Abstract
Currently, there are no quantitative studies of smooth pursuit, a behavior attributed to the fovea, in individuals with macular degeneration (MD). We hypothesize that pursuit in MD patients depends on the relative positions of the scotoma and target trajectory. We tested this hypothesis with a scanning laser ophthalmoscope (SLO), which allows for direct visualization of the target on the damaged retina. Monocular microperimetry and eye movements were assessed in eleven individuals with differing degrees of MD. Observers were asked to visually track a 1.7° target that moved in one of eight radial directions at 5°/s–6°/s. Consistent with our hypothesis, pursuit metrics depended on whether the target moved into or out of scotoma. Pursuit gains decreased with increasing scotoma extent in the target's heading direction (p = 0.017). Latencies were higher when the scotoma was present along the target trajectory (in either starting or heading directions, p < 0.001). Furthermore, an analysis of retinal position shows that targets fell on the fixational locus nearly 50% of the time. The results suggest that MD patients are capable of smooth pursuit eye movements, but are limited by target trajectory and scotoma characteristics.
Keywords: smooth pursuit, macular degeneration, central field loss
Introduction
Smooth pursuit eye movements have traditionally been considered to be a foveal behavior, far more developed in primates than other, afoveate, species (Lisberger & Morris, 1987). A simple question, then, is how do individuals who have lost their fovea adapt to visualize continuously moving objects for an adequate amount of time? It has been shown previously that young observers with healthy retinas can pursue perifoveal targets as far as 6° below the line of sight, albeit with lower gains (Winterson & Steinman, 1978). More recently, investigators showed that healthy participants with artificial scotomas as large as 6° in diameter could pursue periodic and nonperiodic stimuli, with gains dependent on scotoma size and whether the stimulus was periodic (Pidcoe & Wetzel, 2006). The same study also showed that these individuals developed a perifoveal preferred retinal locus (PRL) to which eye movements were rereferenced. However, the effect of losing foveal vision—as in the case of patients with macular degeneration—on smooth pursuit has not been examined in a quantitative manner.
Although these earlier studies suggest that patients lacking foveal vision can use smooth pursuit to follow a moving target, there are some important limitations. First, scotomas can exceed 20° in diameter in individuals with macular degeneration and multiple PRLs are possible, especially with large scotomas (Whittaker, Budd, & Cummings, 1988). Second, the previous work did not assess the relationship between target trajectory and scotoma location relative to the PRL(s). Third, target motion in these studies consisted of continuous, sinusoidal motion along the horizontal axis, which can be pursued with predictive strategies (Bahill & McDonald, 1983). Furthermore, such stimuli are of limited use in assessing pursuit initiation, and provide no information about pursuit of trajectories that are not horizontal or predictable. Lastly, scotoma size, shape, and proximity to the fovea vary widely across individuals.
Therefore, whereas pursuit quality might be expected to degrade when a target is occluded by the scotoma, previous work provides limited insight into the relationship between the PRL and the pursuit target. We hypothesized that pursuit in MD patients would depend on the relative positions of the scotoma and target trajectory—targets starting in the scotoma and heading toward healthy retina would have higher latencies and lower gains (since patients would have trouble locating the target within the scotoma), whereas targets starting in healthy retina and heading toward the scotoma would have lower latencies and higher gains, since patients could more quickly acquire targets and pursue with intact retina. We also expected that the larger the overall lesion, the more pursuit would be degraded, as the target would have lower chances of landing in and traversing intact retina. We used a scanning laser ophthalmoscope (SLO) to directly visualize the target's location on the retina during pursuit, a novel approach to studying pursuit in AMD patients. We first mapped out the scotoma in each patient's eyes. Then we presented the patients with a step-ramp pursuit stimulus (Rashbass, 1961) that was displayed directly in the SLO, thereby allowing visualization of the target's location on the retina during the pursuit movement. Multiple directions of target motion were used to stimulate both damaged and healthy retina. The patients' retinal damage was highly heterogeneous, with the majority of the retinas having diffuse scotomas, oftentimes surrounding the PRL on multiple sides. To control for multiple configurations of scotoma and target direction/location, as well as overall scotoma size and patients' age, we used a linear model that allowed us to look at all of these factors quantitatively.
Our results show that AMD patients can pursue moving targets, albeit with lower gains and longer latencies than their healthy, age-matched counterparts. Surprisingly, overall scotoma size and proximity to the PRL did not systematically affect pursuit gain or latency. However, target motion direction relative to the scotoma did affect both pursuit dynamics, but not along the lines of our initial hypothesis. Performance was poorest (lowest gain, high latency) for targets moving towards the scotoma. Additionally, latency was also increased when retina was compromised in the region where target motion had begun, suggesting that scotoma presence in the first or second half of the target trajectory is related to an increase in latency. The amount of scotoma in the starting and heading regions was related to latency in a complex fashion, where larger scotoma extent in both regions resulted in shorter latency, perhaps as an adaptation to acquire the target with the PRL before the target disappeared. Patients placed the target within their fixation locus about 50% of the time. The results suggest that viable pursuit in AMD patients depends on which direction a target moves relative to their scotoma, and that these patients would benefit from training on how to use pursuit eye movements to keep a moving target visible. Our results also underscore the complexity of the scotoma/target relationship: Although it is simplest and most common to discuss macular degeneration in terms of compact scotomas located in a single portion of the visual field, our subjects demonstrated a range of retinal damage from compact/dense scotomas, to ring scotomas that surrounded the fovea, to patchy scotomas with multiples locations of the visual field affected.
Methods
Data collection
Participants
All research was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at the Smith-Kettlewell Eye Research Institute. Consent was obtained from all participants who were informed of the details of the experiment and their rights. Eleven patients with macular degeneration (six male; five female) and four controls (one male; three female) were tested.
Equipment
Participants were tested in a confocal SLO (SLO 101, Rodenstock, Munich, Germany). All tests for each patient were performed in a single session. Participants were seated with their chins and foreheads resting in the SLO head support, without glasses. The field size used was 40°, at a resolution of 17.7 pixels/°. Both eyes were tested monocularly, with the nontested eye occluded.
Perimetry
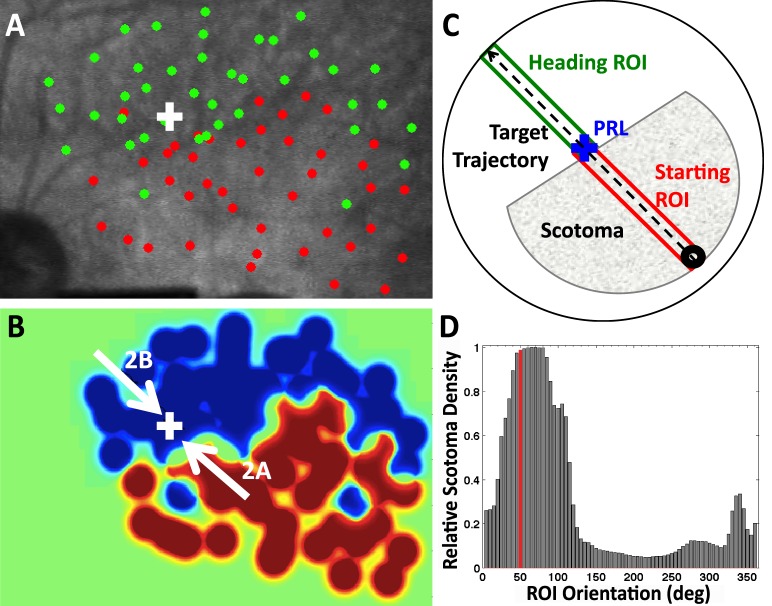
Microperimetry was performed according to the “smart microperimetry” approach of MacKeben and Gofen, 2007. For all tasks, stimulus presentation was gaze contingent, relative to a region of interest on the retina, defined by the experimenter. Participants were presented with a central fixation cross of 2.5° and instructed to maintain fixation on the cross throughout the test. Once the participant visualized the cross, the test was initiated. Participants were presented with bright flashes and were instructed to indicate whether they saw the flashes with the press of a button. Flash position was updated to account for any eye movement, such that stimuli were always presented to the retinal position defined by the experimenter. Detected and nondetected targets were labeled as “hits” and “misses” respectively (Figure 1A). Flashes were presented at random time intervals, and their placement was such that scotoma edges were mapped with particular attention (higher density). For one participant (P10) the optic disc was also mapped, due to its proximity to the PRL.
Figure 1.
(A) SLO perimetry for left eye of P4. (B) Heat map corresponding to the map in A. White arrows represent target directions of trials shown in Figure 2. (C) Schematic for estimating scotoma extent along target trajectory. The shaded area is a cartoon representation of the scotoma in B, and the blue cross is fixation. The dashed arrow is target trajectory. The green and red rectangles represent the Heading region of interest (ROI) where the target is heading and Starting ROI, where target motion originates. (D) Scotoma extent (fraction of red pixels to total number of pixels in an ROI rectangle) for all ROI orientations. The ROI in C is highlighted in red.
Smooth pursuit
Immediately following perimetry, the smooth pursuit experiments began. Participants were instructed to “follow the moving ring as closely as possible for the entirety of the trial.” Each trial started with the fixation cross in the center of the screen. Once the experimenter determined that fixation was acquired (eye position became stable), participants were asked “Are you ready?” and the trial was initiated after an affirmative response. The fixation cross was then replaced by a 1.7° ring, indicating the start of the trial to the participants. All stimuli were shown at 100% contrast. The target was of a sufficient size and contrast to accommodate the visual acuity and contrast sensitivity of the patient population, lowest acuity: 20/480; lowest contrast sensitivity (log): 0.56. Patients fixated the ring target for 1 s. The target then jumped 6° in one of eight possible directions (0°, 45°, 90°, 135°, 180°, 225°, 270°, or 315°; step size and directions designed to probe each patient's scotoma in at least one of the trials) and then moved at a constant velocity (between 5°/s and 6 °/s) back toward fixation in a “step-ramp” fashion (Rashbass, 1961). The trials were approximately 3 s in duration, and the targets traveled approximately 12° of visual angle from the start to the end of movement, traversing its original fixation location, halfway through the movement. The test was repeated for each of the eight directions at least once. The entire experiment was repeated for each eye. Data was sampled at 60 frames per second and recorded in video and text formats for further analysis.
Data analysis
Scotoma map
Continuous heat maps (Figure 1B) were constructed from the discrete points obtained from the perimetry (Figure 1A) using the following method. First, we created two binary images, one using the set of “hit” locations and the other using the “miss” locations. In both images, pixels were assigned value 1 if their (x, y) coordinates corresponded to sampled points in the associated sets, 0 otherwise. Subsequently, the resulting binary images were convolved with a 2D Gaussian low-pass filter (σ = 10 and kernel size = 71 pixels). The resulting two images represented the heat maps for the intact (hit) and scotoma (miss) retina. To avoid interference between adjacent points, the intensity values in the heat maps were clipped to the peak value of a single Gaussian. The intensity values of both images were then rescaled in the [0, 1] range, and the final heat map was computed by subtracting the map representing intact retina from the one representing the scotoma. Points with an intensity value greater than 0 (red when visualizing the heat map using scaled colors) were thus defined as scotoma, and points less than or equal to 0 (blue in the heat map visualization) represented intact retina. The heat map values were normalized across participants in the range [−1, 1], so that the scaled colors representation could be visually comparable, with green representing 0. Flashes were sometimes presented at overlapping locations during perimetry, as deemed necessary by the experimenter, to check for misses due to lapses in patients' attention. Therefore, any miss points whose area (radius of 6 pixels) overlapped by more than 10% with a hit point were excluded.
We computed the relative density of scotoma points in the heat maps as follows. First, we inscribed the heat map in a circle centered at the PRL (Figure 1C). Then, for every direction of target motion, we considered a rectangular region of interest (ROI), with origin in the PRL, length equal to the circle radius, and width equal to 2.26°, sufficient to fit the moving target (1.7°). Within each ROI, we then computed the ratio of scotoma points relative to the total number of points (Figure 1D). The “starting” ROIs represent the region of the retina where smooth target motion was initiated and “heading” ROIs represent the regions of the retina where the target was heading on any given trial (and would end up, if smooth pursuit gains were insufficient, Figure 1C).
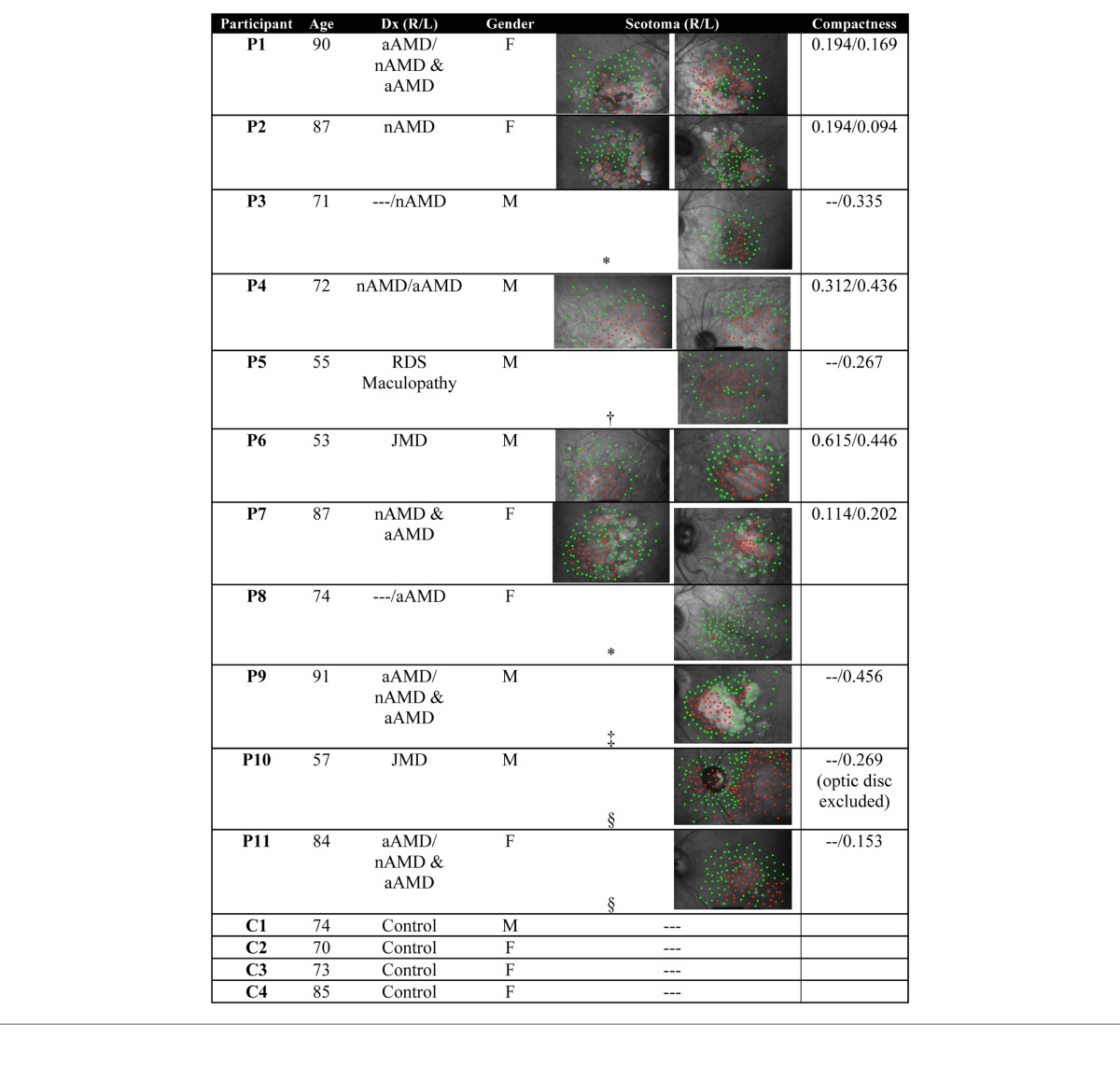
To quantify the heterogeneity in scotoma shape and distribution, we computed a “compactness” ratio (Table 1) as the ratio of the scotoma area to the square of the perimeter of the scotoma, multiplied by 4π, such that a circle has a compactness of 1 and a line approaches a compactness value of 0.
Table 1.
Participant summary. Notes: nAMD—neovascular (wet) AMD, aAMD—atrophic (dry) AMD. Maps included for eyes used in the analysis: *eye not affected; †no fixation stability; ‡strabismic eye (suppressed); §no pursuit.
Smooth pursuit
To provide a reference for the analysis of smooth pursuit data, a feature of the retinal anatomy, such as an intersection of two blood vessels, was chosen in the first available video frame of each trial, and its position was tracked throughout the trial using the technique described by MacKeben and Gofen (2007) for gaze-contingent target presentation. Briefly, the pixel location of the feature was determined in each subsequent frame, and the location of the target and the PRL were calculated with respect to this feature throughout the trial. Horizontal and vertical eye positions were filtered (2-pole Butterworth noncausal filter, cutoff = 25 Hz), differentiated, and geometrically combined to derive eye velocity. Saccades were detected offline when eye velocity exceeded 40°/s, or variance exceeded 150 (°/s)2. All velocity traces were then visually inspected for remaining saccades. Saccades were excised from the traces only for the purpose of calculating smooth velocity and gain, but were left in the records for the eye position analysis. Excised saccades were replaced with a line that interpolated eye velocity before and after the saccade as is standard in the literature (e.g., Heinen, Badler, & Ting, 2005).
We computed gain across the entire period of pursuit for each trial, for patients and controls. For each participant and eye, we computed the mean pursuit latency across all trials, and calculated average eye velocity in each trial from the mean latency time to the end of the trial. Gain was computed per trial as the ratio of the average eye velocity to target velocity. Because pursuit velocity was often highly variable throughout the trial, we devised a secondary means of more accurately measuring gain in this population. We computed gain during the longest continuous period of velocity within ±0.2 of the median velocity after pursuit onset (green boxes, Figure 2). Gain was again computed per sample in that time interval as the ratio of eye to target velocity. Gain outliers were defined as those values that exceeded the mean by ±2 standard deviations of the entire trial, and were excluded. Gain was averaged over an 80 ms sliding window, with 20 ms overlap between windows. Overlapping segments with gains whose medians were within the 25th and 75th percentiles of each other were combined to create larger segments (e.g., Figure 2B). The gain of the longest segment for each trial was then used for analysis. Each trial was visually inspected, and the second longest segment was used if the longest occurred before pursuit onset or if there was a major artifact (e.g., series of saccades or blinks) throughout the longest segment.
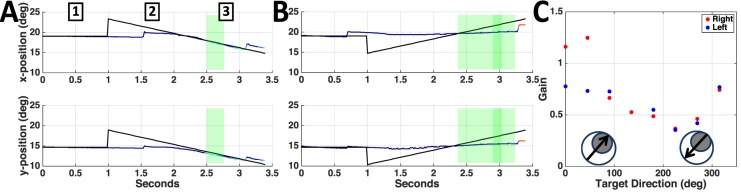
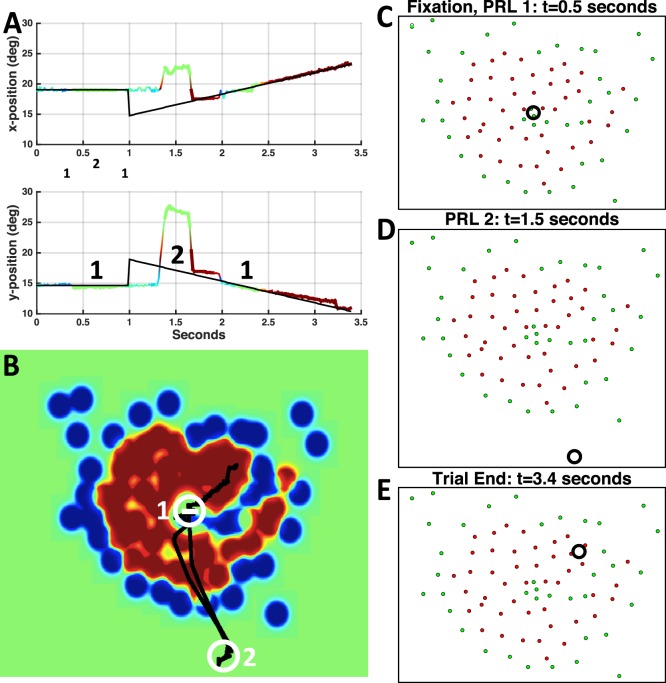
Figure 2.
Horizontal and vertical eye (multicolor) and target (black) position during a trial when the target moved away from (A) and toward the scotoma (B) for patient P4. Green shaded boxes highlight the period of pursuit when gain in C was computed. (C) P4's smooth pursuit eye velocity as a function of target trajectory for left and right eye. Target trajectories relative to the scotoma (right and left eye scotomas are symmetric for this patient) are labeled in the inset circles (grey disc: scotoma, black arrow: target trajectory).
Pursuit latency was computed using a linear regression approach (Heinen et al., 2005). Briefly, the fixation portion of the trial was fit with a horizontal line with a y-intercept set at the average of the fixation velocity (which accounted for any drift). The experimenter defined the approximate start (after end of the fixation period) and end (near peak velocity) points of pursuit initiation, and this region was fit with a line. The intercept of the two lines was defined as pursuit onset and verified by the experimenter. The interval between pursuit initiation and target movement onset was defined as the latency.
Participants' smooth pursuit records were aligned to the perimetry map using their fixation location during perimetry, and at the beginning of each pursuit trial. To estimate the amount of time the target was visible to the participant during a trial, we subdivided each trial into three epochs: (a) fixation (1 s prior to target motion), (b) first half of pursuit, and (c) second half of pursuit. These epochs are labeled in Figure 2A. For each epoch, we calculated the percentage of time the target was located in a region of the heat map with color values <0 (cold), by dividing those frames by the total number of frames in that epoch. Thus, this metric quantified the amount of time a trace was blue (in intact retina)—which in Figure 2A would be 100% of the time for all three epochs.
Statistical analysis
We analyzed the effect of age, overall scotoma size, scotoma density in starting and heading ROIs, amount of time target spent in good retina during fixation, and early and late pursuit on gain and latency by running separate linear mixed effects models for each of these dependent variables. The models included random intercepts for participant (N = 11) and eye (N = 16). We checked for normality and confirmed normality for gain, but found latency to be consistent with a gamma distribution. Therefore, the model for latency was a generalized linear mixed effects model with a gamma distribution and identity link function.
Results
Pursuit characteristics and scotoma location
Figure 1A shows the perimetry map for the left eye of patient P4, where the white cross represents the PRL used for fixation. P4 has a large, upper right visual field scotoma relative to the PRL. (Retinal images in the SLO are vertically flipped relative to the visual field.) Figure 1B depicts the heat map derived from Figure 1A. Figure 2 shows representative traces with different patterns of pursuit when the target moves either out of the scotoma (Figure 2A), or toward it (Figure 2B). When the target moves out of the scotoma, the patient begins moving his eyes at approximately 1.5 s (0.5 s after motion onset), and eye position soon matches the target position for the rest of the trial. When the target moves toward the scotoma, the patient shows negligible smooth pursuit, maintaining a near-constant eye position.
Figure 2C shows gain values plotted as a function of trajectory direction for the patient in Figure 1 (P4). For both right and left eyes, a clear modulation is evident across trajectories. The lowest gain values for both eyes correspond to the trajectories where the target started opposite the scotoma and moved toward it (Table 1). Figure 3A shows a representative pursuit trace for a control observer (C3). Gain modulation was not evident in control participants (Figure 3B), as it was in the patients (compare to Figure 2C).
Figure 3.
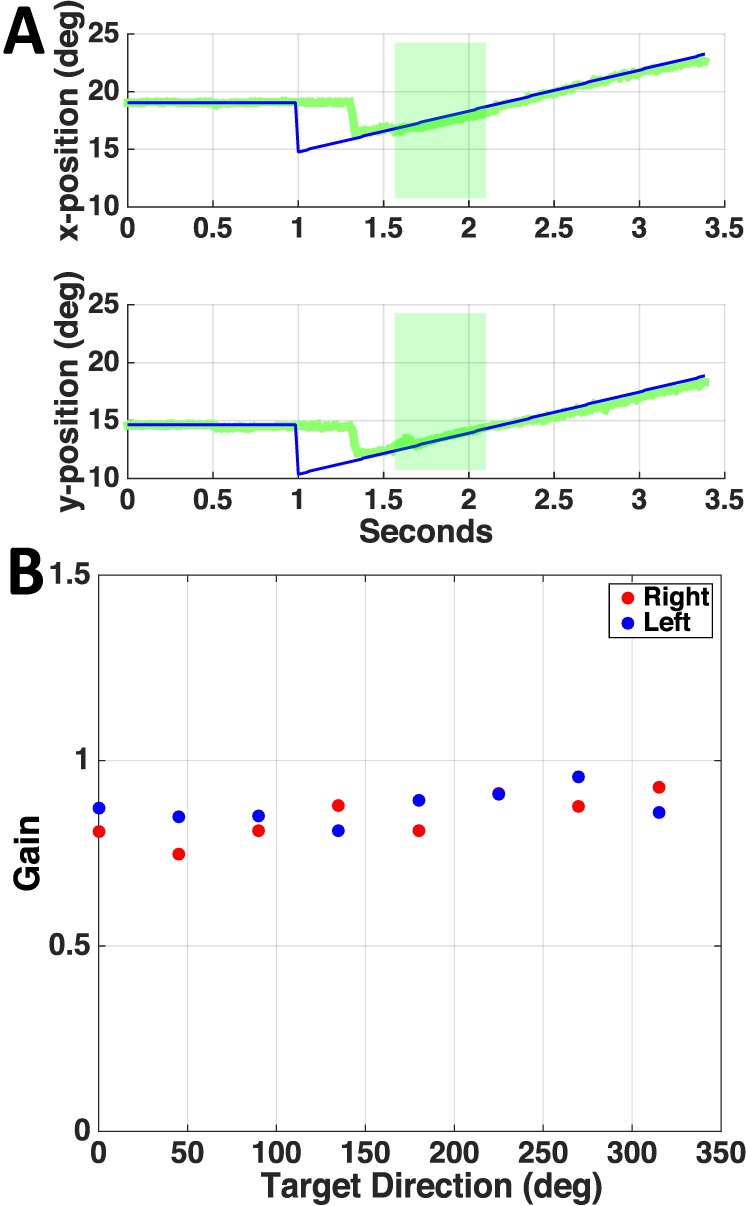
(A) Horizontal and vertical eye (green) and target (black) position for a representative trial for observer C3. (B) C3's smooth pursuit eye velocity as a function of target trajectory for left and right eye.
Across patients, smooth pursuit velocity gains and latencies showed large variation. Patients' gains over the time period from mean pursuit latency to the end of the trial differed significantly from controls (patients' median gain = 0.74; controls' median gain = 0.91; p < 0.0001). To ensure that the differences in pursuit gain between patients and controls were not an artifact of the high variability in pursuit onset times and velocities in patients, we devised a more tailored approach to calculating gain in patients, which involved assessing gain over the longest, continuous, stable-velocity period during a trial (see Methods). Using this method, gain values ranged from virtually no pursuit velocity to eyes leading the target (median = 0.69, min = 0.06, max = 1.39). Patients' latency values ranged from anticipatory (eyes starting to move at the same time as the target) to starting near when the target passed the central fixation (median = 0.33 s, min = 0.02 s, max = 1.04 s). Gains and latencies differed significantly between patients and controls (Gain: median = 0.89, min = 0.63, max = 1.3; Latency: median = 0.29 s, min = 0.18 s, max = 0.66 s; Kruskal-Wallis nonparametric ANOVA, p < 0.001 and p < 0.01 respectively; Figure 4).
Figure 4.
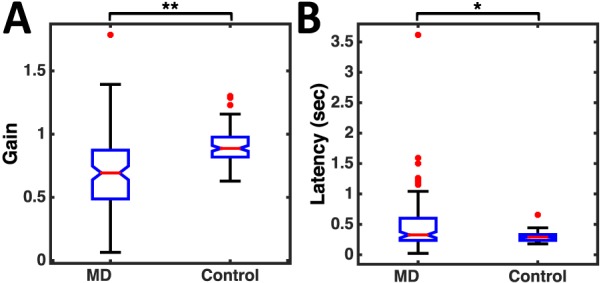
(A) Distribution of gains across all participants with MD versus controls. The gains are significantly higher for control participants (p < 0.001). (B) Distribution of latencies across all MD participants versus controls. The latencies are significantly lower for control participants (p < 0.01).
In general, patients were able to pursue the targets, although pursuit characteristics depended on trajectory direction relative to the scotoma (Figures 2 and 5). Age and overall scotoma size did not affect pursuit characteristics (Table 2). To understand the effect of scotoma and trajectory on pursuit parameters, we first computed the proportion of affected retina along the path of the target for each trial (Figure 1). We divided this computation into two regions of interest—one “starting ROI,” where the target initially appeared and one “heading ROI,” where the target would end up if no pursuit was initiated.
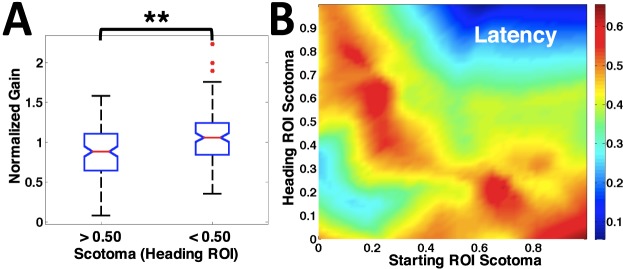
Figure 5.
(A) Normalized pursuit gain distribution for Heading ROIs with scotoma extent greater versus less than 50%. The gains are significantly higher for scotoma extent <50% (p = 0.001). (B) Latency versus scotoma extent in Starting and Heading ROIs. Latency increased with scotoma extent in Starting or Heading ROIs (red) but was low (blue) when scotoma was extensive in both.
Table 2.
Linear model results: Gain and latency. ***extremely significant; **very significant; *significant.
Gain
Gains were highest when the scotoma extent in the heading ROI was small, and were lowest when targets moved toward extensive scotoma (Figure 2). The effects of scotoma in the heading ROI could be confounded with effects of scotoma in starting ROI, particularly in cases where scotomas are compact. In our linear model analysis, we found that the amount of scotoma in the heading ROI had a significant effect on gain, but there was no significant relationship between gain and scotoma extent in the starting ROI (Table 2). The effect of heading ROI on gain can also be visualized using a simple comparison across all participants. Figure 5A plots normalized gains (gaintrial/gainave) in trials with more than 50% scotoma in the heading ROI, compared to those with less than 50% scotoma in the heading ROI (Kruskal-Wallis nonparametric ANOVA, p = 0.001).
Latency
Latencies were high when the targets moved toward or out of scotoma regions and low when little scotoma was present. Latencies also decreased when scotoma was present in both the starting and heading directions of the target trajectory (Figure 5B). From our linear model, we did indeed confirm that both the starting and heading ROIs had a significant effect (Figure 5B). In addition, latencies were also significantly decreased when scotoma was present in both the starting and heading directions of the target (Figure 5B, interaction term in Table 2). Three eyes contributed to the data in this condition: P1, left; P5, left; and P10, left. P1 exhibited very little pursuit and made erratic eye movements throughout the trial, suggesting a searching behavior. P5 and P10 had strong pursuit (gains near 1) with short latencies for all trials where there were extensive scotomas in both the heading and starting ROIs. Their eye movements suggest these patients acquired the target quickly and then maintained it on healthy retina throughout the trial. All three eyes represented some of the most disperse scotomas in the population, as quantified by the compactness measure (Table 1).
Gain and latency were significantly, negatively correlated (Spearman's ρ = −0.25, t(129) = −2.9, p = 0.004). To ensure that the model outcomes were not confounded by this correlation, a linear model of gain based on age, overall scotoma size, eye, and participant did not significantly improve by adding latency, F(1) = 1.31, p = 0.255, nor did the adjusted R2 (both models R2 = 0.31) improve with the addition of latency.
Epoch-based pursuit analysis
The above results are consistent with our hypothesis that scotoma location, relative to the trajectory, is important to pursuit quality. However, because the preceding analysis looks at scotoma placement relative to fixation, it does not provide information regarding the dynamic interaction of target placement relative to scotoma after pursuit initiation, when both the target and potentially the eye are moving. Since the amount of scotomatous retina traversed by the target varied from trial to trial based on the amount of smooth pursuit initiated and the portion of the eye used to track the target, we used raw eye position data to compute the proportion of time that the target was in healthy retina throughout each trial, as an estimate of when the target was visible. The trial was divided into three epochs (fixation, early, and late pursuit, as shown in Figure 2), and the proportion was calculated for each epoch.
The visibility of the target at the start of the trial may determine how well an individual is able to maintain that target on intact retina throughout the trial. Because the target makes a step in an unpredictable direction, whether it continues to be on intact retina after the step could determine how quickly the patient is able to initiate pursuit (latency) and how well pursuit is maintained (gain). We therefore included the visibility during the three epochs defined above in the linear model. There was no significant effect of any of these factors on gain or latency.
One potential confound in this analysis is that if an individual was not able to sufficiently visualize the target at the start of the trial, or after the unpredictable step, the participant might be less likely to visualize the target later in the trial, potentially making the epochs nonindependent. To test for independence, we performed a Spearman's rho correlation between all possible epoch pairs for each eye of each patient. The correlations were not significant across patients, justifying their individual inclusion in the model.
Relation between retinal health and pursuit gain
In contrast to the epoch-based analysis across the entire trial, we also examined the possibility that gain during the period of continuous pursuit may be related to the health of the retina used during that period. To evaluate this possibility, we calculated the proportion of intact retina used in the period over which gain was computed with the same method described for the epoch analysis above. We then correlated the percent intact retina during continuous pursuit with the gain in two ways. First we performed a linear regression for all patients as a group between the % intact retina and normalized gain values (gaintrial/gainave) within each eye of each patient, for all patients as a group. No significant relationship was found. We also performed a linear regression between % intact retina and gain for each patient and eye separately. Although several patients had a significant relationship between the two, the majority did not show significance, and the relationship was not consistent among patients (30% were negatively correlated).
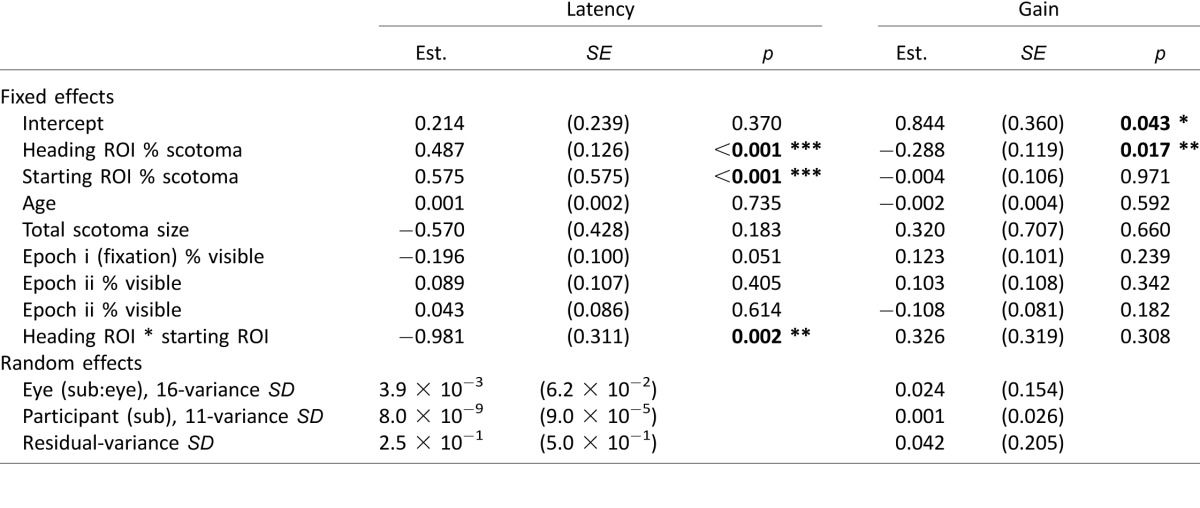
PRL use and placement during pursuit
Use of fixational PRL
To investigate whether patients consistently used the fixational PRL during pursuit, we looked at eye position error relative to target position during the periods of continuous gain used above. During these periods, we calculated the distance between the eye and the moving target relative to the position of the eye during fixation (PRL), per frame. We compared these values to the 95% bivariate contour ellipse area (BCEA) (Crossland, Sims, Galbraith, & Rubin, 2004) used to quantify the participants' fixation stability. For patients, 42% (2351/5618) of all frames had the target within the BCEA (e.g., Figures 6A through B). Points outside the BCEA were distributed at a number of distances and locations, with no systematic patterns that might indicate the consistent use of a second PRL during the periods of continuous gain analyzed. Control participants had 45% (720/1616) of all frames within their BCEAs, likely due to the large target size (e.g., Figure 6C, note difference in BCEA size, scaling is constant across Figures 6A through C). Due to patients' lower gains, the consecutive frames do show a greater degree of lag between the eye and the target (as indicated by the streak-like appearance of subsequent points) in the patient versus control location distributions (compare Figures 6A and B to 6C).
Figure 6.
Distribution of target locations relative to the fixation location (95% BCEA, black oval) on the retina in a patient with a perifoveal PRL (A), an eccentric PRL (B), and a control (C).
Use of multiple PRLs
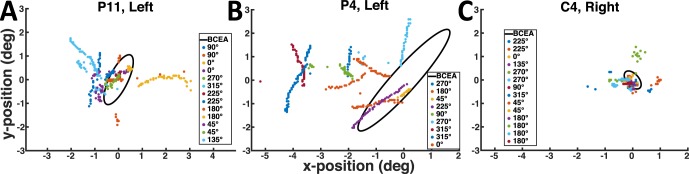
In two participants (P5 and P10) we saw clear evidence for two distinct PRLs, with switching during pursuit. Figure 7 shows a representative pursuit trace and scotoma heat map for participant P5. The patient has a large (>20° diameter) scotoma, with a small foveal island at the center in the left eye, and no foveal sparing in the right eye. Although we saw evidence of PRL switching in both eyes, we only present the trajectory analysis for the left eye, as perimetry in his right eye was not possible due to poor fixation stability. Interestingly, despite this poor fixation stability, the participant was able to pursue with his right eye, with gains varying between 0.3 and 1.4 (mean gain = 0.65 ± 0.37). This result is consistent with the finding that overall scotoma size did not affect smooth pursuit performance (Table 2), given that he has a highly diffuse, large scotoma (Table 1).
Figure 7.
(A) Representative horizontal (top) and vertical (bottom) traces from P5. Regions labeled 1 represent period where fixational PRL was on target. Region labeled 2 represents a second PRL, labeled in B. (B) Heat map showing P5's scotoma region (red) with both PRLs labeled. Black line shows target trajectory across the eye. (C–E) Location of the target (Black Circle, to scale) relative to the scotoma (Red Dots) during fixation (C), pursuit with a peripheral PRL (D), and pursuit at the end of the trial (E).
Using his left eye, participant P5 appeared to use a peripheral (right of center, upper visual field, Figure 7D and circle 2 in Figure 7B) and a central PRL (fovea, Figure 7C and circle 1 in Figure 7B) in several trials to achieve pursuit (Figure 7A). This strategy is likely due to the very small, but high acuity nature of his foveal region, which allows precise visualization of a small target but not visualization of the entire screen. Patient P10 also has an extensive central scotoma in his left eye, with a fixational PRL located near the optic disc. The patient made a vertical saccade near the time of pursuit onset on the majority of the trials and pursued the target with a location approximately 1° above the fixational PRL.
Discussion
Here, we present the first quantitative assessment of smooth pursuit performance in individuals with central visual field loss. We used a novel SLO approach that allowed us to look at target placement directly on the retina during smooth pursuit, thereby allowing us both to describe smooth pursuit characteristics in our patient population and to determine the retinal area used to follow the target in the presence of central field loss.
The eleven individuals with macular degeneration we tested could smoothly pursue a slowly moving (5°/s–6°/s) target. Interestingly, performance did not depend on overall scotoma size, even though in several patients, scotoma size exceeded 20° of visual angle. Performance on the smooth pursuit task, in terms of gain and latency, was significantly worse in patients than in age-matched controls—a result consistent with previous findings in healthy participants with a small (≤6°), dense, artificial scotoma (Pidcoe & Wetzel, 2006) and those pursuing perifoveal targets (Winterson & Steinman, 1978). The present study is an important extension of the work by Pidcoe and Wetzel, which focused on the formation and use of PRLs in young (ages 28–37), healthy controls. In that study, the participants were presented with periodic (sinusoidal) and nonperiodic (sum of sines) targets moving between 4°/s and 16°/s. The authors did not compute smooth pursuit metrics, such as latency and gain, but instead focused on the offset of eye position to determine PRL formation. Qualitatively, the authors reported poorer pursuit in the nonperiodic condition. The across-patient variability of pursuit in our study is in agreement with previous qualitative observations of Schuchard, Naseer, and de Castro (1999). Patients in this study reported being able to visualize the target for at least part of the trial, and being able to pursue it, which is consistent with our data.
Some of our patients were able to follow the target even when it fell into their scotoma region for at least part of the trial (Figure 7, end part of the trial). Given the size of the target (1.7°) and variation in the scotoma boundary, we cannot exclude the possibility that a part of the target continued to stimulate healthy retina, facilitating pursuit in these patients. Overall, there was a significant effect of scotoma location relative to the target direction for both pursuit initiation (latency) and pursuit maintenance (steady state gain).
Several studies have shown that the PRL measured during fixation is not always used as an oculomotor reference. For example, White and Bedell (1990) showed that only observers with long-standing central field loss successfully rereferenced saccades to their fixational PRL. Those with more recent macular degeneration used a mixture of the original fovea and the PRL as an oculomotor reference. Furthermore, although monkeys with induced retinal lesions formed a fixational PRL within a day of the lesions, it took much longer for them to use the PRL for saccades (Heinen & Skavenski, 1992). Thus, it was not clear whether observers with CFL would use their PRLs for smooth pursuit. Our data indicate that patients placed the target on their fixational PRLs about 42% of the time analyzed. We saw no correlation between disease duration and use of a fixational PRL; patients with some of the longest disease durations had some of the fewest trials with the target primarily within the PRL region. Perhaps the discrepancy between our pursuit data and previous saccade data is that pursuit can be accomplished successfully in the periphery (Winterson & Steinman, 1978). Furthermore, the size of our target, and its annular shape, may have allowed the patients to follow a peripheral portion of the target. Additionally, the resolution of our analysis method may be limited by some jitter (<0.5°) that would place the target outside the fixation area. However, for both patients and controls, we saw trials that were well outside of the range of the extent of the target and analysis error. Interestingly, we saw evidence of PRL switching in two of our observers. However, in both cases one of the two PRLs used was the fixational PRL (e.g., Figure 7A).
PRL awareness and placement can be important considerations for successful object viewing in patients with AMD (Verghese & Janseen, 2015). Therefore, understanding the effects of PRL (and thus scotoma) placement, relative to target motion, on pursuit performance is important and can lead to potential successful training paradigms, as shown previously for other tasks (Janssen & Verghese, 2015; Seiple, Szlyk, McMahon, Pulido, & Fishman, 2005). To that end, we analyzed target placements and scotoma-PRL configuration in two ways. For the dynamic analysis, we analyzed the position of the target on the moving eye, analyzing performance relative to the health of the retina traversed by the target. For the static analysis, we looked at pursuit performance as a function of target direction relative to the scotoma, taking into account heading and starting directions. Both analyses need to be considered, as it is important to note that scotomas can vary in shape across individuals, and even between eyes within an individual. In some patients, the PRL is located along the edge of a scotoma, whereas in others it can be entirely or partially surrounded by a scotoma. Therefore, simply looking at retinal health in “heading” and “starting” directions individually is insufficient to understand the complex interplay of target motion, target location, and PRL location relative to the target and the scotoma. Conversely, to look exclusively at retinal health at locations corresponding to the moving target does not take into account patients' experience with their scotoma.
For the dynamic analysis we considered the actual retina traversed by the target. We did so by looking at both the trial as a whole (broken up into three epochs), and the portion of the trial for which gain was computed. We were surprised to find that the health of starting and traversed retina did not have an effect on the latency or gain. Furthermore, whether the target started in healthy retina did not predict whether the patient could maintain the target on healthy retina for the majority of the trial. This outcome is surprising and may require further investigation. In our experiment, we probed only the absolute scotoma, as our flash stimuli were well above threshold in brightness and were therefore easy to detect. The measure of retinal health in a given location and its relationship to pursuit quality may be more nuanced, and the region of relative scotoma may have to be taken into account for future investigations.
For the static analysis, we looked at the heading direction of the target relative to the scotoma. For this analysis we considered two parts of the trajectory, the starting and heading ROI, relative to the PRL. Our initial hypothesis was that patients would perform most poorly when the target motion initiated in the scotoma, i.e., when the starting ROI was in unhealthy retina (Figure 8B). We anticipated low gain and an increase in latency due to the initial disappearance of the target in the scotoma. Our results show that a high proportion of scotoma in the starting ROI increased latency. However, pursuit gain was not affected by the starting ROI (Table 2). Interestingly, both gain and latency were adversely affected by the presence of scotoma in the heading direction (Figure 8A), suggesting several possible interpretations. One possible explanation is that in a situation where pursuit gain is less than one, and therefore the PRL falls behind the target; the target will approach and eventually be occluded by the scotoma (Figure 8A). If the participant does not reacquire the target, the gains should diminish towards the end of these trials (as is the case in Figure 7A, E). Previous research suggests that when a smooth pursuit target is occluded, smooth pursuit eye velocity starts to decelerate approximately 190 ms after target disappearance. The eyes continue to move at a much lower “residual velocity” that develops after as little as 300 ms of target presentation and persists for an extended period of time (<4 s) (Becker & Fuchs, 1985; Bennett & Barnes, 2006). However, we did not consistently observe a decrease in gain towards the end of the trial. This discrepancy could be due to participants refixating the target several times throughout the trial; however, we find this possibility unlikely, as the majority of the participants did not exhibit searching behavior, such as saccades in different directions, at later stages of the trial. Interestingly, the best strategy in this target-PRL configuration might be to maintain a gain of 1 or slightly above—values that are present in some participants for certain directions, but seldom when the target is heading into the scotoma.
Figure 8.
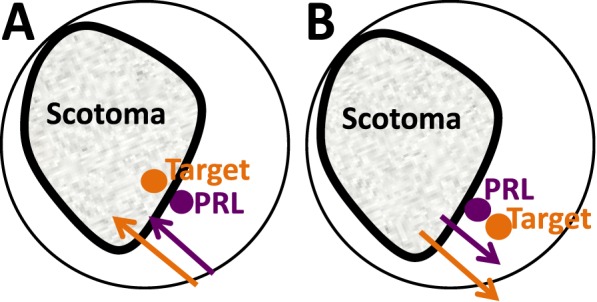
Schematic representation of target motion relative to scotoma. (A) Target moving towards a scotoma. If the eye velocity (purple arrow) is less than target velocity (gain <1), the PRL will lag the target, which will eventually disappear behind the scotoma. (B) Target moving away from the scotoma. If PRL lags the target, the target will move across healthy retina.
The health of the retina may also have been an important factor. When the target is moving toward the scotoma (Figure 8A), it is moving into progressively less healthy retina. The longer latencies suggest that the target could be very close to the scotoma by the time pursuit begins. Conversely, when the target is moving into healthy retina (Figure 8B), as the eye lags, the target moves away from the scotoma, which may be especially important as the PRL is frequently close to the scotoma border (Fletcher & Schuchard, 1997). Our results indicate that this interpretation on its own is unlikely, as retinal health at the location of the target did not directly predict changes in gain, indicating that an investigation that includes relative and dense scotoma boundaries (Ergun et al., 2003) in the analysis is warranted. Finally, our finding may be related to the physiological constraints on patients' eye movements, with movement into the scotoma requiring a more eccentric eye position than is comfortably possible. However, several of our participants had close-to-central PRLs and nonetheless exhibited gain variations based on scotoma location.
For latency, one would easily expect an increase when the target steps into the scotoma at trial onset, as the patient would require a longer time to find the target. However, the influence of heading direction on latency is more surprising. A possible explanation is a change in the patients' strategy: While the target is visible, the patients may not be motivated to immediately move their eyes to start tracking it, as that may introduce additional position error. Patients may even delay making the initial saccade toward the target since vision is suppressed during saccades, reducing the amount of time patients have to visualize the target. As the target approaches the scotoma, patients would then initiate pursuit, albeit with less intact retina and lower gains. An alternative explanation is a measurement artifact: Because gains were lower in these trials, latencies were more difficult to detect precisely. We believe this explanation unlikely, since we saw this phenomenon across patients who had differing ranges of gain modulation, i.e., lowest gains of one patient were comparable to some of the higher gains for another, but they all exhibited this trend in latency. Interestingly, we also found a negative interaction between latency and the extent of scotoma in both ROIs. This interaction indicates that as the amount of scotoma in both regions increases, pursuit latency decreases. One potential explanation is that participants are more likely to move their eyes quickly when the target is likely to disappear into their scotoma, soon after it becomes visible. This behavior suggests that prior experience with their scotoma affects pursuit strategy.
Smooth pursuit can be an important tool for individuals with vision loss, as it can afford additional time for target visualization, analysis, and identification, as in the case of trying to identify the number of a passing bus. The current study is the first to examine smooth pursuit in individuals with macular degeneration, and it provides key first steps to understanding this behavior. However, because of the limitations of the SLO, the study was performed monocularly using above-threshold viewing conditions. To extend our findings to smooth pursuit in the real world for persons with central field loss, we need a greater range of target velocites under binocular viewing. Therefore, future investigation of smooth pursuit under more natural viewing conditions and at a variety of target speeds can provide important information about successful strategies that individuals with central visual field loss can employ. These eye tracker-based studies can be related to the monocular measurements presented here to frame the behavior in the context of each patient's individual pursuit locus.
Supplementary Material
Acknowledgments
The authors thank Val Morash for help with the design and execution of the linear mixed effects models. This research was funded by the Rachel C. Atkinson Postdoctoral Fellowship, and NIH grant F32 EY025151 to Natela Shanidze, and NIH grant R01 EY022394 and PVF grant 09007101 to Preeti Verghese.
Commercial relationships: none.
Corresponding author: Natela M. Shanidze.
Email: natela@ski.org.
Address: Smith-Kettlewell Eye Research Institute, San Francisco, CA, USA.
Contributor Information
Natela Shanidze, natela@ski.org, http://www.ski.org/users/natela-shanidze.
Giovanni Fusco, giofusco@ski.org, http://www.ski.org/users/giovanni-fusco.
Elena Potapchuk, Email: elena@ski.org.
Stephen Heinen, heinen@ski.org, http://www.ski.org/users/steve-heinen.
Preeti Verghese, preeti@ski.org, http://www.ski.org/users/preeti-verghese.
References
- Bahill, A. T.,, McDonald J. D. (1983). Smooth pursuit eye movements in response to predictable target motions. Vision Research, 23 (12), 1573–1583. [DOI] [PubMed] [Google Scholar]
- Becker W.,, Fuchs A. F. (1985). Prediction in the oculomotor system: Smooth pursuit during transient disappearance of a visual target. Experimental Brain Research, 57 (3), 562–575. [DOI] [PubMed] [Google Scholar]
- Bennett S. J.,, Barnes G. R. (2006). Smooth ocular pursuit during the transient disappearance of an accelerating visual target: The role of reflexive and voluntary control. Experimental Brain Research, 175 (1), 1–10, doi.org/10.1007/s00221-006-0533-4. [DOI] [PubMed] [Google Scholar]
- Crossland M. D.,, Sims M.,, Galbraith R. F.,, Rubin G. S. (2004). Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vision Research, 44 (13), 1537–1546, doi.org/10.1016/j.visres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ergun E.,, Maár N.,, Radner W.,, Barbazetto I.,, Schmidt-Erfurth U.,, Stur M. (2003). Scotoma size and reading speed in patients with subfoveal occult choroidal neovascularization in age-related macular degeneration. Ophthalmology, 110 (1), 65–69. [DOI] [PubMed] [Google Scholar]
- Fletcher D. C.,, Schuchard R. A. (1997). Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology, 104 (4), 632–638, doi.org/10.1016/S0161-6420(97)30260-7. [DOI] [PubMed] [Google Scholar]
- Heinen S. J.,, Badler J. B.,, Ting W. (2005). Timing and velocity randomization similarly affect anticipatory pursuit. Journal of Vision, 5 (6): 1 493–503, doi:10.1167/5.6.1 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Heinen S.,, Skavenski A. (1992). Adaptation of saccades and fixation to bilateral foveal lesions in adult monkey. Vision Research, 32 (2), 365–373. [DOI] [PubMed] [Google Scholar]
- Janssen C. P.,, Verghese P. (2015). Stop before you saccade: Looking into an artificial peripheral scotoma. Journal of Vision, 15 (5): 1 1–19, doi:10.1167/15.5.7 [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger S.,, Morris E. (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neuroscience, 10 (1), 97–129. [DOI] [PubMed] [Google Scholar]
- MacKeben M.,, Gofen A. (2007). Gaze-contingent display for retinal function testing by scanning laser ophthalmoscope. Journal of the Optical Society of America A, Optics, Image Science, and Vision, 24 (5), 1402–1410. [DOI] [PubMed] [Google Scholar]
- Pidcoe P. E.,, Wetzel P. A. (2006). Oculomotor tracking strategy in normal subjects with and without simulated scotoma. Investigative Ophthalmology & Visual Science, 47 (1), 169–178. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Rashbass C. (1961). The relationship between saccadic and smooth tracking eye movements. The Journal of Physiology, 159, 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchard R. A.,, Naseer S.,, de Castro K. (1999). Characteristics of AMD patients with low vision receiving visual rehabilitation. Journal of Rehabilitation Research and Development, 36 (4), 294–302. [PubMed] [Google Scholar]
- Seiple W.,, Szlyk J. P.,, McMahon T.,, Pulido J.,, Fishman G. A. (2005). Eye-movement training for reading in patients with age-related macular degeneration. Investigative Ophthalmology & Visual Science, 46 (8), 2886–2896. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Verghese P.,, Janssen C. P. (2015). Scotoma awareness and eye movement training in age-related macular degeneration. Presented at the Association for Research in Vision and Ophthalmology, Session 310, Program No. 2620.
- White J. M.,, Bedell H. E. (1990). The oculomotor reference in humans with bilateral macular disease. Investigative Ophthalmology & Visual Science, 31 (6), 1149–1161. [PubMed] [Article] [PubMed] [Google Scholar]
- Whittaker S. G.,, Budd J.,, Cummings R. W. (1988). Eccentric fixation with macular scotoma. Investigative Ophthalmology & Visual Science, 29 (2), 268–278. [PubMed] [Article] [PubMed] [Google Scholar]
- Winterson B. J.,, Steinman R. M. (1978). The effect of luminance on human smooth pursuit of perifoveal and foveal targets. Vision Research, 18 (9), 1165–1172, doi.org/10.1016/0042-6989(78)90100-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.