Abstract
Myxofibrosarcoma is a soft tissue neoplasm composed of malignant fibroblasts with a myxoid matrix. It is commonly found in patients during their 6th through 8th decades of life with a slight male predominance. Myxofibrosarcomas are classified as low- to high-grade tumors that are differentiated by hypercellularity, variation of mitotic activity and degree of necrosis. The most common sites are the extremities (77%) and trunk (12%), with the retroperitoneum and mediastinum being very rare. In this case report, we describe a patient presenting with myxofibrosarcoma of the mediastinum, a rare site for the development of myxofibrosarcoma. This case of primary mediastinal myxofibrosarcoma appears to be only the second described in the English-language literature.
Key Words: Myxofibrosarcoma, Primary mediastinal myxofibrosarcoma, Thoracic location
Case Report
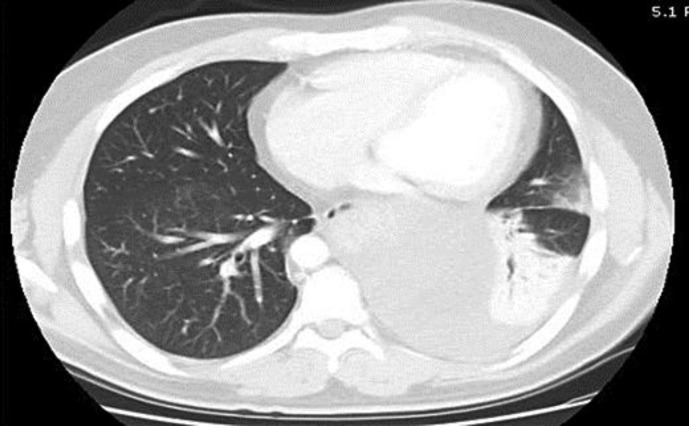
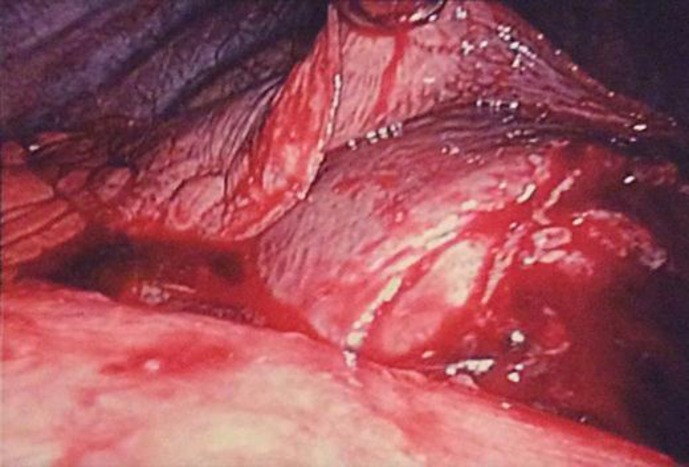
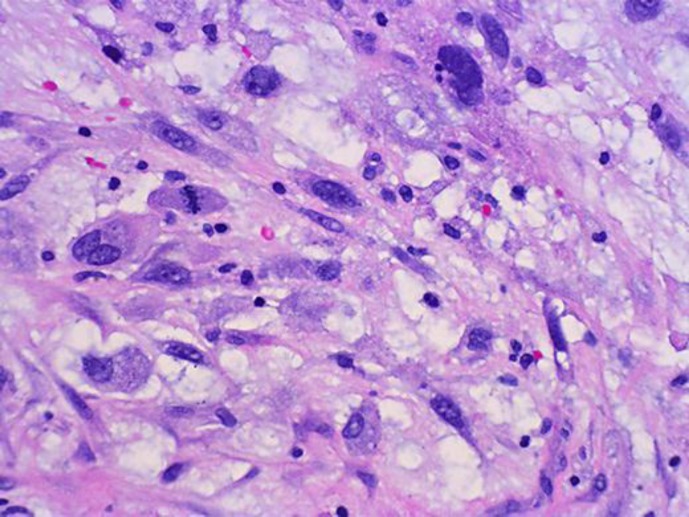
A 38-year-old man presented to his primary care physician with complaints of dry cough, left-sided pleuritic chest pain and left-sided back pain of 2 weeks duration. He was found by chest radiography to have an infiltrate in the left lower lobe and treated for community-acquired pneumonia with moxifloxacin for 10 days. After completing the course of antibiotics, he presented again with worsening symptoms of left-sided chest pain and attesting to a weight loss of approximately 5 lbs over the past month. A repeat chest radiograph showed worsening of the left lower lobe infiltrate and an enlarging effusion. A CT scan of the chest showed a large effusion on the left with some loculation inferomedially (fig. 1). His laboratory analysis revealed a decrease in hemoglobin from 14.0 to 10.4 g/dl within the past 10 days as well as a leukocyte count of 10.6 × 109/l. Laboratory work-up also demonstrated normal platelets and a normal metabolic profile. With a high suspicion for hemorrhagic effusion, he underwent video-assisted thoracoscopic surgery which revealed a large 12.5 × 8.0 cm gelatinous mediastinal mass with bleeding into the pleural cavity (fig. 2). The mass was noted to be compressing the posterior aspect of the left atrium. The origin of the mass was unclear. The patient had a negative work-up for tumor markers including alpha-fetoprotein, CA-125, CA 15-3, CA 19-9 and carcinoembryonic antigen. A biopsy of the mass revealed myxoid stroma with pleomorphic cells and atypical nuclei with high mitotic activity most consistent with myxofibrosarcoma (fig. 3). Pleural fluid cytology was negative for malignancy and the patient was staged as T2bN0M0. He was evaluated for surgical resection of the mass and was advised preoperative radiation therapy. Unfortunately, the mass continued to progress even while he was receiving radiation therapy. He was then started on chemotherapy with a regimen of doxorubicin, ifosfamide and mesna. His clinical course was complicated by multiple episodes of neutropenic fevers, pancytopenia requiring transfusions as well as pericardial and pleural effusions. In addition to this, in subsequent imaging it was found that the mass was infiltrating the pericardium. Follow-up scans showed progression of his cancer and the development of liver metastases. His condition deteriorated over a period of a few months with worsening respiratory symptoms due to progression of the mass in size.
Fig. 1.
CT scan showing large heterogeneous left-sided pleural effusion.
Fig. 2.
Large gelatinous mass with bleeding into the pleural cavity as seen on video-assisted thoracoscopic surgery.
Fig. 3.
Myxoid stroma with high mitotic activity and pleomorphism. Tumor cells are seen at 400× magnification.
Discussion
Treatment of soft tissue sarcomas, including myxofibrosarcomas, is generally based on surgery combined with radiotherapy and/or chemotherapy. Surgical resection is the mainstay of treatment for soft tissue sarcomas [1]. Surgery has evolved greatly throughout the years, shifting from whole limb amputation in the early years to selective tumor excision with flap tissue reconstruction in recent years [2]. However, despite complete resection, recurrence remains common. A retrospective study was done at Memorial Sloan-Kettering Cancer Center to assess for prognostic indicators of local and distant recurrences in patients with chest wall soft tissue sarcomas. The study showed that high tumor grade and tumor size >5 cm were statistically associated with an increased risk of recurrence [2]. Of these two factors, tumor grade was the most important prognostic factor, with a high grade conferring a hazard ratio of about 3–4 times that of patients with low-grade tumors.
Studies have shown varying rates of recurrence, ranging from 16 to 54% [3]. Given this high rate of recurrence, researchers have investigated strategies for decreasing the rate of recurrence and improving mortality. With this goal in mind, radiotherapy is often added to surgery as it has been shown to decrease the rate of recurrence. Preoperative radiotherapy (PreRX) and postoperative radiotherapy (PORT) have been studied. Radiotherapy plays an important role in limb-sparing therapy. These two modalities have been shown to decrease the risk of local recurrence [4, 5].
PORT was shown in a single-center trial to significantly decrease the risk of local recurrence; however, overall survival (OS) was not impacted [5]. This is due to the association of PORT with significant morbidity, including edema and reduced strength and joint motion.
PreRX was developed with the hypothesis that limiting the radiated area would decrease the side effects. However, a study where PreRX was compared to PORT had to be stopped early when patients who received PreRX were found to have a higher incidence of surgical wound complications [6].
Brachytherapy has also been utilized and, similarly, found to decrease the risk of local recurrences, but with no impact on OS [6]. This is due to increased morbidity and reoperation rates. High-dose radiation has been studied in patients with unresectable disease; nevertheless, this should always be used as a last resort [7].
Chemotherapy is usually added to patients who have high-grade tumors, and is used mostly to decrease the rate of distant metastases and to increase OS. A study conducted in Italy randomized patients to standard therapy (surgery plus radiotherapy) or standard therapy plus epirubicin and ifosfamide. The investigators found an improvement in disease-free survival (DFS) and OS at a median follow-up of 59 months [8]. Patients randomized to chemotherapy had a hazard ratio of 0.59 for overall DFS. OS in the intervention group was improved by 48%. However, a later follow-up on this same study at a median of 89 months found that the statistical significance in DFS and OS had disappeared [9].
A meta-analysis of these two studies and several other trials of doxorubicin-based chemotherapy plus ifosfamide chemotherapy compared with doxorubicin alone found that use of any chemotherapy decreased the risk of local and distant recurrences significantly (by about 30–40%); however, only doxorubicin plus ifosfamide affected OS [10].
Conclusions
Most of the research in the treatment of soft tissue sarcomas was done in patients with limb or girdle masses. Nevertheless, we can extrapolate that these patients’ treatment should include wide excision surgery with PORT and chemotherapy with doxorubicin and ifosfamide.
However, the location of the tumor in our patient poses a challenge for treatment. The patient's tumor is located adjacent to vital structures, namely the heart, great vessels and lungs. In addition to this, in subsequent imaging, it was found that the mass was infiltrating the pericardium. This makes resection by surgery technically difficult and wide resection with the recommended 5 cm tumor-free margin [3] impossible. PreRX was attempted in the hope that it might improve resectability. Unfortunately, this was unsuccessful. These factors would classify our patient's disease as unresectable, raising the question of what is the best course of treatment.
As discussed previously, unresectable disease is typically treated with high-dose radiation. According to studies, a dose of 63 Gy or more is recommended to improve DFS and OS [7]. However, it was noted in this same study that patients who received >68 Gy of radiation had significantly higher morbidity compared to those who received a lower dose. These two factors become extremely important in this patient, in whom location of the mass would put the heart and lungs in the trajectory of the radiation beam. It is known from previous experience with radiotherapy of breast tumors and Hodgkin lymphomas that radiation to the heart poses an increased risk of complications. These complications include, but are not restricted to, pericarditis and pericardial disease, which can happen early or late in the treatment, valvular abnormalities and myocardial infarction [11, 12].
In addition to the adverse effects of radiotherapy, the recommended chemotherapy for this patient, which is doxorubicin plus ifosfamide, has also been associated with adverse cardiovascular effects [11]. This is especially true when large doses of radiation are given concomitantly.
Despite the potential adverse effects of radiotherapy and chemotherapy, the fact that this patient has an unresectable mass stresses the need for palliative therapies. In a young patient who has no cardiovascular disease, the potential short-term benefits of radiotherapy and chemotherapy would outweigh the long-term risks and as such, aggressive treatment should be sought after discussion with the patient of potential benefits and complications.
This case of primary mediastinal myxofibrosarcoma appears to be only the second described in the English-language literature. We can also say that, despite appropriate guidelines, the existing literature does not apply to every patient in the same way. Personalization of treatment, especially in patients with rare tumors and atypical presentation like in our patient, is often necessary. Involvement of the patient in the decision on the final therapy becomes even more important in cases like this.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.McMillan RR, Sima CS, Moraco NH, Rusch VW, Huang J. Recurrence patterns after resection of soft tissue sarcomas of the chest wall. Ann Thorac Surg. 2013;96:1223–1228. doi: 10.1016/j.athoracsur.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer. 2002;94:2256–2264. doi: 10.1002/cncr.10419. [DOI] [PubMed] [Google Scholar]
- 3.Hambleton C, Noureldine S, Gill F, Moroz K, Kandil E. Myxofibrosarcoma with metastasis to the lungs, pleura, and mediastinum: a case report and review of literature. Int J Clin Exp Med. 2012;5:92–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 5.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 6.Alektiar KM, Zelefsky MJ, Brennan MF. Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 2000;47:1273–1279. doi: 10.1016/s0360-3016(00)00587-3. [DOI] [PubMed] [Google Scholar]
- 7.Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63:852–859. doi: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti G, Barbieri E, Apice G, Zmerly H, Serraino D, Picci P. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 9.Frustaci S, De Paoli A, Bidoli E, La Mura N, Berretta M, Buonadonna A, Boz G, Gherlinzoni F. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65(suppl 2):80–84. doi: 10.1159/000073366. [DOI] [PubMed] [Google Scholar]
- 10.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 11.Byrd BF, 3rd, Mendes LA. Cardiac complications of mediastinal radiotherapy. The other side of the coin. J Am Coll Cardiol. 2003;42:750–751. doi: 10.1016/s0735-1097(03)00760-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112:1688–1696. doi: 10.1016/j.amjcard.2013.07.031. [DOI] [PubMed] [Google Scholar]