Abstract
BACKGROUND
Prednisone and other corticosteroids can provide palliation and tumor responses in patients with prostate cancer. The combination of docetaxel and prednisone was the first treatment shown to prolong survival in men with metastatic castration-resistant prostate cancer (mCRPC). Since the approval of docetaxel in 2004, additional treatments are available, including abiraterone, which is also administered with prednisone. Therefore, patients are increasingly likely to have prednisone therapy several times throughout their disease course, and the contribution of prednisone to the efficacy of docetaxel is unknown.
METHODS
We conducted a retrospective study of patients with mCPRC treated with docetaxel at our institution between 2004–2014. Patients were divided into 2 cohorts based upon whether prednisone was co-administered with docetaxel. Cohorts were further stratified based upon prior prednisone (with abiraterone) or hydrocortisone (with ketoconazole) use. The primary endpoint was clinical/radiographic progression-free survival (PFS). The secondary endpoints were >50% PSA response rate and PSA progression-free survival (PSA-PFS). A multivariable cox regression model was constructed to determine if prednisone use was independently predictive of PFS.
RESULTS
We identified 200 consecutive patients for inclusion in the study: 131 men received docetaxel with prednisone and 69 received docetaxel alone. The docetaxel-prednisone cohort had superior PFS compared to the docetaxel-alone cohort (median PFS: 7.8 vs 6.2 months, HR 0.68 [95% CI 0.48–0.97], p=0.03). Prednisone was associated with a reduced risk of progression on docetaxel in the propensity score-weighted multivariable Cox model (p=0.002). Among abiraterone- or ketoconazole-pretreated patients, no difference in PFS was observed between prednisone-containing and non-prednisone containing cohorts (median PFS: 7.1 vs 6.3 months, HR 0.96 [95% CI 0.59–1.57], p=0.87).
CONCLUSIONS
The incorporation of prednisone potentially augments the efficacy of docetaxel in patients with mCRPC. We hypothesize that this advantage is limited to patients who have not previously received corticosteroids. Prospective confirmation is needed.
Keywords: prednisone, docetaxel, taxane, chemotherapy, metastatic prostate cancer
Introduction
Prednisone and other corticosteroids are used frequently in the treatment of advanced prostate cancer. Corticosteroids are sometimes prescribed to alleviate pain from bone metastases,1 for management of cancer-related fatigue,2 or to potentially reduce chemotherapy-related toxicity.3 Beyond these palliative uses, corticosteroids have also been associated with favorable antitumor responses.4 In addition, a number of randomized trials in advanced prostate cancer have used corticosteroids (namely prednisone) as the backbone or the control arm of these studies. This includes the study of Tannock et al. comparing mitoxantrone plus prednisone vs. prednisone alone which led to FDA approval of the combination for palliation of symptomatic castration-resistant prostate cancer (CRPC).5 Thus, data on efficacy of many drugs in prostate cancer is interpreted in the context of concurrent corticosteroid use.
Docetaxel was the first chemotherapy agent shown to prolong survival in men with metastatic CRPC. In the pivotal TAX327 study, 75mg/m2 of docetaxel given intravenously every 3 weeks was compared to mitoxantrone given every 3 weeks. Since the control group in this study consisted of mitoxantrone and prednisone, patients on the docetaxel arm also received the same dose of 5mg of prednisone administered orally twice daily.6 The arm receiving every-3-week docetaxel (plus prednisone) demonstrated superior survival, resulting in FDA approval of docetaxel plus prednisone in 2004 for metastatic CRPC,7 and quickly replacing the prior standard-of-care consisting of mitoxantrone plus prednisone. Notably, a non-prednisone containing regimen of docetaxel plus estramustine was also shown to be superior to mitoxantrone plus prednisone,8 but this regimen has fallen out of favor due to the significant toxicities of estramustine and the questionable added benefit.9
Since 2004, docetaxel has been a cornerstone of treatment for men with advanced prostate cancer. In modern clinical practice, however, prednisone is not always co-administered with docetaxel, for a number of reasons.10 First, some oncologists have concerns about the sequelae of chronic prednisone use, such as glucose intolerance, osteopenia, fluid retention and peptic ulcers, among other risks.11 Furthermore, there is a theoretical risk of activating the androgen receptor (AR) with prednisone, leading to growth of prostate cancer.12 Patients experiencing progression on antiandrogen therapy occasionally have responses to antiandrogen withdrawal;13 one basis for this observation is changes in AR signaling leading to paradoxical AR agonism with antiandrogens.14 Similarly, other AR mutations may allow activation by glucocorticoids. For example, although the wild-type AR does not engage glucocorticoids, the T878A and L702H mutations in AR allow glucocorticoid binding to the ligand-binding domain,15 leading to glucocorticoid-mediated activation of downstream androgen-response elements causing growth of cancer cells.16
Although docetaxel is frequently given in combination with prednisone based on the results of the TAX327 study, there is no compelling biological evidence for synergy between glucocorticoids and taxanes. Thus, whether prednisone contributes to the efficacy of docetaxel or is merely a vestige of docetaxel’s approval process remains unclear.17 Two recently presented studies that tested docetaxel in metastatic castration-sensitive disease employed different strategies regarding prednisone. In STAMPEDE, docetaxel and prednisolone were added to androgen deprivation therapy (ADT) and compared to ADT alone. The patients receiving up to 6 cycles of chemotherapy had significantly improved survival (77mo vs 67mo, HR 0.76 [95%CI 0.63–0.91]).18 In CHAARTED, patients who received up to 6 cycles of docetaxel plus ADT—without prednisone—also demonstrated significantly improved survival compared to patients receiving ADT alone (52.7mo vs 42.3mo, HR= 0.63 [95%CI 0.48–0.82]).19 The efficacy of both CHAARTED and STAMPEDE protocols casts some doubt to prednisone’s added value in this setting.
This begs the question of whether prednisone is required for patients receiving docetaxel for metastatic CRPC. Since there are no prospective trials comparing docetaxel/prednisone with docetaxel-alone, we conducted a retrospective analysis to investigate the independent contribution of prednisone on the clinical efficacy of docetaxel. This study was enabled by the fact that not all clinicians using docetaxel to treat prostate cancer at our institution routinely prescribed it with prednisone. We also examined whether prior use of abiraterone (which is also given with prednisone) or ketoconazole (which is given with hydrocortisone) influenced the effect of prednisone on docetaxel. We hypothesized that prednisone would augment the efficacy of docetaxel, but only in men who had not received prior corticosteroids in combination with either abiraterone or ketoconazole.
Patients and Methods
We conducted an IRB-approved retrospective study involving consecutive patients treated with first-line docetaxel chemotherapy for metastatic CRPC at our institution between 2004 and 2014. Only patients who received every-3-weekly docetaxel (at a planned dose of 75mg/m2) were included; those receiving weekly docetaxel were not studied. Patients who received additional concurrent therapies (e.g. abiraterone, enzalutamide, radium-223), those who had small cell/neuroendocrine histologies, and those without follow-up information were excluded.
Patients were divided into two cohorts: those who received docetaxel with concurrent prednisone, and those who received docetaxel alone. These two groups emerged due to variations in physician practice with respect to routinely prescribing prednisone with docetaxel. We also gathered further clinical data on age, year of docetaxel initiation, Gleason score, number and types prior hormonal therapies received (with particular attention to prior abiraterone or enzalutamide use), presence of visceral metastases, ECOG performance status, presence of pain, baseline hemoglobin, creatinine, alkaline phosphatase, and prostate-specific antigen (PSA) value. The number of cycles of docetaxel administered, date of PSA progression, and date of clinical/radiographic progression were recorded. We defined PSA progression as an increase from nadir of 25% (and at least 2ng/mL), without requiring a confirmatory PSA measurement. Clinical/radiographic progression was defined as the first of soft tissue progression, bone scan progression, or clinical progression. In patients with measureable disease, radiographic progression was determined by applying RECIST criteria (i.e. >20% increase in the sum of target lesions). Progression on the basis of bone scan was defined as the development of 2 new osseous lesions that were not related to a flare phenomenon, similar to Prostate Cancer Working Group 2 (PCWG2) criteria;20 however, confirmatory imaging was not required. Progression on clinical grounds was defined as an escalation of bone pain, a referral for palliative radiation to bone, a referral for surgical stabilization of bone, or any other cancer-related complication (e.g. obstructive uropathy, myelophthisic bone marrow failure). Data was censored at the time of initiation of further therapy if that date occurred prior to PSA or clinical/radiographic progression.
The primary endpoint was clinical/radiographic progression-free survival (PFS). We chose PFS as the endpoint instead of overall survival because most patients received multiple subsequent therapies that may have confounded survival estimates. Secondary endpoints included >50% PSA response rates and PSA progression-free survival (PSA-PFS); PSA response rates correlate with overall survival in meta-analyses of docetaxel-based trials.21 We also performed subgroup analyses investigating the effect of prednisone on docetaxel’s efficacy in 2 substrata: men who had previously received abiraterone or ketoconazole and men who had not received abiraterone or ketoconazole before.
Statistical considerations: A comparison of baseline characteristics between the docetaxel/prednisone group and the docetaxel-alone group was performed using Fisher’s exact test for categorical variables and Wilcoxon-Mann Whitney test for continuous variables. Best PSA responses were depicted using standard waterfall plots; PSA response rates were compared using Fisher’s exact test. PFS and PSA-PFS were displayed using Kaplan-Meier curves, and differences between groups were sought with the log-rank test. To adjust for baseline clinical characteristics, univariate and propensity score-weighted multivariable regression models were constructed to determine if prednisone use was independently predictive of clinical outcomes. To estimate whether the influence of prednisone on docetaxel efficacy differed according to prior abiraterone or ketoconazole use, multivariable Cox regression analyses were performed with the interaction terms of ‘prednisone’ and ‘abiraterone’/’ketoconazole’, and simultaneously adjusted for the other clinical variables.
Results
We identified 200 consecutive patients for inclusion in the study: 131 men received docetaxel with prednisone, and 69 received docetaxel alone, reflecting different practice patterns at our institution. Summary statistics for each cohort are listed in Table 1. Groups were generally balanced with respect to baseline characteristics. More patients had missing performance status data in the docetaxel-alone group. Prior abiraterone use was significantly more common in the docetaxel/prednisone cohort. Enzalutamide treatment prior to chemotherapy22 was uncommon in both groups during this time. Notably, all patients who received prior enzalutamide also received abiraterone.
Table 1.
Baseline characteristics.
| Characteristic | All Patients (n = 200) | Docetaxel Alone (n = 69) | Docetaxel + Prednisone (n = 131) | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 68 (45 – 85) | 68 (52 – 85) | 69 (45 – 85) | 0.45 |
|
| ||||
| Performance Status | ||||
| 0 | 75 (38%) | 22 (32%) | 53 (40%) | 0.001 |
| 1 – 2 | 86 (43%) | 21 (30%) | 50 (50%) | |
| Missing | 39 (20%) | 26 (38%) | 13 (10%) | |
|
| ||||
| Hemoglobin (g/dL) | 11.9 (7.7 – 15.9) | 11.9 (7.7 – 15.9) | 11.9 (8.1 – 15.3) | 0.49 |
|
| ||||
| Creatinine (mg/dL) | 0.9 (0.5 – 3.5) | 1.0 (0.5 – 3.5) | 0.9 (0.5 – 2.1) | 0.08 |
|
| ||||
| Alkaline Phosphatase (IU/L) | 137 (39 – 2109) | 125 (44 – 684) | 149 (39 – 2109) | 0.22 |
|
| ||||
| PSA (ng/dL) | 153 (1.2 – 5327) | 131.1 (2.9 – 4861) | 155.7 (1.2 – 5327) | 0.96 |
|
| ||||
| Gleason score sum | ||||
| 6 | 13 (6%) | 5 (7%) | 8 (6%) | 0.56 |
| 7 | 50 (25%) | 17 (25%) | 33 (25%) | |
| 8 – 10 | 116 (58%) | 37 (54%) | 79 (60%) | |
| Missing | 21 (10%) | 10 (14%) | 11 (8%) | |
|
| ||||
| Presence of visceral metastasis | ||||
| Liver | 30 (15%) | 10 (14%) | 20 (15%) | 0.99 |
| Lung | 24 (12%) | 6 (9%) | 18 (14%) | 0.36 |
|
| ||||
| Presence of pain | 82 (41%) | 26 (38%) | 56 (43%) | 0.55 |
|
| ||||
| Number of prior hormonal therapies | 3 (1 – 5) | 2 (1 – 5) | 3 (1 – 5) | 0.45 |
|
| ||||
| Prior Abiraterone – Prednisone | 46 (23%) | 9 (13%) | 37 (28%) | 0.02 |
|
| ||||
| Prior Ketoconazole – Hydrocortisone | 77 (38%) | 26 (38%) | 51 (39%) | 0.88 |
|
| ||||
| Prior Enzalutamide | 14 (7%) | 2 (3%) | 12 (9%) | 0.15 |
Data reported as median (range) or percentages. P-values for categorical variables are based on Fisher’s Exact test, and for continuous variables are based on Wilcoxon-Mann Whitney test.
Clinical/radiographic PFS for the entire docetaxel-treated population (depicted in Figure 1) was the primary endpoint. In an unadjusted analysis, PFS was superior in the docetaxel/prednisone group compared to the docetaxel-alone group (median PFS: 7.8 vs 6.2 months, HR 0.68 [95% CI 0.48–0.97], p=0.03). The clinical/radiographic PFS advantage for the docetaxel/prednisone cohort was supported by the difference in the number of chemotherapy cycles received. On average, the docetaxel/prednisone cohort received 7.3 cycles of docetaxel compared to 5.7 cycles for the docetaxel alone cohort. These numbers corresponded to a median of 7 cycles (range 1 – 19) versus 6 cycles (range (1 – 13) for docetaxel/prednisone and docetaxel alone cohorts, respectively.
Figure 1.
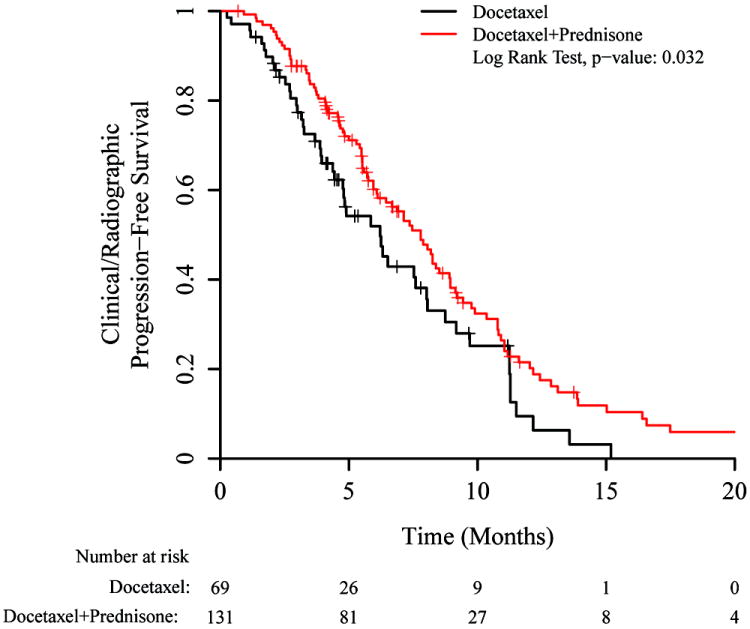
Kaplan-Meier plot for clinical/radiographic progression-free survival (PFS), based on whether or not prednisone was co-administered with docetaxel. Hazard ratio = 0.68 (0.48 – 0.97).
We next constructed a propensity score-weighted multivariable Cox model to determine factors independently associated with PFS (Table 2). In this model, prednisone use was significantly associated with a reduced risk of clinical/radiographic progression on docetaxel, after adjusting for other factors (HR 0.53 [95% CI 0.35–0.80], p=0.002). Performance status 0 (vs 1-2) was also associated with reduced risk for clinical/radiographic progression. Gleason score 7 or 8-10 appeared to be more favorable than Gleason score 6, an unexpected finding, although only 6% of patients had Gleason 6 disease. Prior data has shown that the incremental benefit for high-grade tumors is greater with docetaxel compared to lower-grade tumors, but patients with lower-grade tumors have longer survival.23 Prior abiraterone or ketoconazole use was not significantly associated with inferior PFS in this model; however, the interaction test between treatment with prednisone and prior abiraterone or ketoconazole use was significant.
Table 2.
Propensity Score-Weighted Multivariable Cox Model for Clinical/Radiographic Progression-Free Survival (PFS).
| Hazard Ratio (95% C.I.) | P-value | |
|---|---|---|
|
| ||
| Treatment | ||
| Docetaxel Alone | 1.0 [Ref] | |
| Docetaxel + Prednisone | 0.53 (0.35 – 0.80) | 0.002 |
|
| ||
| Performance Status | ||
| 0 | 1.0 [Ref] | |
| 1 – 2 | 1.37 (1.003 – 1.87) | 0.05 |
|
| ||
| Presence of Pain | ||
| No | 1.0 [Ref] | |
| Yes | 1.15 (0.86 – 1.55) | 0.36 |
|
| ||
| PSA | 1 [Based upon 1 unit change] | 0.91 |
|
| ||
| Alkaline Phosphatase | 1 | 0.83 |
|
| ||
| Gleason Score | ||
| 6 | 1.0 [Ref] | |
| 7 | 0.57 (0.33 – 0.99) | 0.04 |
| 8 – 10 | 0.60 (0.36 – 0.99) | 0.05 |
|
| ||
| Visceral Metastasis: Liver | ||
| No | 1.0 [Ref] | |
| Yes | 1.22 (0.80 – 1.84) | 0.36 |
|
| ||
| Visceral Metastasis: Lung | ||
| No | 1.0 [Ref] | |
| Yes | 1.07 (067 – 1.69) | 0.78 |
|
| ||
| Prior Abi/Keto. | ||
| No | 1.0 [Ref] | |
| Yes | 0.73 (0.46 – 1.16) | 0.18 |
|
| ||
| Interaction | ||
| Treatment * Prior Abi/Keto | 1.79 (1.001 – 3.19) | 0.05 |
In a prespecified analysis, we examined clinical/radiographic PFS stratified by prior abiraterone or ketoconazole use (abiraterone is prescribed with 10mg of prednisone daily and ketoconazole is prescribed with 30mg of hydrocortisone daily, so patients with prior abiraterone or ketoconazole exposure also had prior corticosteroid exposure). Among corticosteroid-naïve patients (i.e. no prior abiraterone or ketoconazole), docetaxel/prednisone was superior to docetaxel alone (Figure 2A; median PFS: 8.9 vs 5.9 months, HR 0.49 [95% CI 0.29–0.84], p=0.009). For abiraterone- or ketoconazole-pretreated patients, a difference in PFS was not seen between docetaxel/prednisone and docetaxel-alone arms (Figure 2B; median PFS: 7.1 vs 6.3 months, HR 0.96 [95% CI 0.59–1.57], p=0.87).
Figure 2.
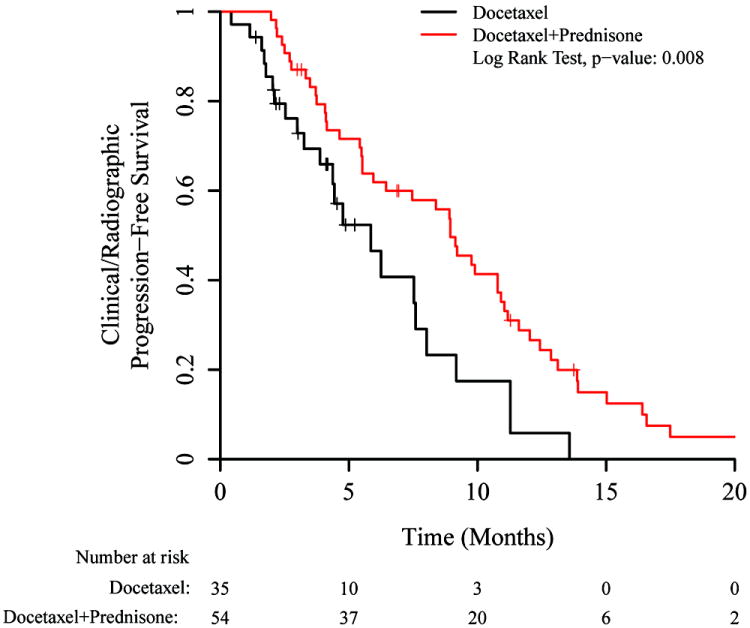
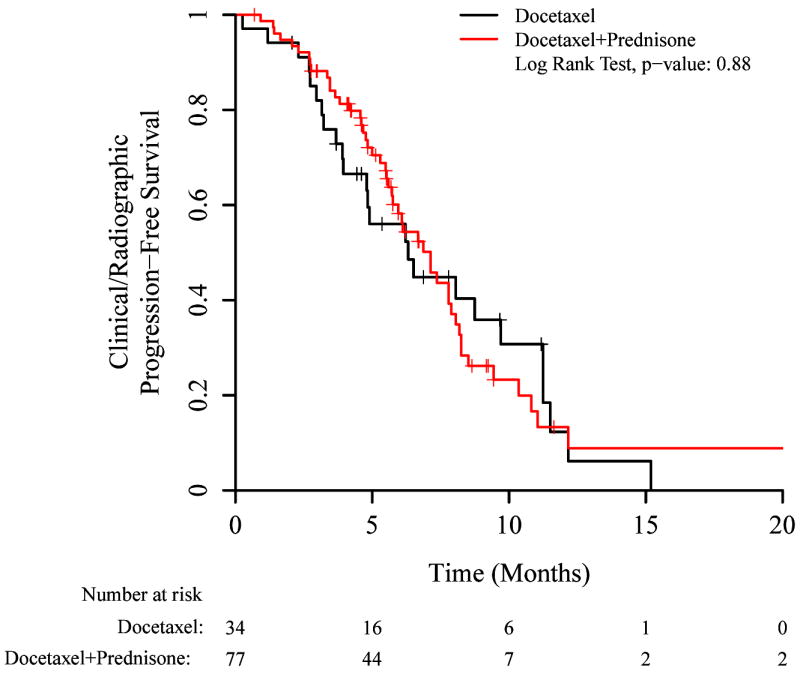
Kaplan-Meier plots for clinical/radiographic PFS, showing subsets according to prior use of abiraterone or ketoconazole. A) No prior use of abiraterone or ketoconazole (hazard ratio 0.49 [0.29–0.84]), B) Prior use of abiraterone or ketoconazole (hazard ratio 0.96 [0.59–1.57]).
PSA responses were also analyzed in the 2 patient cohorts. Best PSA responses are illustrated in the waterfall plots in Figure 3; >50%PSA responses were numerically higher in the docetaxel-prednisone cohort. Table 3 shows >50% PSA response rates stratified by prior abiraterone or ketoconazole use; superior PSA responses in the docetaxel/prednisone group were only observed in those who had not previously received abiraterone or ketoconazole. In a propensity score-weighted multivariable logistic regression model, poor performance status and liver metastases were independently associated with a poor PSA response.
Figure 3.
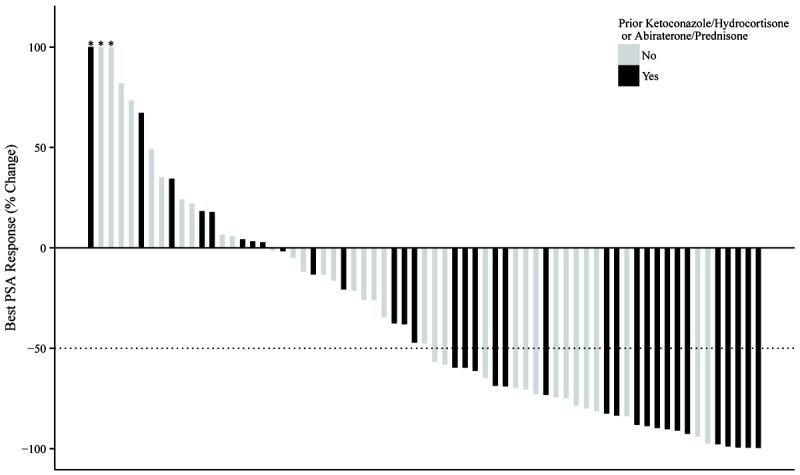
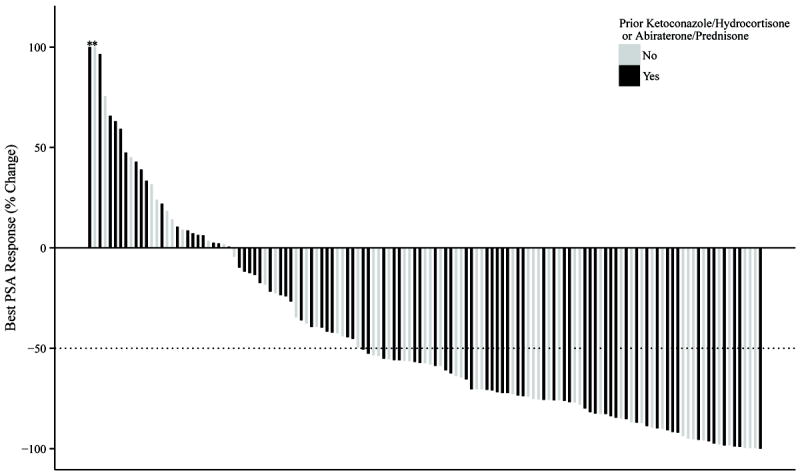
Waterfall plots depicting best PSA response, according to whether or not prednisone was co-administered with docetaxel. The proportion of men with >50% PSA responses was 48% for those receiving docetaxel alone (A) and was 60% for those receiving docetaxel plus prednisone (p = 0.14) (B). Dark bars indicate patients who had received prior abiraterone or ketoconazole; light bars indicate those who had not received prior abiraterone or ketoconazole. Values >100% are truncated (as depicted by the asterisks).
Table 3.
Proportion of Patients Achieving a >50% PSA Response, According to Prior Abiraterone or Ketoconazole Use.
| All Patients (n = 200) | Docetaxel Alone (n = 69) | Docetaxel + Prednisone (n = 131) | P-value | |
|---|---|---|---|---|
| No Prior Abi/Keto (n = 89) | 49 / 89 (55%) | 14 / 35 (40%) | 35 / 54 (65%) | 0.03 |
| Prior Abi/Keto (n = 111) | 62 / 111 (56%) | 19 / 34 (56%) | 43 / 77 (56%) | >0.99 |
P-values are based on Fisher’s Exact test.
We also compared PSA progression-free survival (PSA PFS) between cohorts. Median PSA PFS was similar in the docetaxel/prednisone and docetaxel-alone cohorts in an unadjusted analysis (5.5 vs 5.0 months, HR 0.80 [95% CI 0.57–1.13], p=0.20). For the subgroup of patients without prior abiraterone or ketoconazole use, prednisone use was significantly associated with a reduced risk for PSA progression (HR 0.59 [95% CI 0.36–0.99], p=0.04). For abiraterone- or ketoconazole-treated patients, however, the differences were non-significant (HR 1.09 ]95% CI 0.68–1.75], p=0.71). In the propensity score-weighted multivariable Cox model, concurrent prednisone use was associated with a decreased risk of PSA progression (HR 0.53 [95% CI 0.35–0.79], p=0.002), and the interaction test between prednisone use and prior abiraterone or ketoconazole use was significant (p=0.04).
Discussion
Both prednisone and docetaxel are active agents in the treatment of advanced prostate cancer. In our study, we postulated that docetaxel plus prednisone would be more effective than docetaxel alone. Indeed, patients in the docetaxel/prednisone cohort had superior outcomes (longer PFS, longer PSA PFS, higher PSA repose rates) than those receiving docetaxel alone. One possible explanation for this result is that patients received additive benefit from two drugs that are each known to be effective in prostate cancer through different mechanisms.
The activity of prednisone as a single agent has been well characterized. For example, in a randomized phase III trial of mitoxantrone/prednisone versus prednisone-alone, patients receiving 10mg of prednisone daily had >50% PSA response rates of 24% and a PFS of 4.1 months.24 Comparable responses were reported in the control arm of the pre-chemotherapy COU-AA-302 study of abiraterone/prednisone versus prednisone-alone.25 In that study, 24% of patients receiving prednisone 10mg daily achieved a >50% PSA response rate, with a PFS of 8.1 months. The mechanism by which prednisone exerts its activity in prostate cancer is at least partially understood. Corticosteroids can suppress adrenal androgens leading to a more complete androgen blockade. They can also inhibit growth of prostate cancer cells through action on various cellular signals, including upregulation of TGF-beta and downregulation of IL-6.26
Docetaxel, a taxane chemotherapeutic, acts through its stabilization of microtubules, preventing mitosis. When used for prostate cancer, docetaxel is also believed to interfere with androgen receptor trafficking.27 Docetaxel’s efficacy has been observed to be reduced when used after multiple hormonal agents, including abiraterone.28 Because of the proposed cross-resistance between abiraterone and docetaxel, we performed a prespecified subgroup analysis based upon stratification by prior abiraterone or ketoconazole exposure. We believe that these results generate an interesting hypothesis: that prednisone may only enhance the efficacy of docetaxel in those who have not previously received corticosteroids. In current practice, patients are much more likely to have been treated with abiraterone than ketoconazole. Because abiraterone as a single-agent was found to cause symptoms of mineralocorticoid excess through its potent effects on adrenal steroid synthesis,29 abiraterone was subsequently developed to be co-administered with a corticosteroid.30 Therefore, patients with prior abiraterone exposure in this study would have also had prior chronic prednisone exposure.
Data in the literature about the efficacy of prednisone in various contexts support this hypothesis of reduced prednisone efficacy on subsequent re-challenge. While acknowledging the inherent difficulties of cross-study comparisons, the single-agent activity of prednisone appears reduced when used post-docetaxel. Prednisone was minimally effective, for example, in the control arm of the post-chemotherapy COU-AA-301 study of abiraterone/prednisone versus prednisone-alone.31 Patients receiving 10mg prednisone daily in that study had a >50% PSA response rate of only 6% and a PFS of only 3.6 months. One possible explanation for reduced efficacy of prednisone post-docetaxel is the development of generally more aggressive and refractory disease.32 Another explanation is that these patients may have developed a glucocorticoid-resistant phenotype through progression on prior prednisone (co-administered with docetaxel).
The characteristics of corticosteroid-sensitive tumors are not well described. The glucocorticoid receptor (GR) is observed to be overexpressed in pre-treated and advanced prostate cancer.33 However, steroid responsiveness in the setting of overexpressed or underexpressed GR has not been demonstrated. Therefore, a need exists for identification of a biomarker for glucocorticoid responsiveness, similar to ongoing work on biomarkers for AR-directed therapy resistance.34 Such a marker might allow a clinician to decide whether to administer prednisone together with docetaxel or not.
This study has several important limitations. First, this data represents a single institution’s retrospective experience, and prospective study in a multi-institutional manner would provide the strongest confirmation. Although PFS was the most appropriate endpoint for this study due to its timespan with different post-chemotherapy treatment options available, PFS can be confounded by non-standardized radiographic and clinical assessment schedules. In addition, LDH is a prognostic factor for patients with prostate cancer,35 but this was not routinely measured for these patients and thus was unable to be included in our analysis.
In summary, this retrospective analysis suggests that patients who receive docetaxel together with prednisone may have superior outcomes than those receiving docetaxel alone. While this analysis did not address questions of safety, this putative benefit must be weighed against the potential side effects of prednisone in the individual patient. This study also generates a hypothesis that patients who have received prior prednisone (for example, with abiraterone) may not benefit from additional prednisone administration during docetaxel treatment. Judicious use of prednisone has been suggested based upon preclinical work showing reduced efficacy of enzalutamide in the setting of corticosteroid use,36 as well as inferior outcomes for concurrent corticosteroid administration in a trial of enzalutamide.37 Interactions between therapies will become increasingly relevant as the number of drugs available to patients with metastatic CRPC increases further in coming years. In this context, we postulate that prednisone may only be beneficial once during a patient’s treatment course for advanced prostate cancer, and prospective confirmation of this hypothesis is needed.
Acknowledgments
This work was partially supported by NIH grant T32 CA009071 (B.A.T.), a Conquer Cancer Foundation of ASCO Young Investigator Award (B.A.T.), and by NIH grant P30 CA006973 (E.S.A., S.R.D.).
Footnotes
Conflict of interest:
The authors declare no conflicts of interest.
References
- 1.Haywood A, Good P, Khan S, Leupp A, Jenkins-Marsh S, Rickett K, et al. Corticosteroids for the management of cancer-related pain in adults. The Cochrane database of systematic reviews. 2015;4 doi: 10.1002/14651858.CD010756.pub2. CD010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(25):3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 3.Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol. 2013;24(1):31–38. doi: 10.1093/annonc/mds216. [DOI] [PubMed] [Google Scholar]
- 4.Lorente D, Omlin A, Ferraldeschi R, Pezaro C, Perez R, Mateo J, et al. Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone. British journal of cancer. 2014;111(12):2248–2253. doi: 10.1038/bjc.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Dagher R, Ning L, Abraham S, Rahman A, Sridhara R, Pazdur R. Approval summary: Docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10(24):8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9.Machiels JP, Mazzeo F, Clausse M, Filleul B, Marcelis L, Honhon B, et al. Prospective Randomized Study Comparing Docetaxel, Estramustine, and Prednisone With Docetaxel and Prednisone in Metastatic Hormone-Refractory Prostate Cancer. Journal of Clinical Oncology. 2008;26(32):5261–5268. doi: 10.1200/JCO.2008.16.9524. [DOI] [PubMed] [Google Scholar]
- 10.Lafeuille MH, Gravel J, Grittner A, Lefebvre P, Ellis L, McKenzie RS. Real-World Corticosteroid Utilization Patterns in Patients with Metastatic Castration-Resistant Prostate Cancer in 2 Large US Administrative Claims Databases. American health & drug benefits. 2013;6(6):307–316. [PMC free article] [PubMed] [Google Scholar]
- 11.Auchus RJ, Yu MK, Nguyen S, Mundle SD. Use of Prednisone With Abiraterone Acetate in Metastatic Castration-Resistant Prostate Cancer. Oncologist. 2014;19(12):1231–1240. doi: 10.1634/theoncologist.2014-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CY, Walther PJ, McDonnell DP. Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Res. 2001;61(24):8712–8717. [PubMed] [Google Scholar]
- 13.Miyamoto H, Rahman MM, Chang CS. Molecular basis for the antiandrogen withdrawal syndrome. J Cell Biochem. 2004;91(1):3–12. doi: 10.1002/jcb.10757. [DOI] [PubMed] [Google Scholar]
- 14.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan AV, Zhao XY, Swami S, Brive L, Peehl DM, Ely KR, et al. A glucocorticoid-responsive mutant androgen receptor exhibits unique ligand specificity: Therapeutic implications for androgen-independent prostate cancer. Endocrinology. 2002;143(5):1889–1900. doi: 10.1210/endo.143.5.8778. [DOI] [PubMed] [Google Scholar]
- 17.Ndibe C, Wang CG, Sonpavde G. Corticosteroids in the Management of Prostate Cancer: A Critical Review. Curr Treat Option On. 2015;16(2) doi: 10.1007/s11864-014-0320-6. [DOI] [PubMed] [Google Scholar]
- 18.James ND, Sydes MR, Mason MD, Clarke NW, Dearnaley DP, Spears MR, et al. Docetaxel and/or zoledronic acid for hormone-naive prostate cancer: First overall survival results from STAMPEDE ( NCT00268476) ASCO Meeting Abstracts. 2015;33(15_suppl):5001. [Google Scholar]
- 19.Sweeney C, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Eisenberger MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. ASCO Meeting Abstracts. 2014;32(15_suppl):LBA2. [Google Scholar]
- 20.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the prostate cancer clinical trials working group. Journal of Clinical Oncology. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francini E, Petrioli R, Rossi G, Laera L, Roviello G. PSA response rate as a surrogate marker for median overall survival in docetaxel-based first-line treatments for patients with metastatic castration-resistant prostate cancer: an analysis of 22 trials. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(11):10601–10607. doi: 10.1007/s13277-014-2559-8. [DOI] [PubMed] [Google Scholar]
- 22.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. New Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Soest RJ, de Morree ES, Shen LJ, Tannock IF, Eisenberger MA, de Wit R. Initial Biopsy Gleason Score as a Predictive Marker for Survival Benefit in Patients with Castration-resistant Prostate Cancer Treated with Docetaxel: Data from the TAX327 Study. Eur Urol. 2014;66(2):330–336. doi: 10.1016/j.eururo.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Berry W, Dakhil S, Modiano M, Gregurich M, Asmar L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. The Journal of urology. 2002;168(6):2439–2443. doi: 10.1016/S0022-5347(05)64163-8. [DOI] [PubMed] [Google Scholar]
- 25.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. New Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakih M, Johnson CS, Trump DL. Glucocorticoids and treatment of prostate cancer: a preclinical and clinical review. Urology. 2002;60(4):553–561. doi: 10.1016/s0090-4295(02)01741-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-Targeting Chemotherapy Impairs Androgen Receptor Activity in Prostate Cancer. Cancer Res. 2010;70(20):7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweizer MT, Zhou XC, Wang H, Bassi S, Carducci MA, Eisenberger MA, et al. The Influence of Prior Abiraterone Treatment on the Clinical Activity of Docetaxel in Men with Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2014;66(4):646–652. doi: 10.1016/j.eururo.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attard G, Reid AHM, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, et al. Clinical and Biochemical Consequences of CYP17A1 Inhibition with Abiraterone Given with and without Exogenous Glucocorticoids in Castrate Men with Advanced Prostate Cancer. J Clin Endocr Metab. 2012;97(2):507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 30.Kluetz PG, Ning YM, Maher VE, Zhang LJ, Tang SH, Ghosh D, et al. Abiraterone Acetate in Combination with Prednisone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer: US Food and Drug Administration Drug Approval Summary. Clin Cancer Res. 2013;19(24):6650–6656. doi: 10.1158/1078-0432.CCR-13-2134. [DOI] [PubMed] [Google Scholar]
- 31.De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. New Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JJ, Yin B, Christudass CS, Terada N, Rajagopalan K, Fabry B, et al. Acquisition of paclitaxel resistance is associated with a more aggressive and invasive phenotype in prostate cancer. J Cell Biochem. 2013;114(6):1286–1293. doi: 10.1002/jcb.24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yemelyanov A, Bhalla P, Yang XM, Ugolkov A, Iwadate K, Karseladze A, et al. Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells A novel therapeutic modality. Cell Cycle. 2012;11(2):395–406. doi: 10.4161/cc.11.2.18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonarakis ES, Lu CX, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. New Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia XY, et al. Circulating Tumor Cell Biomarker Panel As an Individual-Level Surrogate for Survival in Metastatic Castration-Resistant Prostate Cancer. Journal of Clinical Oncology. 2015;33(12):1348. doi: 10.1200/JCO.2014.55.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid Receptor Confers Resistance to Antiandrogens by Bypassing Androgen Receptor Blockade. Cell. 2013;155(6):1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scher HI, Fizazi K, Saad F, Chi KN, Taplin M-E, Sternberg CN, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor. ASCO Meeting Abstracts. 2013;31(6_suppl):6. [Google Scholar]


