione reduced, and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich, USA. TFA and anhydrous citric acid were bought from Acros Organics. Formic acid and acetic acid used to prepare formate and acetate buffers were purchased from Acros Organics and Thermo Fisher Scientific, respectively. Sulfur dioxide gas used for the synthesis of MMAIIII2 and DMAIIII was acquired from Specialty Gasses of America. Sodium metaarsenite (98%), sodium arsenate (99%), and cacodylic acid (98%) were from Aldrich. Monosodium acid methane arsonate sesquihydrate was obtained from Chem Service, PA, USA. Solid DMAIII(GS), obtained from Ziopharm Oncology, USA, was prepared in solution in our lab along with other arsenic standards used. All reagents used were of analytical grade or better.
Arsenical standards synthesized in house
Due to the lacking availability, the following arsenical standards were synthesized in the laboratory: MMAIIII2, DMAIIII, DMAIII(Cys), AsIII(GS)3, MMAIII(GS)2, DMAIII(GS), DMMTAV, DMDTAV, and DMMTAV(GS). The synthesis of these arsenicals was based on the previously reported procedures with some modifications if necessary, and the identity and purity (if it was possible to purify the arsenicals using currently available procedures) of the synthesized As standards were verified with NMR, HPLC-ESI-MS, and/or HPLC-ICP-MS. The detailed information for the synthesis of these As standards was provided in the Supplementary Information.
2.2 Instrumentation
A Perkin Elmer Series 200 HPLC system equipped with a temperature controlled column compartment was coupled to a Perkin Elmer Elan DRC-e ICPMS. The ICPMS was equipped with a cyclonic spray chamber and a Meinhard nebulizer and was used in the standard mode. ICPMS signal at m/z 75 for 75As was monitored. Data was collected and processed using Elan v. 3.4 and Chromera v. 1.2 software (Perkin Elmer, USA).
2.3 Optimization of HPLC and ICP-MS parameters
Experiments were performed to optimize a variety of parameters, including HPLC column type and dimension, column temperature, mobile phase (composition, pH, and gradient program), and ICP-MS parameters such as nebulizer gas, RF power, analog stage, and lens voltages. It was observed that HPLC mobile phase and ICP-MS nebulizer gas were the key factors influencing the separation and detection of the As compounds of interest. Therefore the experiments were focused on these two parameters to find the most optimal conditions for method performance.
The optimization of mobile phase included selection of aqueous buffer and organic solvent and the gradient elution program. Considering the pKa values of arsenicals, a number of buffers, including trifluoroacetate (TFA, pKa = 0.5), formate (pKa = 3.8), and acetate (pKa = 4.8), were tested. The commonly used organic solvents, such as acetonitrile, methanol, and isopropyl alcohol, were test for separation of the As species of interest. The gradient elution program was optimized by changing the v/v % of the selected organic component from 0-20%.
Nebulizer gas was optimized for its composition and flow rate to investigate its effect on detection of the arsenic metabolites selected in this study. In order to address the issue of plasma stability and instrument contamination, which was caused by high v/v % of organic solvent used here (see Results and Discussion), O2 was added through a T-connector to the nebulizer Ar gas. The flow rate of Ar for nebulization was further optimized to investigate its effect on method performance, by varying the flow rate from 0.80 to 1.04 L min-1.
2.4 Linearity, limit of detection (LOD), and limit of quantitation (LOQ)
The v/v % of organic solvent in mobile phase significantly changes the ionization characteristics in the plasma and thus the sensitivity of the method. Consequently, the linearity, LOQ, and LOD of the method were determined using two compounds, AsIII and AsIII(GS)3, with the former eluting during 1% of organic solvent (ACN was selected, see Results and Discussion) while the latter at 15% of ACN. Linearity, LOQ, and LOD studies were performed under the optimized instrumental conditions.
2.5 Determination of metabolites of DMAIII(GS) in human cells
The developed method was used to analyze the metabolites of DMAIII(GS) in 8226/S multiple myeloma cell line (ATCC, Manassas, VA). The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 on RPMI-1640 media, supplemented with 100 U mL-1 of penicillin, 100 μg mL-1 of streptomycin, 10% heat inactivated fetal bovine serum and 2 mM L-glutamine (all culture reagents from Cellgro, MediaTech, Herndon, VA). Following incubation the cells were harvested by centrifugation at 1000 rpm for 5 minutes, washed with PBS once, spun down again, then the pellets were frozen in liquid nitrogen and stored at -11 °C. Each cell pellet contained 5.0 × 106 cell counts. For the As exposure experiment, the cell pellets were thawed, spiked with 0.26 mM DMAIII(GS) in double deionized water (DDIW) and left for 10 minutes. The cells were then broken up with a sonication probe, and the sample was filtered through a 0.2 μm nylon syringe filter into an HPLC vial and analyzed. Unspiked cells used as control were prepared in the same way, but without spiking the cells with DMAIII(GS).
3 Results and Discussion
Since a number of As metabolites could exist in human cells and some of which (e.g., trivalent arsenicals) are redox sensitive, an efficient separation is required for their determination to maintain integrity of the analytes. We first compared three HPLC columns, namely ion exchange, C18, and C8, and found that C8 column was the most promising for further optimization to achieve better separation of a variety of arsenicals. Further experiments were then performed to optimize the C8 column-based HPLC-ICP-MS method, focusing on mobile phase and nebulizer gas as we observed they are critical in the performance of the method. Since lower temperatures help preserve the oxidation state of trivalent As species and prevent the degradation of As-glutathione complexes, concurring with previous studies [41], all experiments were conducted with the column kept at 10 °C.
3.1 Mobile phase
It was observed that a mixture of aqueous buffer and organic solvent was needed for the separation of the arsenic species of interest. For aqueous mobile phase, a number of buffers were evaluated, particularly such volatile buffers as trifluoroacetate (TFA, pKa = 0.5), formate (pKa = 3.8), and acetate (pKa = 4.8) [43]. The selection of these buffers was primarily based on pH. Ideally, the pH of the mobile phase should be chosen to be at least two units away from the analyte pKa values in order to have all the analytes present in their neutral or fully ionized forms [43]. Considering that the species to be separated have a wide range of pKa values (including unknown pKa for some species) and that many of these species are more stable at lower pH values, the final pH range studied was set from 1.5 to 4.6 based on the available pKa values [41]. In addition to pH, volatility was considered a factor for selection of aqueous mobile phase as the developed separation method is intended to be used not only with ICP-MS, but also with electrospray mass spectrometry (ESI-MS). HPLC-ESI-MS can be used for the unequivocal identification of the species in question. A comparison of the above 3 mobile phases yielded that the best separation can be achieved using 0.05% TFA as the aqueous mobile phase (data not shown).
For organic solvents in the mobile phase, ACN, methanol, and isopropyl alcohol were considered. It was found that ICP did not tolerate isopropyl alcohol well even at a concentration as low as 2-3 % v/v and isopropyl alcohol was not used after the initial trials. Methanol, though evaluated extensively to be used for separation, was not selected due to the decreased stability of certain arsenic species in methanol [42]. ACN was eventually selected, as it has higher eluotropic strength when used at the same volume percentage compared to methanol [43] achieving adequate separation within reasonable time. An additional benefit of acetonitrile addition to the mobile phase was the enhanced signal on ICP-MS detection (data not shown). This was not surprising, as it is known that the presence of organic solvents at 1-5 v/v% would cause signal enhancement of As and other elements with higher ionization potentials due to the modification of equilibrium in the plasma [44]. Since the degree of ionization of carbon is significantly larger than that of Ar, the introduction of organic solvents in the plasma would lead to the increased population of C+ (ionization energy, Ei, 11.26 eV). The transfer of electron to C+ from an element with lower Ei (such as 75As which has an Ei of 9.81 eV) would increase the degree of ionization of that element, resulting in the signal enhancement.
After selection of aqueous and organic mobile phases, the gradient program was optimized by modifying the v/v % of organic component, as this v/v % is the principal factor that governs the retention of analytes in reversed-phase LC separations. It was found that the v/v % needed to be gradually increased and a relative large amount of ACN (20% v/v) at the late stage of separation was necessary to achieve the most optimal separation of all species within reasonable time. Fig. 1 illustrates the separation of major As compounds (25 μg L-1 of As for all species) under the optimized HPLC gradient program and other conditions (Table 2), excluding As-GSH complexes which were separately discussed in section 3.3. The method was able to determine a number of As species, including AsV, AsIII, MMAV, DMAV, MMAIII, and DMAIII, DMMTAV, and DMDTAV. The good separation for DMMTAV, DMDTAV, MMAIII, and DMAIII indicates the applicability of the method in determining thiolated and trivalent methylated human As metabolites. Peak overlapping and broadening could be observed for AsIII, MMAV, and DMAV, but further effort was not made to better resolve these species, as the arsenicals of primary interest for this method were thiolated and trivalent methylated arsenicals. A broad peak was observed for DMAIII, but in human cells DMAIII is likely present in GSH complexed form which can be detected as a separate species (see section 3.3).
Fig. 1.
Typical chromatogram for separation and analysis of As compounds (25 μg L-1) containing no glutathione using HPLC-ICP-MS. The compounds separated are as follows: 1. AsV, 2. DMDTAV, 3. AsIII, 4. MMAV, 5. MMAIII, 6. DMMTAV, 7. DMAV, 12. DMAIII, * Baseline disturbance originating from high concentration acetonitrilegradient program.
Table 2. A. Instrumental parameters after optimization.
| Parameter (unit) | Typical values |
|---|---|
|
| |
| ICP-MS | |
|
| |
| ICP-MS Nebulizer Ar Gas Flow (L min-1) | 0.84-0.90 |
| Nebulizer Oxygen Gas Flow (L min-1) | 0.2 |
| Auxiliary Gas Flow (L min-1) | 1 |
| Plasma Gas Flow (L min-1) | 15 |
| Lens voltage | 9 |
| ICP RF Power (W) | 1350 |
| Analog Stage Voltage | -1525 |
| Pulse Stage Voltage | 950 |
| Quadrupole Rod Offset Std (QRO) | -2 |
| Cell Rod Offset Std (CRO) | -7 |
| Discriminator Threshold | 40 |
| Cell Path Voltage Std (CPV) | -20 |
|
| |
| HPLC | |
|
| |
| Column | Waters Spherisorb 5 μm C8, 4.0×250 mm |
| Column temperature | 10°C |
| Mobile phase temperature | Cooled in ice bath during analysis |
| Sample temperature | Room temperature |
| Mobile phase | 0.05% TFA and acetonitrile gradient |
| B. Final HPLC gradient elution program | ||||||
|---|---|---|---|---|---|---|
| Step | Step type | Step time (min) | Flow (mL min-1) | 0.05% TFA | ACN % | Curve |
| 0 | Equilibration | 6.0 | 1.0 | 99 | 1 | N/A |
| 1 | Run | 5.5 | 0.5 | 99 | 1 | 0.0 |
| 2 | Run | 3.5 | 0.5 | 87 | 13 | 1.0 |
| 3 | Run | 11.0 | 0.5 | 80 | 20 | 0.0 |
| 4 | Run | 10.0 | 0.5 | 95 | 5 | 0.0 |
The flow rate of mobile phase was chosen at 0.5 mL min-1 during the run, and the total run time was set to 30 min with the retention time of the last peak being around 23.4 min. The extra run time (between 26 to 30 min) can be used for the post column injection of a stable internal standard (e.g. AsV), if required, to correct for instrumental drifts. Prior to each run, there was a equilibration stage of 6 min when the mobile phase was 99:1 (v/v) of TFA:ACN at a flow rate of 1 mL min-1 (Table 2). During the last ten minutes of the gradient program, the v/v % of ACN was decreased to 5% from 20% in the previous step. This change of ACN v/v % not only had no significant effect on the retention time and the resolution of the peaks eluted during this period, but also provided a way to reduce the usage of organic solvent and to protect the stability of plasma. If the run was finished with 20% ACN, the plasma would be frequently extinguished once the equilibration stage began when the mobile phase changed to 1% v/v ACN.
3.2 Nebulizer gas
From the optimization of mobile phase, it was seen that high ACN composition (up to 20% v/v) was needed for the separation of As species of interest. The usage of high fraction of organic solvent in the mobile phase caused several problems. First, high fraction of ACN extinguished the plasma frequently during the run. Second, due to the high organic composition in the mobile phase, carbon built up quickly on the ICP sampling interface and torch, necessitating the frequent cleanup or replacement of the torch, the injector, and the cones. Finally, high ACN v/v % in mobile phase produced severe baseline disturbance during gradient elution and caused appearance of ghost peaks, interfering with detection of As species. In order to solve these problems, we did further optimization on the method and found that nebulizer gas was the critical factor. We then optimized nebulizer gas, including composition and flow rate, to solve these problems.
For nebulizer gas composition, we introduced a small amount of O2 to the nebulizer gas flow, aiming to burn the carbon from organic solvent to avoid carbon deposition on ICP hardware. A T-connector was employed to add 0.2 L min-1 oxygen gas to the Ar nebulizer flow, and the O2 flow rate was not further optimized, as the manufacturer-recommended 0.2 L min-1 was found appropriate to prevent the instrument from carbon build up for a long time (e.g., months) and from plasma extinguishing.
During the experiments of introducing O2 into the nebulizer gas flow, it was observed that the intensity of the ICP-MS signal was enhanced when the nebulizer gas was composed of O2 and Ar than was Ar alone, with the total flow rate being the same (Fig. 2). For example, the signal for MMAIII(GS)2, which was eluted at high ACN v/v %, increased by a factor of about 7 when the nebulizer gas was composed of 0.2 L min-1 of O2 and 0.84 L min-1 of Ar, compared to 1.04 L min-1 of Ar (Fig. 2A). Similarly, under the same experimental conditions, the signals for AsV and AsIII, which were eluted at low ACN v/v %, increased when the mixture of O2/Ar was used as nebulizer gas (Fig.2B). This signal increase could be due to the introduction of O2 and/or the difference in Ar flow rates (0.84 L min-1 when O2 was added versus 1.04 L min-1 with Ar only).
Fig. 2.
Demonstration of signal enhancement due to changes in nebulizer gas composition and flow rate for (A) MMAIII(GS)2 eluting during high (20%) v/v% acetonitrile and (B) AsV and AsIII eluting during low (1%) v/v% acetonitrile in the gradient program.
Arsenic standards were run to assess the effect of Ar flow rate on instrumental signal. It was observed that lowering the nebulizer Ar gas flow increased the sensitivity of the instrument for As detection (Fig. 3). Fig. 3A demonstrates an example of the effect of Ar flow on the response of the instrument, with DMAIII(Cys) being detected on ICP-MS. The instrumental intensity for DMAIII(Cys) increased by a factor of 2 when the Ar flow decreased from 0.90 to 0.80 L min-1 with O2 present in the nebulizer gas at 0.20 L min-1. This result is indicative of the signal enhancing effect of lower Ar flow rate, but cannot rule out the possibility that the signal enhancement was caused by the decrease in total flow rate of nebulizer gas (from 1.1 to 1.0 L min-1).
Fig. 3.
The effect of nebulizer gas on instrumental response demonstrated by (A) decreased sensitivity for DMAIII(Cys) detection with increasing Ar flow rate and (B) similar responses of DMAIII(Cys) at the same Ar flow rate regardless of the presence of oxygen. The effect of Ar flow rate on baseline disturbance is also observable in the figure.
Additional experiments were conducted to examine the effect of total flow rate of nebulizer gas (Ar + O2) on instrumental signal, by detecting DMAIII(Cys) at a fixed Ar flow rate with and without the addition of O2. It was observed that the ICP-MS signals were fairly similar when 0.2 L min-1 of O2 was added to 0.86 L min-1 of Ar (making up a total flow of 1.06 L min-1), compared to 0.86 L min-1 of Ar alone being used as nebulizer gas (Fig. 3B). This result suggests that the Ar flow rate in the nebulizer gas remarkably affected the instrumental response, whereas O2 had little effect even if the addition of O2 increased the total flow rate of nebulizer gas.
Baseline disturbance from high ACN v/v % in the mobile phase was another obstacle affecting the performance of the method, and was found to be related to nebulizer gas. Blanks without As compounds were used to check the chromatographic baseline during gradient elution under different flow rates of nebulizer Ar gas. It was found that the ghost peaks showing at 17-25 min can be substantially decreased by increasing slightly the Ar nebulizer flow (Fig. 4). Increasing the Ar flow from 0.80 to 0.90 L min-1 could reduce the baseline disturbance to an acceptable level. It appears that O2 had no influence on baseline disturbance, as indicated by the similarity of baseline signals with or without O2 present in the nebulizer gas when Ar was 0.8 L min-1.
Fig. 4.
Baseline disturbance vs nebulizer flow rate and composition for blank injection.
Increasing Ar flow rate reduced baseline disturbance, but at the same time decreased instrumental response. Therefore, the nebulizer Ar flow rate should be carefully optimized prior to analysis, to achieve adequate sensitivity while minimizing the interference from baseline disturbance. After testing Ar at 0.80-0.90 L min-1 for instrumental sensitivity and baseline disturbance, the final Ar flow rate for sample analysis was set to 0.84 L min-1, and the O2 was 0.2 L min-1 (Table 2). The enhanced signal intensity at lower nebulizer Ar flow rates could be due to more efficient conversion and atomization of sample aerosol, as previously reported [45]. As for O2, it was observed that O2 protected the instrument from carbon buildup and from plasma extinguishing, but did not affect signal intensity. Previous studies have reported inconsistent effects of O2 on instrumental signal, with both suppressed and unchanged signal intensity being observed when O2 was added to nebulizer gas, probably related to the specific plasma conditions [44, 46].
3.3 Analysis of As-GSH complexes
As-GSH complexes represent an important type of intermediates during As metabolism in human body, and thus the developed method was also intended for analysis of As-GSH complexes. For analysis of As-GSH complexes, the first consideration was about the standards. The As-GSH complexes standards were prepared by mixing As standard solutions (AsIII, MMAIII, and DMAIII) with excess GSH, and thus the standards of As-GSH complexes, e.g., AsIII(GS)3, MMAIII(GS)2, and DMAIII(GS), were always present in excess GSH. The standard solution of As-GSH complexes could not mixed with the non-GSH complexed As standards, because, once mixed, the non-GSH complexed arsenicals (free AsIII, MMAIII, and DMAIII) would be complexed by GSH from the solution of As-GSH complexes. Therefore we prepared two sets of standards, one for As-GSH complexes and the other for non-GSH complexed arsenicals, and these two types of As standards need to be injected separately. For samples, when GSH and non-GSH complexed arsenicals are present in a sample, equilibrium will be established between the GSH complexes and their non-GSH counterparts depending on the reduced GSH concentration in the sample. When injected, the relative amounts of each arsenic species can be determined based on the two sets of As standards.
Fig. 5A shows a typical chromatogram with good separation for sulfur-containing As compounds (25 μg L-1), including As-GSH complexes, such as AsIII(GS)3, DMMTAV(GS), MMAIII(GS)2, and DMAIII(GS), and thiolated arsenicals, such as DMDTAV and DMMTAV. An overlay of the chromatograms for non-GSH complexes As standards and As-GSH complexes shows the separation of major As metabolites of interest (Fig. 5B), suggesting that the developed method is indeed able to detect both sulfur-containing and non-sulfur-containing arsenicals if they are both present in the sample. In particular, the method was able to analyze thiolated arsenicals, trivalent methylated As species, and As-GSH complexes, including DMMTAV, DMDTAV, AsIII(GS)3, DMMTAV(GS), MMAIII(GS)2, and DMAIII(GS), suggesting the validity of the method in analyzing human As metabolites.
Fig. 5.
Separation of As metabolites demonstrated by (A) injection of glutathione complexes and thiolated arsenicals and (B) overlay of the chromatograms for injection of sulfur-containing and non-sulfur-containing arsenicals (25 μg L-1). The compounds separated are as follows: 1. AsV, 2. DMDTAV, 3. AsIII, 4. MMAV, 5. MMAIII, 6. DMMTAV, 7. DMAV, 8. AsIII(GS)3, 9. DMMTAV(GS), 10. MMAIII(GS)2, 12. DMAIII, 13. DMAIII(GS), * Baseline disturbance originating from high concentration acetonitrilegradient program.
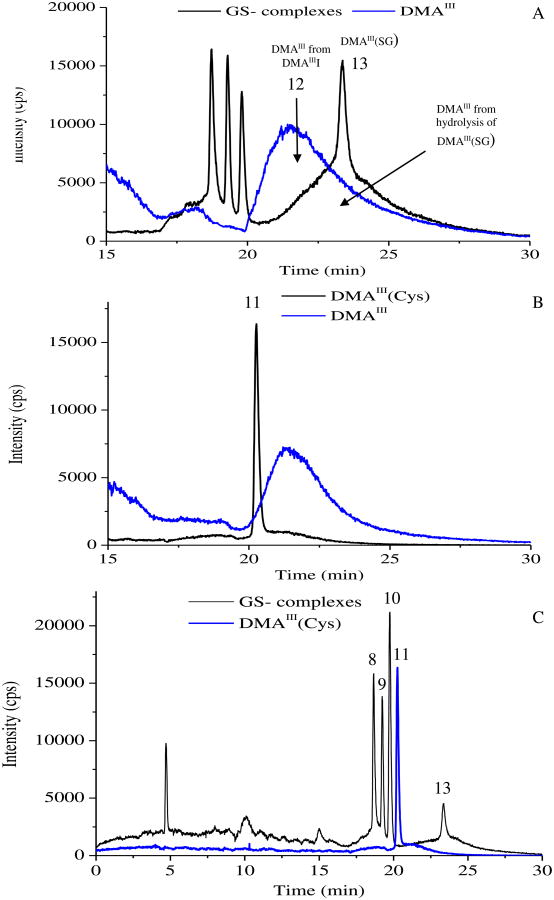
Another consideration for analyzing As-GSH complexes was related to specific form of DMAIII species. The DMAIII peak (#12 in the chromatogram, Fig. 5B) corresponded the injection of DMAIIII synthesized in our lab, which could be hydrolyzed to DMAIII(OH) upon dilution in aqueous solutions. When a high concentration of DMAIII(GS) (#13 in the chromatogram, Fig. 5) was injected, it partially dissociated to DMAIII during the run, as shown by the fronting peak of DMAIII(GS) (Fig. 6). The decomposition of DMAIII(GS) to DMAIII during analysis, along with the lack of availability of pure DMAIII standard and DMAIII being extremely prone to oxidation during storage and sample preparation, make it nearly impossible to accurately determine the native DMAIII concentration in a sample. As the preparation of DMAIIII is rather difficult, DMAIII(Cys) has been used as standard for DMAIII in a number of studies, which further complicated the issue related to identification and detection of the specific form of DMAIII species. By using the developed method, we observed that DMAIIII (probably should be labeled as DMAIII as this species usually refers to the non-conjugated form) and DMAIII(Cys) gave two completely different peaks, with a very broad peak for DMAIII and a sharp one for DMAIII(Cys) (Fig. 6). Therefore, DMAIII(GS), DMAIII(Cys), and DMAIII (non-conjugated form) should be treated as three different As species and caution should be exercised to avoid confusion. We further demonstrated that the developed method was able to separate all these three forms of the trivalent dimethylated arsenical (Fig. 6), if they are present as As metabolites in a sample.
Fig. 6.
Resolution of DMAIII, DMAIII(Cys), and DMAIII(GS) demonstrated by (A) generation of DMAIII during high concentration of DMAIII(GS) injection, (B) separation of DMAIII(Cys) (#11) and DMAIIII (#12), and (C) separation of DMAIII(Cys) from As-GSH complexes.
3.4 Linearity, LOQ and LOD
Linearity, LOQ, and LOD were determined using AsIII and AsIII(GS)3 standards under the optimized conditions, with a nebulizer flow consisting of 0.2 L min-1 O2 and 0.84 L min-1 Ar giving a total flow of 1.04 L min-1. Because AsIII and AsIII(GS)3 were two peaks eluted at low and high acetonitrile v/v %, respectively, the results obtained for these two species are indicative of the method performance for all As species. The method was found to be linear between the tested range of 1.25 to 2500 μg L-1 for both AsIII and AsIII(GS)3 (r2 > 0.999). Limit of detections of AsIII and AsIII(GS)3 were experimentally determined to be 1.25 μg L-1 with an average of signal to noise ratio (S/N) of 3 for three injections for both compounds (at 5 μg L-1). The limits of quantitation for AsIII and AsIII(GS)3 were set to be 2.5 μg L-1 for each, based on the low percent relative standard deviations of the peak areas (4.2 for AsIII and 7.0 for AsIII(GS)3, respectively).
3.5 Application of method
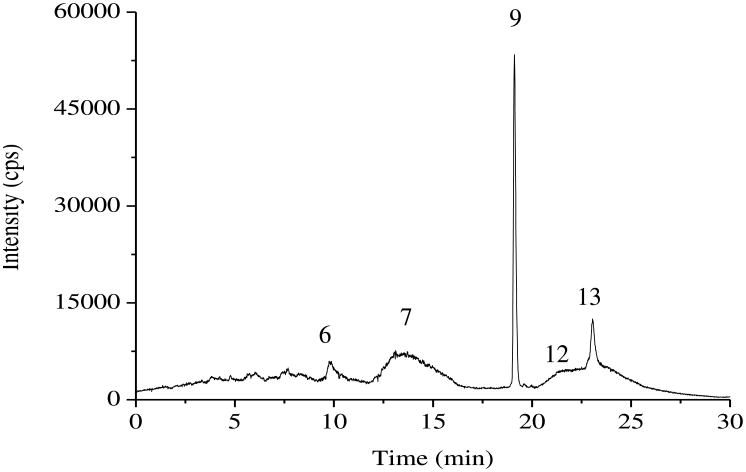
The analysis of the 8226/S multiple myeloma cells exposed to DMAIII(GS) was performed using the developed method, following the separate injection of non-GSH complexed and GSH-complexed As standards. The results showed that, in addition to the parent DMAIII(GS), its dissociation product, DMAIII, along with the further oxidation product, DMAV, were detected (Fig. 7). The thiol-containing arsenical, DMMTAV, was detected, and more importantly its GSH conjugate, DMMTAV(GS), was found to be the major metabolite of DMAIII(GS) metabolism in the cells. These results suggest that thiol-containing arsenicals may play an important role in the metabolism of DMAIII(GS) and these thiolated arsenicals could be eventually related to the anticancer activity of DMAIII(GS). The simultaneous determination of thiol-containing and non-thiol-containing arsenicals (e.g., DMMTAV(GS), DMMTAV, and DMAIII) demonstrates the robustness of the developed method, as a combination of two HPLC-ICP-MS methods had to be used to run the same sample twice for the separation of the metabolites of interest in our previous studies [25]. Even running both previous methods (on two different columns) could not separate all possible As intermediates during Darinaparsin metabolism, as indicated by the missing of DMMTAV in the previous studies. It was also observed that the method developed had better resolution of As species separation.
Fig. 7.
Results of human cells analysis using the developed method after incubation with darinaparsin. The compounds separated are as follows: 6. DMMTAV, 7. DMAV, 9. DMMTAV(GS), 12. DMAIII, 13. DMAIII(GS)
3.6 Potentials and challenges
The HPLC separation method developed in this study can be used for ICP-MS (as reported here) or ESI-MS detection, as the mobile phases used in the HPLC separation are compatible with ESI-MS. It is possible to use the separation method on ESI-MS for the identification of unknown As species, with the flow rate of mobile phase being adjusted to 0.5 mL/min during equilibration and the equilibration time being extended due to the reduced flow rate. Fortunately, these changes during equilibration stage will not affect chromatographic separation of As species. This dual suitability of the separation method for both ICP-MS and ESI-MS can help us in the unequivocal identification of As species. It should be noted that not all As species could be easily detected on ESI-MS, as some As species do not have high ionization efficiency and/or could get oxidized during the electrospray ionization.
When identifying and quantifying As metabolites, the unavailability of some species as stable high purity standards poses a significant problem. AsIII, AsV, MMAIII, MMAV, DMAV, AsIII(GS)3, MMAIII(GS)2, DMAIII(Cys), and DMAIII(GS) can be purchased or synthesized as high purity standards, but DMDTAV, DMMTAV, DMAIII, and DMMTAV(GS) cannot, making the quantification of these species difficult. For example, DMAIII has the high potency of being oxidized to DMAV without the presence of relatively high concentrations of GSH, so it is difficult to accurately quantify this species in the absence of GSH. However, it is also difficult to detect DMAIII in the presence of GSH, as in this case DMAIII is present in the form of DMAIII(GS) and not free DMAIII.
4 Conclusions
An HPLC-ICP-MS method was developed and optimized in an attempt to analyze for the above mentioned thirteen confirmed and plausible human As metabolites and intermediates of As metabolism in one chromatographic run. Results showed that while it is difficult to separate all these species due to their very different chemical properties, it is indeed achievable. The HPLC-ICP-MS technique described here is applicable to the analysis of these arsenic metabolites from cells as demonstrated with the incubation experiments of human cancer cells with DMAIII(GS). While ICP-MS is supposed to be element specific, significant baseline disturbance was observed due to high ACN v/v% present in the gradient elution program. Adjustment of Ar nebulizer flow rate could reduce the problem. Under optimum conditions, the detection limits were shown to be 1.25 μg L-1 and the quantitation limit 2.5 μg L-1. The method was found to be linear between the tested ranges of 1.25 to 2500 μg L-1. This method could be also suitable for use on HPLC-ESI-MS for the identification of unknown As metabolites.
Supplementary Material
Highlights.
Development of an HPLC-ICP-MS method for the detection of 13 human arsenic metabolites.
Analysis of multiple sulfur-containing arsenicals and glutathione complexes.
Identification of arsenic metabolites in human cancer cells exposed to dimethylarsinous glutathione.
Acknowledgments
This work was made possible through NIH (R01CA129968) and NIEHS ARCH (S11ES11181) program. Szabina Stice thanks the FIU MBRS RISE program for their financial support. This is the contribution # of Southeast Environmental Research Center at FIU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konkola K. More Than a Coincidence? The Arrival of Arsenic and the Disappearance of Plague in Early Modern Europe. J Hist Med All Sci. 1992;47:186–209. doi: 10.1093/jhmas/47.2.186. [DOI] [PubMed] [Google Scholar]
- 2.Nelson L. Goldfrank's toxicologic emergencies. McGraw-Hill Medical; New York: 2011. [Google Scholar]
- 3.Mandal B. Arsenic round the world: a review. Talanta. 2002;58:201–235. [PubMed] [Google Scholar]
- 4.Smedley P, Kinniburgh DG. Arsenic in groundwater and the environment. Springer; Netherlands: 2013. [Google Scholar]
- 5.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annu Rev Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 6.Brammer H, Ravenscroft P. Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int. 2009;35:647–654. doi: 10.1016/j.envint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC. Dietary arsenic exposure in Bangladesh. Environ Health Perspect. 2007;115:889. doi: 10.1289/ehp.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meharg AA, Rahman MM. Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Tech. 2003;37:229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- 9.Francesconi KA. Arsenic species in seafood: Origin and human health implications. Pure Appl Chem. 2010;82:373–381. [Google Scholar]
- 10.Bhattacharjee P, Chatterjee D, Singh KK, Giri AK. Systems biology approaches to evaluate arsenic toxicity and carcinogenicity: An overview. Int J Hyg Envir Heal. 2013 doi: 10.1016/j.ijheh.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee M, Bhattacharjee P, Giri AK. Arsenic-induced cancers: a review withspecial reference to gene, environment and their interaction. Gene Environ. 2011;33:128–140. [Google Scholar]
- 12.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 13.Antman KH. Introduction: The History of Arsenic Trioxide in Cancer Therapy. Oncologist. 2001;6:1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 14.Waxman S, Anderson KC. History of the Development of Arsenic Derivatives in Cancer Therapy. Oncologist. 2001;6:3–10. doi: 10.1634/theoncologist.6-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 15.Aronson S. Arsenic and old myths. Rhode Island medicine. 1994;77:233. [PubMed] [Google Scholar]
- 16.Haller JS. Therapeutic mule: the use of arsenic in the nineteenth century materia medica. Pharmacy in History. 1975;17:87–100. [PubMed] [Google Scholar]
- 17.Liu JX, Zhou GB, Chen SJ, Chen Z. Arsenic compounds: revived ancient remedies in the fight against human malignancies. Curr Opin Chem Biol. 2012;16:92–98. doi: 10.1016/j.cbpa.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 19.Tsimberidou AM, Camacho LH, Verstovek S, Ng C, Hong DS, Uehara CK, Gutierrez C, Daring S, Stevens J, Komarnitsky PB, Schwartz B, Kurzrock R. A Phase I Clinical Trial of Darinaparsin in Patients with Refractory Solid Tumors. Clin Cancer Res. 2009;15:4769–4776. doi: 10.1158/1078-0432.CCR-08-2984. [DOI] [PubMed] [Google Scholar]
- 20.Matulis SM, Morales AA, Yehiayan L, Croutch C, Gutman D, Cai Y, Lee KP, Boise LH. Darinaparsin induces a unique cellular response and is active in an arsenic trioxide-resistant myeloma cell line. Mol Cancer Ther. 2009;8:1197–1206. doi: 10.1158/1535-7163.MCT-08-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Henderson C, Feun L, Van Veldhuizen P, Gold P, Zheng H, Ryan T, Blaszkowsky LS, Chen H, Costa M, Rosenzweig B, Nierodzik M, Hochster H, Muggia F, Abbadessa G, Lewis J, Zhu AX. Phase II study of darinaparsin in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2010;28:670–676. doi: 10.1007/s10637-009-9286-9. [DOI] [PubMed] [Google Scholar]
- 22.Lossos I, Craig M, Tallman R, Boccia P, Conkling C, Becerra P, Komarnitsky B, Hamilton Lewis J, Miller W. Novel organic arsenic molecule darinaparsin: development of IV and oral forms. J Clin Onc, Proc Am Soc Clin Onc. 2009;27 A# 8501. [Google Scholar]
- 23.Raml R, Rumpler A, Goessler W, Vahter M, Li L, Ochi T, Francesconi KA. Thio-dimethylarsinate is a common metabolite in urine samples from arsenic-exposed women in Bangladesh. Toxicol Appl Pharmacol. 2007;222:374–380. doi: 10.1016/j.taap.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki KT, Iwata K, Naranmandura H, Suzuki N. Metabolic differences between two dimethylthioarsenicals in rats. Toxicol Appl Pharmacol. 2007;218:166–173. doi: 10.1016/j.taap.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Yehiayan L. Ph D Dissertation. Florida International University; Miami, Florida, USA.: 2010. Interactions of different arsenic species with thols: Chemical and biological implications. [Google Scholar]
- 26.Naranmandura H, Suzuki N, Iwata K, Hirano S, Suzuki KT. Arsenic metabolism and thioarsenicals in hamsters and rats. Chem Res Toxicol. 2007;20:616–624. doi: 10.1021/tx700038x. [DOI] [PubMed] [Google Scholar]
- 27.Naranmandura H, Suzuki KT. Formation of dimethylthioarsenicals in red blood cells. Toxicol Appl Pharmacol. 2008;227:390–399. doi: 10.1016/j.taap.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Yehiayan SSL, Liu G, Matulis S, Boiseb LH, Cai Y. Dimethylarsinothioyl glutathione as a metabolite in human multiple myeloma cell lines upon exposure to Darinaparsin. Chem Res Toxicol. 2014 doi: 10.1021/tx400386c. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehman K, Naranmandura H. Arsenic metabolism and thioarsenicals. Metallomics. 2012;4:881–892. doi: 10.1039/c2mt00181k. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 31.Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD. Methylated Trivalent Arsenic Species Are Genotoxic. Chem Res Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- 32.Naranmandura H, Iwata K, Suzuki KT, Ogra Y. Distribution and metabolism of four different dimethylated arsenicals in hamsters. Toxicol Appl Pharmacol. 2010;245:67–75. doi: 10.1016/j.taap.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Kojima C, Sakurai T, Waalkes MP, Himeno S. Cytolethality of Glutathione Conjugates with Monomethylarsenic or Dimethylarsenic Compounds. Biol Pharm Bull. 2005;28:1827–1832. doi: 10.1248/bpb.28.1827. [DOI] [PubMed] [Google Scholar]
- 34.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 35.Hirano S, Kobayashi Y, Cui X, Kanno S, Hayakawa T, Shraim A. The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol Appl Pharmacol. 2004;198:458–467. doi: 10.1016/j.taap.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Gong Z, Lu X, Ma M, Watt C, Le XC. Arsenic speciation analysis. Talanta. 2002;58:77–96. doi: 10.1016/s0039-9140(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 37.Chungang Y, Le XC. Arsenic Speciation Analysis. Prog Chem. 2009;21:467–473. [Google Scholar]
- 38.Hsu KC, Sun CC, Huang YL. Arsenic speciation in biomedical sciences: Recent advances and applications. Kaohsiung J Med Sci. 2011;27:382–389. doi: 10.1016/j.kjms.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.B'Hymer C, Caruso JA. Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. J Chromatogr A. 2004;1045:1–13. doi: 10.1016/j.chroma.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Francesconi KA, Kuehnelt D. Determination of arsenic species: A critical review of methods and applications, 2000-2003. Analyst (London) 2004;129:373. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 41.Raab A, Meharg AA, Jaspars M, Genney DR, Feldmann J. Arsenic-glutathione complexes: their stability in solution and during separation by different HPLC modes. J Anal At Spectrom. 2004;19:183. [Google Scholar]
- 42.Yehiayan L, Membreno N, Matulis S, Boise LH, Cai Y. Extraction tool and matrix effects on arsenic speciation analysis in cell lines. Anal Chim Acta. 2011;699:187–192. doi: 10.1016/j.aca.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazakevich YV, Lobrutto R. HPLC for Pharmaceutical Scientists. John Wiley & Sons; New York: 2007. [Google Scholar]
- 44.Kralj P, Veber M. Investigations into nonspectroscopic effects of organic compounds in inductively coupled plasma mass spectrometry. Acta chimica slovenica. 2003;50:633–644. [Google Scholar]
- 45.Stewart II, Olesik JW. The effect of nitric acid concentration and nebulizer gas flow rates on aerosol properties and transport rates in inductively coupled plasma sample introduction. J Anal At Spectrom. 1998;13:1249–1256. [Google Scholar]
- 46.Lam JWH, Horlick G. A comparison of argon and mixed gas plasmas for inductively coupled plasma-mass spectrometry. Spectrochim Acta B. 1990;45:1313–1325. [Google Scholar]
- 47.Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2012:1–11. doi: 10.1007/s00204-012-0904-5. [DOI] [PubMed] [Google Scholar]
- 48.Le XC, Lu X, Ma M, Cullen WR, Aposhian HV, Zheng B. Speciation of Key Arsenic Metabolic Intermediates in Human Urine. Anal Chem. 2000;72:5172–5177. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- 49.Mandal BK, Ogra Y, Suzuki KT. Identification of Dimethylarsinous and Monomethylarsonous Acids in Human Urine of the Arsenic-Affected Areas in West Bengal, India. Chem Res Toxicol. 2001;14:371–378. doi: 10.1021/tx000246h. [DOI] [PubMed] [Google Scholar]
- 50.Mandal B, Ogra Y, Anzai K, Suzuki KT. Speciation of arsenic in biological samples. Toxicol Appl Pharmacol. 2004;198:307–318. doi: 10.1016/j.taap.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Zhou J, Lu X, Gong Z, Le XC. Arsenic Speciation in Urine from Acute Promyelocytic Leukemia Patients undergoing Arsenic Trioxide Treatment. Chem Res Toxicol. 2004;17:95–103. doi: 10.1021/tx0341714. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, Del Razo LM. Urinary Trivalent Methylated Arsenic Species in a Population Chronically Exposed to Inorganic Arsenic. Environ Health Perspect. 2004;113:250–254. doi: 10.1289/ehp.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshino Y, Yuan B, Miyashita Si, Iriyama N, Horikoshi A, Shikino O, Toyoda H, Kaise T. Speciation of arsenic trioxide metabolites in blood cells and plasma of a patient with acute promyelocytic leukemia. Anal Bioanal Chem. 2009;393:689–697. doi: 10.1007/s00216-008-2487-9. [DOI] [PubMed] [Google Scholar]
- 54.Devesa V, Del Razo LM, Adair B, Drobná Z, Waters SB, Hughes MF, Stýblo M, Thomas DJ. Comprehensive analysis of arsenic metabolites by pH-specific hydride generation atomic absorption spectrometry. J Anal At Spectrom. 2004;19:1460–1467. [Google Scholar]
- 55.Xie R, Johnson W, Spayd S, Hall GS, Buckley B. Arsenic speciation analysis of human urine using ion exchange chromatography coupled to inductively coupled plasma mass spectrometry. Anal Chim Acta. 2006;578:186–194. doi: 10.1016/j.aca.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 56.Cui X, Kobayashi Y, Hayakawa T, Hirano S. Arsenic speciation in bile and urine following oral and intravenous exposure to inorganic and organic arsenics in rats. Toxicol Sci. 2004;82:478–487. doi: 10.1093/toxsci/kfh265. [DOI] [PubMed] [Google Scholar]
- 57.Kala SV, Kala G, Prater CI, Sartorelli AC, Lieberman MW. Formation and urinary excretion of arsenic triglutathione and methylarsenic diglutathione. Chem Res Toxicol. 2004;17:243–249. doi: 10.1021/tx0342060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.