Summary
Liver metastases are the most frequent malignant liver lesions. Besides colorectal carcinoma, gastric carcinoma, pancreatic carcinoma, breast cancer, lung cancer, and neuroendocrine tumors are the most common entities that metastasize to the liver. The morphology of these metastases depends on the primary tumor. For morphologic and functional imaging of non-colorectal liver metastases, multiple imaging techniques such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography coupled with CT or MRI are available. This review summarizes morphologic and functional characteristics of different non-colorectal liver metastases.
Keywords: Computed tomography, CT; Liver metastases; Magnetic resonance imaging, MRI; Positron emission tomography, PET; Ultrasonography
Introduction
Liver metastases are the most common malignant liver lesions and arise about 18-40 times more frequent than primary liver tumors [1]. In previous autopsy studies, approximately 55% of patients with diagnosed malignant tumors were suffering from liver metastases [2]. Since metastatic disease has a major impact on the morbidity and prognosis of the patient, the detection of potential liver metastases must be considered of utmost importance. Therefore, the identification of liver metastases is one of the most frequent indications for diagnostic imaging of the liver.
Besides colorectal carcinoma, gastric carcinoma, pancreatic carcinoma, breast cancer, lung cancer, and neuroendocrine tumors (NETs) are the most common entities that metastasize to the liver [3]. The morphologic characteristics of liver metastases depend on the primary tumor. Typically, metastases from adenocarcinomas are centrally hypovascularized and may show a hypervascularized peripheral rim encircling the hypovascularized central zone [4]. In contrast, metastases from NETs, thyroid cancers, renal cell carcinoma, and malignant melanomas are usually predominantly hypervascularized compared to the adjacent liver parenchyma. Thus, the imaging protocol needs to address different contrast-enhancing phases. For morphologic and functional imaging of non-colorectal liver metastases, different imaging techniques are available.
Imaging Techniques
Ultrasonography
Ultrasonography (US) plays a pivotal role in morphologic and functional imaging of non-colorectal liver metastases. Due to its ubiquitous and rapid availability, the lack of radiation exposure, and its low cost, US is the most frequently used imaging technique in clinical routine.
Depending on the primary tumor, non-colorectal liver metastases can show a great variety of appearances with different echogenicity on gray-scale US compared to the adjacent liver tissue. Tumors may appear hypo-, iso-, or hyperechoic on non-contrast-enhanced gray-scale US. Additional sonographic patterns include cystic, calcific, or mixed echogenic appearance. The presence of a hypoechoic halo surrounding a liver mass is suggestive of liver metastases [5]. However, there is no typical appearance of a liver metastasis on non-contrast-enhanced gray-scale ultrasound. Apart from the wide range of appearances, detection of liver metastases may be influenced by the experience of the examiner [6]. Thus, compared to computed tomography (CT) and magnetic resonance imaging (MRI), conventional non-contrast-enhanced gray-scale US suffers from a lower detection rate of focal liver lesions [7]. The development of US contrast agents accompanied by the use of contrast-specific imaging modes significantly improved both sensitivity and specificity in the detection of hepatic metastases [8,9].
Liver metastases have similar characteristics on contrast-enhanced US as compared with contrast-enhanced CT and MRI (table 1). Contrast-enhanced US offers the detection and characterization of liver lesions by assessing their contrast dynamics. A major limitation of contrast-enhanced US is the limited field of view that depends on the US probe being used [10]. Typically, only one or a limited number of liver lesions can be assessed dynamically.
Table 1.
Imaging characteristics of different non-colorectal liver metastases
| Hypovascularizeda |
| Pancreatic adenocarcinoma |
| Gastric carcinoma |
| Lung carcinoma |
| Genitourinary (prostate, bladder) |
| Hypervascularized |
| Neuroendocrine tumors |
| Renal cell carcinoma |
| Thyroid carcinoma |
| Breast carcinoma |
| Malignant melanoma |
| Sarcoma |
| Calcified |
| Breast carcinoma |
| Testicular carcinoma |
| Renal cell carcinoma |
| Neuroblastoma |
| Mucinous carcinoma from pancreas, gastrointestinal, ovary |
| Osteosarcoma |
| Thyroid carcinoma |
Sometimes with hypervascular rim.
Computed Tomography
Due to its wide availability CT is the preferred modality for liver imaging in most centers [10]. In particular, modern multidetector contrast-enhanced CT is the standard imaging technique for liver imaging. Depending on the primary tumor, non-colorectal liver metastases may present with a different appearance on CT imaging. Therefore, the use of a triphasic contrast-enhanced CT protocol is advantageous for the characterization of primary metastases. The non-enhanced phase may detect calcifications inside metastases, whereas with the arterial and portal venous contrast phases the vascularization of liver metastases can be evaluated. Most of the non-colorectal liver metastases are hypovascularized and therefore appear hypodense compared to healthy liver tissue in the portal venous or venous contrast-enhancing phase (fig. 1). However, metastases from hypervascular tumors demonstrate an early contrast enhancement in the arterial contrast phase. These hypervascular metastases typically demonstrate with a wash-out in the portal venous or venous contrast-enhancing phase [3]. This wash-out either leads to a hypodense appearance compared with normal liver parenchyma or to an isodense appearance in the portal venous or venous contrast-enhancing phase. In cases of an isodense appearance, metastases may be masked by their similar appearance as compared to the surrounding liver parenchyma and may be overlooked if only a portal venous or venous phase are acquired (fig. 2). Therefore, an arterial contrast-enhancing phase is mandatory when suspecting hypervascular liver metastases. Non-colorectal primaries that are associated with hypervascularized hepatic metastases are listed in table 1.
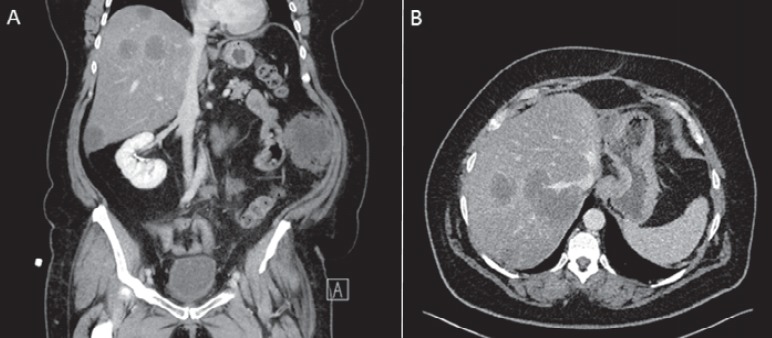
Fig. 1.
A Coronal and B transversal abdominal CT scan of a 51-year-old female patient suffering from sarcoma of the uterus. Both images show multiple hypodense liver metastases in the right liver lobe during the portal venous contrast phase.
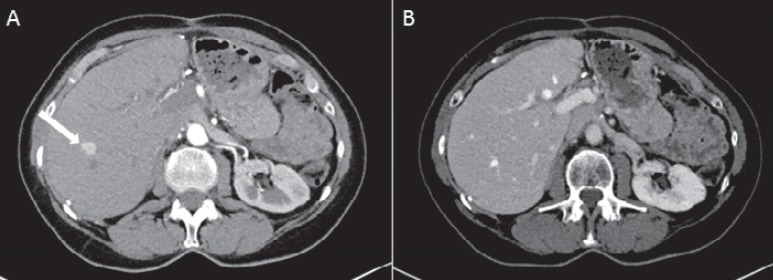
Fig. 2.
CT scan of the liver of a 75-year-old female patient with a histologically proven NET of the pancreas. A Arterial contrast enhancement of a metastasis in segment 8 of the liver (white arrow). B In the portal venous contrast-enhancing phase, there is similar enhancement of the metastasis and the surrounding liver parenchyma which leads to masking of the metastasis.
In order to optimize tumor delineation of hypervascularized liver lesions and to better delineate vascular structures, Kamel et al. [11] have demonstrated the superiority of multiplanar volume rendering and maximum intensity projection of multislice CT compared with routine axial images. Regarding detection rates of hepatic metastases, CT offers a sensitivity of about 85% [12], but has lower sensitivity in the detection of small lesions with a diameter of <1 cm.
Magnetic Resonance Imaging
Due to the lack of ionizing radiation and the high sensitivity caused by the good lesion-to-liver contrast, MRI is rapidly evolving as the imaging modality of choice for the characterization and detection of hepatic lesions [13]. One of the most characteristic MRI features for the detection and characterization of focal liver metastases is based on the typical appearance in T1- and T2-weighted pulse sequences [14]. Usually, most non-colorectal liver metastases show a hypo- to isointense appearance in T1-weighted images and an iso- to slightly hyperintense appearance in T2-weighted images. However, some liver metastases provide a hyperintense signal on T1-weighted images, e.g. liver metastases from malignant melanoma caused by melanin and extracellular methemoglobin [13] (fig. 3). As with CT, a gadolinium-based MRI contrast agent can be applied to acquire a dynamic liver MRI in order to differentiate between hypo- and hypervascularized focal liver lesions. The intravenous use of hepatocyte-specific contrast agents can further increase the diagnostic accuracy of MRI for the detection and characterization of focal liver lesions. Besides imaging of the hepatic arterial and portal venous phases, hepatocyte-specific contrast agents allow the visualization of the delayed hepatocyte uptake and partial excretion of the contrast agent into the biliary system. This combination of hepatocyte uptake and biliary excretion results in the additional so-called hepatocellular phase of imaging [15]. As hepatic metastases do not usually contain functioning hepatocytes or bile ducts, they do not enhance in the hepatocellular phase (also called the liver-specific phase) and therefore appear hypointense compared to healthy liver parenchyma [15] (fig. 4).
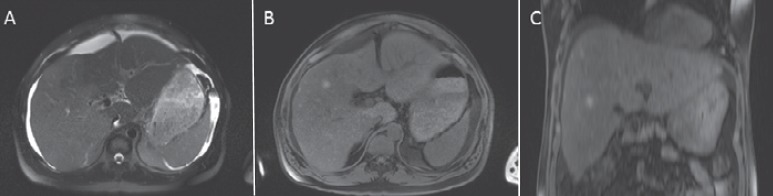
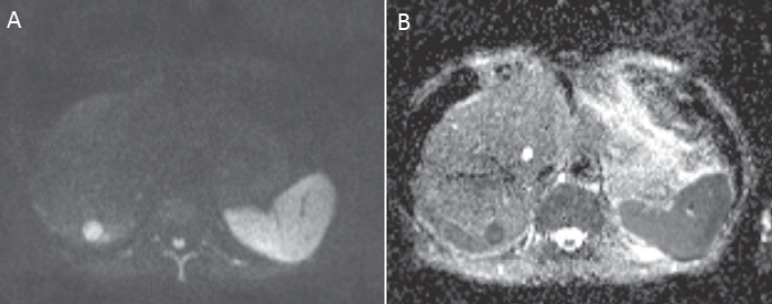
Fig. 3.
A MRI of the liver in a 42-year-old male patient with a liver metastasis from malignant melanoma in the right liver lobe, not clearly visible in the T2-weighted scan. In the non-contrast-enhanced T1-weighted scan the metastasis appears hyperintense compared with the adjacent liver tissue. Hyperintensity is caused by melanin. B Axial image; C coronal image.
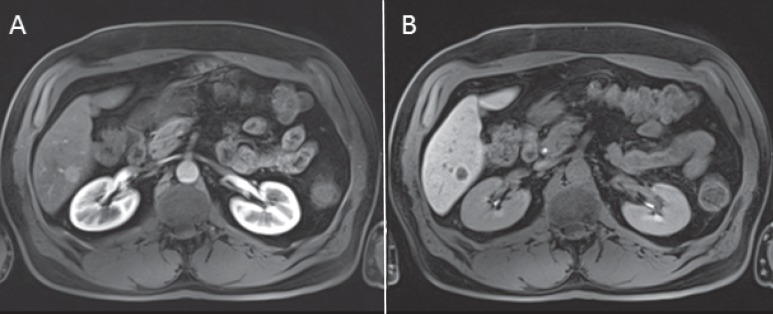
Fig. 4.
A MRI scan of the liver of a 75-year-old male patient with a NET of the pancreas shows arterial contrast enhancement of a hepatic metastasis in segment 6 of the liver. B In the liver-specific contrast-enhancing phase 40 min after i.v. contrast injection using a hepatocyte-specific contrast agent, the metastasis appears hypointense compared with the adjacent liver parenchyma.
Apart from liver-specific contrast, MRI offers other functional parameters that are frequently used in the diagnosis of liver disease, e.g. diffusion-weighted imaging (DWI). DWI is based on the Brownian motion of water and enables quantification of the apparent diffusion coefficient (ADC). Malignant lesions provide lower ADC values due to a combination of higher cellularity and tissue disorganization, contributing to reduced motion of water. DWI has high sensitivity for detection of liver metastases and has been adopted as part of the protocol for MRI of the liver by many centers [16]. Due to the bright appearance of malignant liver lesions on high-b-value DWI images, DWI can serve as a ‘search-sequence’ in MRI protocols of the liver (fig. 5). However, DWI suffers from its low specificity in characterizing focal liver lesions [17,18]. Hence, there is still an ongoing debate whether DWI can be seen as a reasonable alternative to contrast-enhanced MRI [19] or may just serve as an add-on to the contrast-enhanced data set [20].
Fig. 5.
A High-b-value DWI (b = 800 s/mm2) and B corresponding ADC map of a metastasis in segment 7 of the liver in a 67-year-old male patient with NET of the small bowel. Increased signal on high-b-value images and decreased signal on ADC will be expected in a liver metastasis.
PET Imaging
Positron emission tomography (PET) with (18F)-fluorodeoxyglucose (FDG) is frequently used in the setting of a whole-body PET/CT or PET/MRI for tumor staging of FDG-PET-positive tumors (table 2). In this setting, FDG-PET may allow for a more accurate characterization of focal liver lesions due to the added molecular information on glucose metabolism [21] (fig. 6). However, the sensitivity of FDG-PET in small liver metastases (<1 cm in diameter) is limited due to liver motion while acquiring the PET, PET resolution, and the rather non-specific radiopharmaceutical FDG [22,23]. Thus, if the primary clinical question is to detect or exclude liver metastases, MRI must be considered the imaging modality of choice (fig. 7).
Table 2.
Tumors frequently FDG-PET-positive
| FDG-avid tumors |
|---|
| Malignant melanoma |
| Non-small cell lung cancer |
| Lymphoma |
| Colorectal cancer |
| Esophageal cancer |
| Stomach cancer / GIST |
| Head and neck cancer |
| Thyroid cancer |
| Cervical cancer |
| Breast cancer |
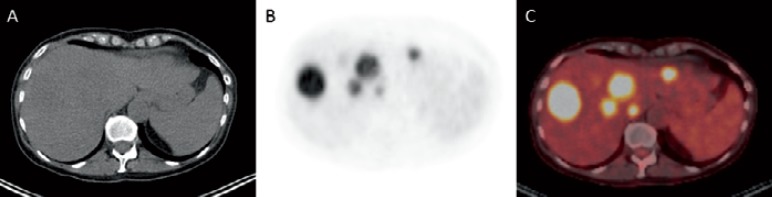
Fig. 6.
A (18F)-FDG-avid liver metastases in both liver lobes in a 53-year-old female patient with malignant melanoma of the forehead on non-enhanced CT image, B PET, and C on fused FDG-PET/CT data set.
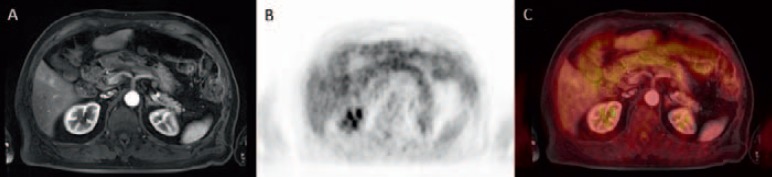
Fig. 7.
A Small contrast-enhancing liver metastasis in segment 5 of the liver in a 63-year-old male patient suffering from uveal melanoma. B, C The metastasis does not show on FDG-PET mainly based on its small size.
PET/CT has a clear indication in the diagnosis of NETs and their metastases. The detection and evaluation of NET metastases benefits from overexpression of somatostatin receptors, reflecting a specific target for molecular imaging techniques using somatostatin analogs such as octreotide or lanreotide. Scintigraphy or single-photon emission CT with (111In-diethylenetriamine pentaacetic acid-0-)octreotide are basic tools for the detection of NETs, though with a weakness in delineating anatomical detail [24]. PET/CT or PET/MRI with 68-Gallium (68Ga)-labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) peptides such as DOTA-D-Phe1-Tyr3-octreotide (DOTATOC), DOTA-1-Nal3-octreotide (DOTANOC), or DOTA-D-Phe1-Tyr3-octreotate (DOTATATE) have shown a higher diagnostic accuracy than scintigraphy-based examinations due to a higher affinity for the somatostatin receptor subtype 2 (SSTR2), an increased spatial resolution, and a more distinct detail delineation based on the corresponding CT [25,26]. Using PET/CT or PET/MRI with specific somatostatin-based radiopharmaceuticals, small NETs and their metastases can be easily detected [27] (fig. 8). The specific radionuclide offers good lesion-to-background contrast which allows detection of liver metastases even below 1 cm. However, even in this setting with a specific somatostatin-based radiopharmaceutical, MRI has been found to be more sensitive for detection of very small liver metastases. An MRI of the liver is therefore performed preoperatively (before the resection of liver metastases) in all our patients to exclude further metastases which may alter patient management. Those patients that are eventually operated on undergo intraoperative ultrasound additionally.
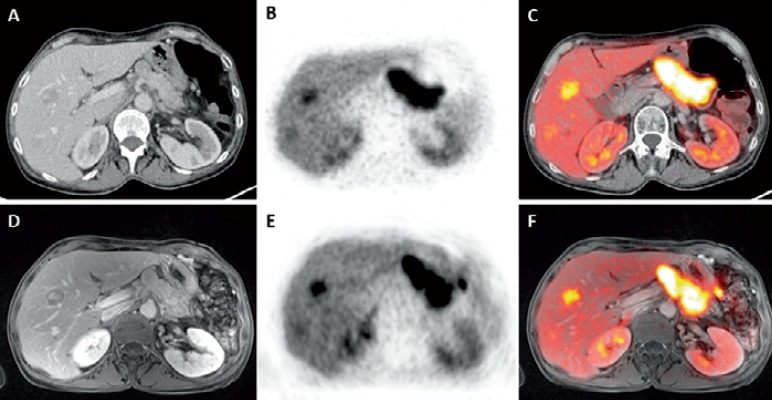
Fig. 8.
A 62-year-old female patient with a NET liver metastasis in the right liver lobe on CT. B This liver lesion shows increased (68Ga)-DOTATOC signal on PET and C on the fused DOTATOC-PET/CT. D The same liver metastasis of the same patient on contrast-enhanced T1w MR image, E clearly visible on PET, and F on fused (68Ga)-DOTATOC-PET/MRI.
In some institutions PET/MRI has been available. This imaging modality is currently being evaluated for potential indications. The addition of MRI to PET offers a superior soft tissue contrast of MRI as compared with CT. Based on this superior soft tissue contrast of MRI, the accuracy of detecting liver metastases will probably be higher with PET/MRI than with PET/CT. For the evaluation of liver metastases of NETs, studies on retrospective PET/MRI fusion have already shown promising results. For example, Schreiter et al. [28] demonstrated that the high soft tissue contrast of MRI, as well as the information provided by DWI, enabled a higher detection rate and diagnostic confidence when compared to PET/CT, especially for metastases <1 cm in size. By now, only a few studies focusing on the detection or characterization of liver lesions using a real hybrid PET/MRI are available. Beiderwellen et al. [29] demonstrated a higher lesion conspicuity and diagnostic confidence of FDG-PET/MRI compared to FDG-PET/CT. In this study, FDG-PET/MRI allowed for the depiction of all lesions, whereas nine benign liver lesions were not visualized by PET/CT. However, additional malignant liver lesions were not found using PET/MRI [29]. Still, due to the small number of prospective studies with only small sample sizes, PET/MRI studies on non-colorectal liver metastases have to be considered as preliminary. It is important to know that an advantage of PET/MRI over MRI has not been found to date when assessing the liver, making MRI the unchallenged imaging modality of choice for liver metastases.
Disclosure Statement
Gerald Antoch is a speaker for Siemens Medical Solutions, Bayer Healthcare, and BGT. Philipp Heusch declares no conflict of interest.
References
- 1.Imam K, Bluemke DA. MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am. 2000;8:741–756. [PubMed] [Google Scholar]
- 2.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Prokop M, Galanski M, Schaefer-Prokop CM, Van der Molen AJ. Ganzkörper-Computertomographie: Spiral- und Multislice-CT. Stuttgart: Thieme; 2007. [Google Scholar]
- 4.Danet IM, Semelka RC, Leonardou P, Braga L, Vaidean G, Woosley JT, Kanematsu M. Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol. 2003;181:809–817. doi: 10.2214/ajr.181.3.1810809. [DOI] [PubMed] [Google Scholar]
- 5.Choi J. Imaging of hepatic metastases. Cancer Control. 2006;13:6–12. doi: 10.1177/107327480601300102. [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg A, Hohmann J, Foert E, Skrok J, Hoffmann CW, Frericks B, Wolf KJ, Albrecht T. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med. 2005;26:277–284. doi: 10.1055/s-2005-858526. [DOI] [PubMed] [Google Scholar]
- 7.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M, Campani R, Hoffmann CW, Cosgrove DO, LeFevre F. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–370. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 9.Hohmann J, Albrecht T, Oldenburg A, Skrok J, Wolf KJ. Liver metastases in cancer: detection with contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:669–681. doi: 10.1007/s00261-004-0175-6. [DOI] [PubMed] [Google Scholar]
- 10.Gaa J, Wieder H, Schwaiger M, Rummeny EJ. Modern imaging for liver metastases from colorectal tumors (Article in German) Chirurg. 2005;76:525–526. doi: 10.1007/s00104-005-1031-0. 528-534. [DOI] [PubMed] [Google Scholar]
- 11.Kamel IR, Georgiades C, Fishman EK. Incremental value of advanced image processing of multislice computed tomography data in the evaluation of hypervascular liver lesions. J Comput Assist Tomogr. 2003;27:652–656. doi: 10.1097/00004728-200307000-00038. [DOI] [PubMed] [Google Scholar]
- 12.Cantisani V, Grazhdani H, Fioravanti C, Rosignuolo M, Calliada F, Messineo D, Bernieri MG, Redler A, Catalano C, D'Ambrosio F. Liver metastases: contrast-enhanced ultrasound compared with computed tomography and magnetic resonance. World J Gastroenterol. 2014;20:9998–10007. doi: 10.3748/wjg.v20.i29.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer Imaging. 2007;7:2–9. doi: 10.1102/1470-7330.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ba-Ssalamah A, Fakhrai N, Matzek WK, Herneth AM, Stadler A, Bastati N, Herold CJ, Schima W. Magnetic resonance imaging of liver malignancies. Top Magn Reson Imaging. 2007;18:445–455. doi: 10.1097/rmr.0b013e3181614374. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin MD, Dobson JE, Sirlin CB, Lim BG, Stella DL. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics. 2011;31:1547–1568. doi: 10.1148/rg.316115528. [DOI] [PubMed] [Google Scholar]
- 16.Kanematsu M, Goshima S, Watanabe H, Kondo H, Kawada H, Noda Y, Moriyama N. Diffusion/perfusion MR imaging of the liver: practice, challenges, and future. Magn Reson Med Sci. 2012;11:151–161. doi: 10.2463/mrms.11.151. [DOI] [PubMed] [Google Scholar]
- 17.Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Aisen AM. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad Radiol. 2009;16:1208–1214. doi: 10.1016/j.acra.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Miller FH, Hammond N, Siddiqi AJ, Shroff S, Khatri G, Wang Y, Merrick LB, Nikolaidis P. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. J Magn Reson Imaging. 2010;32:138–147. doi: 10.1002/jmri.22235. [DOI] [PubMed] [Google Scholar]
- 19.Hardie AD, Naik M, Hecht EM, Chandarana H, Mannelli L, Babb JS, Taouli B. Diagnosis of liver metastases: value of diffusion-weighted MRI compared with gadolinium-enhanced MRI. Eur Radiol. 2010;20:1431–1441. doi: 10.1007/s00330-009-1695-9. [DOI] [PubMed] [Google Scholar]
- 20.Shimada K, Isoda H, Hirokawa Y, Arizono S, Shibata T, Togashi K. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur Radiol. 2010;20:2690–2698. doi: 10.1007/s00330-010-1842-3. [DOI] [PubMed] [Google Scholar]
- 21.Frankel TL, Gian RK, Jarnagin WR. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J Gastrointest Oncol. 2012;3:11–18. doi: 10.3978/j.issn.2078-6891.2012.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil HI, Patterson SA, Panicek DM. Hepatic lesions deemed too small to characterize at CT: prevalence and importance in women with breast cancer. Radiology. 2005;235:872–878. doi: 10.1148/radiol.2353041099. [DOI] [PubMed] [Google Scholar]
- 23.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol. 2011;197:W256–259. doi: 10.2214/AJR.10.6331. [DOI] [PubMed] [Google Scholar]
- 24.Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 25.Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schafer M, Schilling T, Haufe S, Herrmann T, Haberkorn U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 26.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52:1864–1870. doi: 10.2967/jnumed.111.091165. [DOI] [PubMed] [Google Scholar]
- 27.Klasen J, Heusner TA, Riegger C, Reichelt D, Kuhlemann J, Antoch G, Blondin D. Modern CT and PET/CT imaging of the liver (Article in German) Radiologe. 2011;51:671–679. doi: 10.1007/s00117-010-2125-3. [DOI] [PubMed] [Google Scholar]
- 28.Schreiter NF, Nogami M, Steffen I, Pape UF, Hamm B, Brenner W, Rottgen R. Evaluation of the potential of PET-MRI fusion for detection of liver metastases in patients with neuroendocrine tumours. Eur Radiol. 2012;22:458–467. doi: 10.1007/s00330-011-2266-4. [DOI] [PubMed] [Google Scholar]
- 29.Beiderwellen K, Gomez B, Buchbender C, Hartung V, Poeppel TD, Nensa F, Kuehl H, Bockisch A, Lauenstein TC. Depiction and characterization of liver lesions in whole body (18F)-FDG PET/MRI. Eur J Radiol. 2013;82:e669–675. doi: 10.1016/j.ejrad.2013.07.027. [DOI] [PubMed] [Google Scholar]