Summary
Background
The purpose of this review is to demonstrate the clinical indications, technical developments, and outcome of liver-directed therapies in interventional oncology of non-colorectal liver metastases.
Methods
Liver-directed therapies are classified into vascular transarterial techniques such as chemoperfusion (TACP), chemoembolization (TACE), radioembolization (selective internal radiation therapy (SIRT)), and chemosaturation, as well as thermal ablation techniques like microwave ablation (MWA), radiofrequency ablation (RFA), laser-induced thermotherapy (LITT), cryotherapy, and irreversible electroporation (IRE). The authors searched the database PubMed using the following terms: ‘image-guided tumor ablation’, ‘thermal ablation therapies’, ‘liver metastases of uveal melanoma’, ‘neuroendocrine carcinoma’, ‘breast cancer’, and ‘non-colorectal liver metastases’.
Results
Various combinations of the above-mentioned therapy protocols are possible. In neuroendocrine carcinomas, oligonodular liver metastases are treated successfully via thermal ablation like RFA, LITT, or MWA, and diffuse involvement via TACE or SIRT. Although liver involvement in breast cancer is a systemic disease, non-responding nodular metastases can be controlled via RFA or LITT. In ocular or cutaneous melanoma, thermal ablation is rarely considered as an interventional treatment option, as opposed to TACE, SIRT, or chemosaturation. Rarely liver-directed therapies are used in pancreatic cancer, most likely due to problems such as biliary digestive communications after surgery and the risk of infections. Rare indications for thermal ablation are liver metastases of other primary cancers like non-small cell lung, gastric, and ovarian cancer.
Conclusion
Interventional oncological techniques play a role in patients with liver-dominant metastases.
Keywords: Radiofrequency ablation, Laser ablation, Microwave ablation, Non-colorectal liver metastases
Introduction
The liver is the second most common site of metastases after lymph nodes. Liver metastases are found in 30-70% of patients who die of cancer. In the Western world, metastases to the liver are more common than primary liver cancer. The most frequent origins of liver metastases are: colon/rectum, pancreas, breast, and lung. In 25-50% of patients with malignancies, liver metastases are observed in autopsies. Cancer commonly spreads to the liver because it provides a suitable environment for the growth of tumor cells due to dual blood supply. For patients with non-colorectal liver metastases several treatment options are available such as resection (metastectomy, lobectomy), extremely rarely liver transplantation, systemic chemotherapy, and currently also methods of interventional oncology. In interventional oncology, two different therapeutic approaches are used for the treatment of liver metastases: i) transvascular, transarterial procedure with the administration of chemotherapeutic agents or radioactive material, or ii) chemoperfusion combined with occlusion of the vascular supply, chemoembolization, or applications of radioactive material. Other local therapeutic approaches are thermal ablation techniques such as laser-induced thermotherapy (LITT), radiofrequency ablation (RFA), microwave ablation (MWA), cryotherapy, or irreversible electroporation (IRE). All methods of transvascular or thermal ablation techniques are limited by the size of the ablation zones developing after the procedure and by the number of lesions. A safety margin of at least 1 cm around the tumor is necessary for achieving complete ablation. Thus, the maximum size of a lesion that can be successfully ablated is 4-5 cm in diameter.
In metastases which are too large for ablation therapy alone, downsizing can be achieved via transarterial chemoperfusion (TACP) and transarterial chemoembolization (TACE) in order to meet the indications for ablative therapies. Radioembolization techniques are also able to downsize liver metastases. The latest technology using chemosaturation is furthermore intended to downsize, devascularize, and stabilize in the presence of liver metastases. The goal of any type of ablative therapies is to achieve an A0 ablation, which means complete ablation also in the imaging follow-up.
Pancreatic neuroendocrine tumors (PNET) are a rare subgroup of tumors found in the pancreas. Most of the PNET are already metastatic by the time they are diagnosed, and the liver is the most common site of metastases [1,2,3]. Regional lymph node spread is also common. PNET are non-functional in the majority of cases, and the absence of a distinct functional syndrome as well as their indolent course and subsequent delay in diagnosis is mainly responsible for the advanced stage at the time of diagnosis [3,4]. PNET have a 5-year survival that can range from 97% in benign insulinomas to as low as 30% in non-functional metastatic PNET [3,4]. In addition, more recent data demonstrate that poorly differentiated PNET can have a similar prognosis like adenocarcinomas of the gastrointestinal tract [3]. Surgery as well as other forms of local treatment such as TACE or RFA can also improve the prognosis of patients with liver metastases [2,3,4].
Tumor Ablation Techniques
The term ‘tumor ablation’ is currently defined as the direct application of non-energy (chemical) or energy-based (thermal and non-thermal) therapies to eradicate or substantially destroy focal tumors [5]. Thermal ablation has to be distinguished from other applicator-based therapies which are applied via an intravascular or peripheral venous route.
Important concepts for defining the methods in thermal ablation are image guidance and planning. Image guidance is currently performed by means of imaging modalities like ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and fluoroscopy. All of these methods are defined as minimally invasive or percutaneous [5]. In the following, energy-based ablation modalities are presented.
Laser-Induced Thermotherapy
In LITT, coagulation is accomplished by using neodymium-yttrium aluminum garnet laser light. Laser delivery is possible with flexible thin fibers or a water-cooled laser applicator. A point source from a bare-tip fiber will produce a sphere of necrosis, whereas a diffuser fiber will produce an elliptical ablation [6,7,8].
The multi-applicator technique involves the treatment of one lesion with multiple (up to 5) laser applicators simultaneously [9].
Microwave Ablation
As with RFA, MWA allows flexible approaches to treatment, including percutaneous, laparoscopic, and open surgical access. A microwave antenna is introduced directly into the tumor. When the antenna is attached to the microwave generator with a coaxial cable, an electromagnetic microwave is emitted from the exposed, non-insulated portion of the antenna (fig. 1). Microwaves can produce coagulative necrosis with an ultra-high-speed (900-2,450 MHz) alternating electric field, causing the rotation of water molecules. Intratumoral temperatures can be measured with a separately placed thermocouple [8,10].
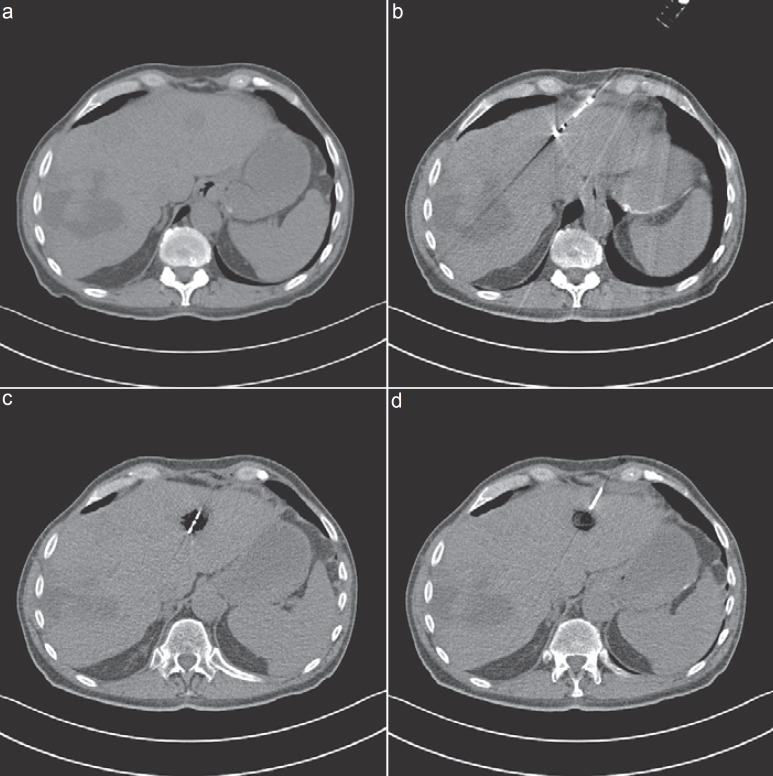
Fig. 1.
CT-guided microwave ablation in a patient with an intrahepatic local recurrence of a leiomyosarcoma. a Unenhanced CT with localization of a tumor recurrence in segment 2 with 14 mm in diameter. b Introduction of a microwave antenna (Emprint™ system; Covidien Deutschland GmbH, Neustadt/Donau, Germany). MWA with 100 W for 7 min. c Complete vaporization of the metastasis depicted as area of air-containing space. d Final result after MWA. Complete vaporization of the metastasis.
Radiofrequency Ablation
In RFA, a needle is inserted directly into the target tissue, normally the liver, usually under US or CT guidance, and one or more electrodes are deployed from the end of the needle into the tissue. Once placed within the tumor, a generator is used to deliver a rapidly alternating current. The agitation and friction of ions created by radiofrequency energy through the needle produce heat and destroy the tumor tissue. Heat is generated at the site of the lesion through frictional heat and causes the destruction of the tumor. Tiny thermometers (thermocouples) incorporated into the tips of the electrodes allow continuous monitoring of tissue temperatures. Power is automatically adjusted so that the target temperatures remain constant. As tissue temperature increases above 50 °C, cell protein is permanently damaged and coagulation necrosis starts. Above 60 °C, cell death occurs almost instantly. Approximately 15-30 min are required to perform a 3- to 5-cm ablation [11]. Multiple ablations can be overlapping in order to decrease the chance of local tumor recurrence. The size of the ablated area is largely determined by the size of the electrode needle, the temperature of the tissue, and the duration of time the energy is applied. A sharp boundary separates dead tissue and unaffected surrounding tissue [11,12].
Various radiofrequency devices are used for tissue ablation but none of them is specifically standardized for the ablation of hepatic tumors. However, these devices have recently been approved for the treatment of unresectable liver tumors [13,14]. RFA has been performed either percutaneously or with laparoscopic surgery. The procedure has been used with a cooled-tip or a non-cooled-tip electrode; however, the first system is normally preferred in cases of larger lesions as it allows a larger area of ablation due to reduced charring at the tip [8] (table 1).
Table 1.
Metastases: results of RFA
| Authors | Patients, n | Technique | Local control rate | Survival rate | Complications |
|---|---|---|---|---|---|
| Park et al. [21] | 34 | ultrasound-guided RFA | 15 months (range 3–65 months) | median 14 months | |
| Kyildiz et al. [22] | 89 | laparoscopic RFA | 30 ± 3 months | overall survival: 6 years | |
| Mazzaglia et al. [23] | 63 | laparoscopic RFA | 1.6 ± 0.3 years | 3.9 years post first RFA | |
| De Baere et al. [24] | 68 | RFA (intraoperative and percutaneous) | 4–23 months (mean 13.7 months) | 1-year survival 70% | one bilioperitoneum and two abscesses |
Imaging techniques including US, CT, MRI, and PET-CT are first of all used to help determine whether the patients are suitable candidates for these procedures. Imaging aspects including tumor size, shape, number, and location within the organ and relative to the blood vessels, as well as critical structures that might be at risk for injury during the ablative procedure are enormously important. Additionally, imaging of non-target areas helps to perform disease-specific cancer staging. The following different steps during ablative procedure are important:
– First, targeting involving the placement of an applicator into the tumor.
– Second, monitoring. This helps controlling the zone of ablation. Here, US, CT, or MRI can be used depending on the underlying technology.
– Third, procedure modification allows adapting the treatment protocol to the current tumor situation.
– Fourth, subsequent assessment of immediate treatment response. Immediately after the treatment or 24 h later the first imaging control helps to determine whether a treatment response can be found.
Ancillary procedures are procedures which try to lower the risk of injuring non-target structures, e.g. injection of fluid (so-called ‘hydrodissection’), injection of air, creation of artificial ascites, or pneumothorax.
Treatment Effect
The treatment effect describes the gross pathological changes due to ablation. The gross pathological periods of treated tissue should be referred to as coagulation. This is associated with high temperature thermal injury. This term is preferred to the term necrosis. The zone of ablation induces a central ‘white zone’ which presents the coagulated tissue and a variable ‘red zone’ of hyperemia.
The treatment efficacy on non-colorectal liver metastases after ablation is defined (i) as the primary efficacy rate and (ii) as the percentage of target tumors successfully eradicated following initial procedure. The term ‘retreatment’ is reserved for describing ablation of locally progressive tumors. Outcome refers to the following definitions: overall survival (OS), time-to-progression (TTP), and progression-free survival (PFS). Additional parameters are symptom-free survival and cancer-specific survival.
Results
Thermal ablation in non-colorectal liver metastases has to be judged as an alternative to liver resection or mostly as an add-on. It can be stated that in liver metastases up to 5 cm, thermal ablation achieves equal local tumor control in comparison to liver resection with a lower risk of major complications due to a lower interventional morbidity.
Further indications are the failure of systemic chemotherapy or non-effective systemic chemotherapy, especially with a neighborhood to capsular structures and an adequate status.
Contraindications for all these treatments are poor performance status (Karnofsky status <75%), extensive liver involvement with more than 75%, poor hepatic synthesis (serum albumin < 2 mg/dl), high serum bilirubin level > 2.5 mg%, and current florid infections.
Neuroendocrine Carcinoma Liver Metastases
In neuroendocrine carcinoma liver metastases, different results are reported for interventional oncological treatment. Currently, the response to therapy is calculated on the basis of clinical parameters, including symptomatic response (SR), biological response (BR), morphological response (MR), PFS, and survival periods (SP). TACE has been associated with SR rates of 60-95%, a BR of 50-90%, an MR of 33-80%, an SR of 20-80 months, and a 5-year survival of 50-65%. PFS was also reported to be between 18 and 24 months [15]. In the transarterial embolization (TAE) group, SR was similar to the TACE group, MR was between 32 and 82%, and survival was between 18 and 88 months with a survival rate of 40-67% and a BR of 50-69%. RFA, either percutaneous or during surgery, has been associated with an SR of 71-95% for a mean duration of 8-10 months, a BR of 65%, and a mean SP of 1.6 years after ablation. The mean survival following surgical resection for operable cases is 4.26 ± 1.1 (SD) years [15]. Thus, the interventional protocol for the management of liver metastases from neuroendocrine tumors for oligonodular liver metastastic deposits, local resection, or RFA, MWA, and/or LITT are recommended (table 2, fig. 2) while in multinodular diseases with higher tumor load, TACE or TAE is recommended [15].
Table 2.
Liver metastases of neuroendocrine carcinoma: role of LITT
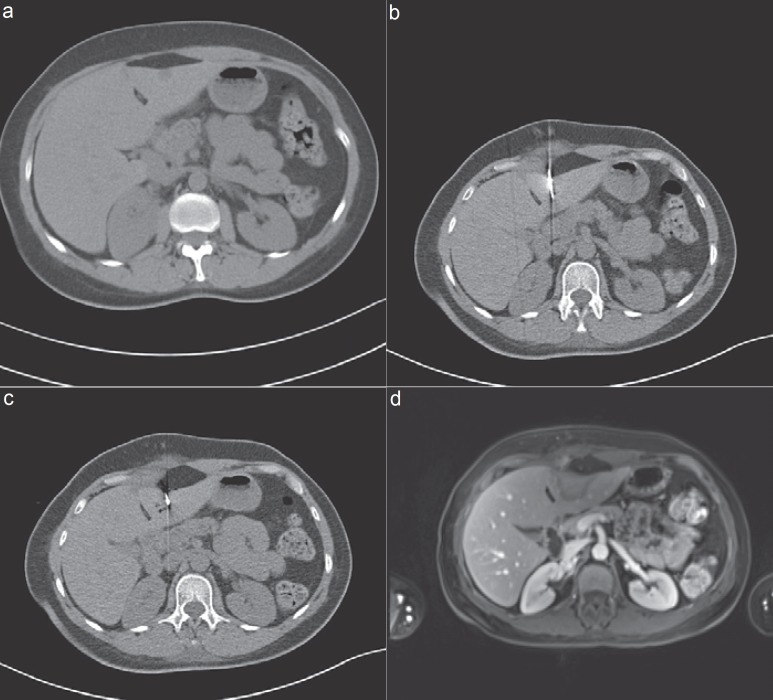
Fig. 2.
Curative thermal ablation of a patient with oligonodular liver metastases of a neuroendocrine cancer of the colon. a Documentation of an unenhanced T1w sequence. First metastasis 10 mm in size, second 5 mm large. b Insertion of the microwave antenna into the area of the liver metastasis. c Documentation of the area of tissue necrosis, carbonization, and ablation in segment 5 measuring 35 mm, thus demonstrating a 1-cm safety margin surrounding the lesion. d MRI follow-up 6 years post thermal ablation of an oligonodular liver metastasis. Complete A0 ablation. No local tumor recurrence.
Breast Cancer Liver Metastases
Due to the complex role of liver metastases in breast cancer only a limited number of patients are candidates for thermal ablation. In 2013, Vogl et al. [16] documented a positive response rate of 63-97% in RFA lesions, of 98.2% in LITT-treated lesions, and of 34.5-62.5% in MWA lesions. The median survival was documented to be 10.9-60 months when using RFA, 51-54 months after LITT, and 41.8 months when applying MWA. 5-year survival rates were 27-30, 35, and 29%, respectively. Local tumor progression amounted to 13.5-58% when using RFA, 2.9% with LITT, and 9.6% with MWA. Ablation therapies of breast cancer liver metastases either as a single therapy or combined with other local regional therapies are a good alternative as an adjunction to resection in patients with resectable lesions or with positive response using chemotherapy [16].
Additional data currently show that patients using a combination of TACE and LITT have better palliative treatment results; here, we achieved a median survival of 21.1 months [17].
By definition, manifestation of liver metastases in patients with breast cancer is regularly considered to be a regional manifestation of a systemic involvement. Regularly, patients undergo updated chemotherapies, hormone therapies, and nowadays immunotherapeutic therapy options. For liver-directed therapies, two indications for interventional oncology procedures can be considered. In cases of oligonodular, metachronous, and liver-only or liver-dominant metastases, regional interventional oncology techniques are an option. In these rare clinical situations in liver metastases with a size ≤ 5 cm and a number of ≤5, thermal ablation techniques such as LITT, RFA, or MWA are possible therapy options. These clinical conditions also mean that thermal ablation can be a successful alternative to liver resection. Among the thermal ablation techniques, data are currently available for LITT showing a median survival of 37.6 months and a PFS of 12.2 months in the treatment of 401 patients [18]. Similar data are available for the use of image-guided RFA and MWA. Interventional oncology ablation techniques thus provide similar results to liver resection (fig. 3).
Fig. 3.
Liver metastases of breast cancer. Curative thermal ablation. a Localized metastasis in segment 3. Documentation of a liver metastasis of breast cancer of 11 mm in size. b Solitary breast cancer liver metastases. Insertion of a microwave antenna (AMICA™ system; HS Medical Inc., Boca Raton, FL, USA) into the tumor. Thermal ablation for 11 min post ablation. c CT-guided documentation of vaporization in the area between the liver surface and the peritoneal cavity. d MRI follow-up post thermal ablation demonstrating two ablation necroses of 2 cm each in the left liver lobe.
Other clinical conditions are the presence of a high tumor load of breast cancer as well as clinical problems such as bile duct compression or capsular pain. Here, liver-directed vascular procedures are a possible therapy option, i.e. TACP, TACE, selective internal radiation therapy (SIRT), or nowadays chemosaturation. Unfortunately, mainly retrospective data are available in this regard.
Comparative indications for TACP and TACE allow a median survival of 21.1 months [17]. The data for the use of radioembolization are currently not conclusive.
Liver Metastases from Malignant Melanoma
In patients with malignant melanoma two etiologies for the presence of liver metastases have to be considered: one is a group of patients with cutaneous malignant melanoma. Rarely there is a liver-dominant metastasis, while a systemic distribution of cancer is mostly detected. In rare cases of solitary or oligonodular liver metastases from malignant melanoma, thermal ablation is a possible option in higher tumor load, i.e. TACE, TACP, and radioembolization. Newer data also prove that chemosaturation (Delcath) is a possible option in patients with liver-dominant metastases of malignant melanoma [19]. This will also be discussed in the following for a group of patients with ocular melanoma. In patients with ocular melanoma, there can be a synchronous or metachronous manifestation of malignant melanoma metastases in the liver. Exclusively, these tumors demonstrate either oligonodular or multinodular and diffuse dominant liver metastases. Regional therapies have been established in the form of thermal ablation in rare cases of oligonodular tumors or of transarterial therapies in cases with multinodular or diffuse liver metastases.
All data when using LITT in 18 patients with uveal melanoma liver metastases showed a mean survival rate of 3.6 years for all treated patients, with a median survival of 1.83 years and a 5-year survival of 17%. After the first LITT treatment the median survival of 2.8 years could be proven. An additional 10 patients were treated with a combination of TACE (table 3) and LITT. In summary, thermal ablation shows high local tumor control in patients with liver metastases of malignant melanoma and a respectable survival rate [20].
Table 3.
TACE with uveal melanoma
| Authors | Patients, n | Technique | Mean number of sessions | Survival |
|---|---|---|---|---|
| Schuster et al. [33] | 25 | fotemustine-based or cisplatin-based TACE | median overall survival was 6 months, 15% of patients alive at 1 year | |
| Huppert et al. [34] | 14 | 100 mg/m2 of cisplatin + embolization by polyvinyl alcohol particles | 2.4 treatments | median survival after first TACE was 11.5 months |
| Fiorentini et al. [35] | 10 | TACE + DC beads + irinotecan | 6.5 months (range 4–9 months) | 5 months |
| Vogl et al. [36] | 12 | mitomycin C, lipiodol, resorbable microspheres | 3-month intervals | mean survival following primary tumor treatment was 32.9 months |
| Patel et al. [37] | 30 | 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) dissolved in ethiodized oil; gelatine sponge | 24 | median overall survival of the entire intention-to-treatment group of patients was 5.2 months |
Liver Metastases of Different Primaries (Pancreatic Cancer, Renal Cell Carcinoma, Lung Cancer)
Thermal ablation only plays a minor role in patients suffering from pancreatic neoplasms with liver metastases. Only in oligonodular tumor infiltrations, limited hepatic infiltration, and metachronous liver metastases, thermal ablation could be an alternative. LITT, RFA, or MWA are the treatment techniques of choice. The current role of thermal ablation in improving survival is still questionable.
Vogl et al. [18] included 401 patients with liver metastases from different primary tumors who were treated with LITT. The median survival was 37.6 months from the start of LITT. The mean PFS was 12.2 months and the 5-year PFS rate was 17%. LITT in the treatment of hepatic metastases of different primaries showed good results concerning long-term survival and PFS.
Liver metastases from lung cancer are a rather rare finding, and mostly it is also a sign of complete systemic involvement. In rare cases of oligonodular liver metastases of lung cancer (non-small cell lung cancer (NSCLC)), thermal ablation is a treatment option and should be intensively discussed in a specific tumor board. There is only a limited number of studies available for the use of LITT, RFA, or MWA.
Rarely, thermal ablation techniques are used in pancreatic cancer, especially regarding the problem of hepatobiliary anastomosis. Rare indications are oligonodular liver metastases of primary cancer such as NSCLC, esophagus cancer, gastric cancer, ovarian cancer, and others.
In colorectal liver metastases the indication for performing thermal ablation is rather limited and should be restricted to oligonodular liver metastases. The number should be <5 and the size should be <5 cm. Under certain circumstances, an improved survival time can also be achieved by means of these techniques.
TACE or radioembolization are rarely used, particularly in clinical scenarios of high tumor load and compression syndromes (tables 4, 5).
Table 4.
Pancreatic tumor and metastases: role of TACP and TACE
| Authors | Patients, n | Technique | Duration of treatment | Survival rate |
|---|---|---|---|---|
| Vogl et al. [26] | 40 | gemcitabine (1,000 mg/m2), mitomycin C (8.5 mg/m2) | 12 months | median 6.4 months |
| Vogl et al. [27] | 16 | mitomycin C (8.5 mg/m2), gemcitabine (500 mg/m2) | 5 months | median 25 months |
| Akahori et al. [28] | 5 | cisplatin mixed with degradable starch microspheres | 24 sessions of TACE | median 36 months |
| Azizi et al. [29] | 32 | chemoembolization with lipiodol | 2 months | 1, 3, and 5 years: 60, 25, and 11%, respectively |
| Jarzabek et al [30] | 15 | hepasphere 50–100 μm + 100 mg doxorubicin | 3.1 months | median 5.8 months |
| De Baere et al. [31] | 20 | TACE with drug-eluting beads (500–700 μm) + doxorubicin | 15 months | 5-year survival rates of 73–85% |
| Kress et al. [32] | 26 | TACE octreotide and/or α-interferon, lipiodol | 15.5 months | 5-year survival rates of 48% |
Table 5.
Liver metastases: role of radioembolization
| Author | Patients, n | Technique | Local control rate, months | Survival rate |
|---|---|---|---|---|
| Peker et al. [38] | 30 | 90Y radioembolization | 3.0 ± 19.4 | 1- and 2-year survival rates of 71 and 45%, respectively |
| Devcic et al. [39] | 156 | (90)Y resin radioembolization | 2.78 | |
| Michl et al. [40] | 19 | (90)Y radioembolization | 3.4 | 1-year survival 24% |
| Paprottka et al. [41] | 42 | Yttrium 90 (Y-90) | 16.2 | 95.2% |
Conclusion
While in colorectal liver metastases the current role of thermal ablation in oligonodular liver metastases is established, no major significant scientific data are available for non-colorectal metastases. Studies have proven that the initial number of metastases and their volume are the most important prognostic factors. However, the status of the lymph nodes, the existence of other extrahepatic metastases, the location of the primary tumor, and different neoadjuvant therapies are of non-prognostic value [18]. Meanwhile, LITT, RFA, and MWA are established as ablation techniques. After intensive discussion in an interdisciplinary oncological tumor board these treatment methods can be effectively used.
Disclosure Statement
None of the authors has anything to disclose.
References
- 1.Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–175. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165:424–437. doi: 10.1016/j.chemphyslip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spalding AC, Lawrence TS. New and emerging radiosensitizers and radioprotectors. Cancer Invest. 2006;24:444–456. doi: 10.1080/07357900600705706. [DOI] [PubMed] [Google Scholar]
- 4.Grieco CA, Simon CJ, Mayo-Smith WW, Di Petrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17:1117–1124. doi: 10.1097/01.RVI.0000228373.58498.6E. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd GD, 3rd, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 7.Cline HE, Hynynen K, Watkins RD. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 8.Vogl TJ, Mack MG, Balzer JO, et al. Liver metastases: neoadjuvant downsizing with transhepatic arterial chemoembolization before laser-induced thermotherapy. Radiology. 2003;229:457–464. doi: 10.1148/radiol.2292021329. [DOI] [PubMed] [Google Scholar]
- 9.Vogl TJ, Straub R, Eichler K, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions) Radiology. 2002;225:367–377. doi: 10.1148/radiol.2252011171. [DOI] [PubMed] [Google Scholar]
- 10.Hibata T, Limuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 11.Bilal Shafi BB, Bilal RH, Evans JC, et al. Percutaneous radiofrequency ablation of liver tumors. http://emedicine.medscape.com/article/1390475 (accessed Jan 7, 2014).
- 12.McGahan JP, Dodd GD., 3rd Radiofrequency ablation of the liver. AJR Am J Roentgenol. 2001;176:13–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2:399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion. Acad Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 15.Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72:517–528. doi: 10.1016/j.ejrad.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Vogl TJ, Farshid P, Naguib NN, Zangos S. Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol. 2013;23:797–804. doi: 10.1007/s00330-012-2662-4. [DOI] [PubMed] [Google Scholar]
- 17.Vogl TJ, Kreutzträger M, Gruber-Rouh T, Eichler K, Nour-Eldin NE, Zangos S, Naguib NN. Neoadjuvant TACE before laser-induced thermotherapy (LITT) in the treatment of non-colorectal non-breast cancer liver metastases: feasibility and survival rates. Eur J Radiol. 2014;83:1804–1810. doi: 10.1016/j.ejrad.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Vogl TJ, Freier V, Nour-Eldin NE, Eichler K, Zangos S, Naguib NN. Magnetic resonance-guided laser-induced interstitial thermotherapy of breast cancer liver metastases and other non-colorectal cancer liver metastases: an analysis of prognostic factors for long-term survival and progression-free survival. Invest Radiol. 2013;48:406–412. doi: 10.1097/RLI.0b013e31828328d7. [DOI] [PubMed] [Google Scholar]
- 19.Vogl TJ, Zangos S, Scholtz JE, Schmitt F, Paetzold S, Trojan J, Orsi F, Lotz G, Ferrucci P. Chemosaturation with percutaneous hepatic perfusions of melphalan for hepatic metastases: experience from two European centers. Rofo. 2014;186:937–944. doi: 10.1055/s-0034-1366081. [DOI] [PubMed] [Google Scholar]
- 20.Eichler K, Zangos S, Gruber-Rouh T, Vogl TJ, Mack MG. MR-guided laser-induced thermotherapy (LITT) in patients with liver metastases of uveal melanoma. J Eur Acad Dermatol Venerol. 2014;28:1756–1760. doi: 10.1111/jdv.12405. [DOI] [PubMed] [Google Scholar]
- 21.Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, Kim PN, Han DJ. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012;23:635–641. doi: 10.1016/j.jvir.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 22.Kyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288–1293. doi: 10.1016/j.surg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 24.De Baere T, Elias D, Dromain C, Din MG, Kuoch V, Ducreux M, Boige V, Lassau N, Marteau V, Lasser P, Roche A. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 25.Perälä J, Klemola R, Kallio R, Li C, Vihriälä I, Salmela PI, Tervonen O, Sequeiros RB. MRI-guided laser ablation of neuroendocrine tumor hepatic metastases. Acta Radiol Short Rep. 2014;3:2047981613499753. doi: 10.1177/2047981613499753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogl TJ, Zangos S, Heller M, Hammerstingl R, Böcher E, Jacob U, Bauer RW. Transarterial chemoperfusion with gemcitabine and mitomycin C in pancreatic carcinoma: results in locally recurrent tumors and advanced tumor stages (Article in German) Rofo. 2007;179:1181–1188. doi: 10.1055/s-2007-963568. [DOI] [PubMed] [Google Scholar]
- 27.Vogl TJ, Heller M, Zangos S, Schwarz W, Eichler K, Mack MG, Berger D, Balzer JO. Transarterial chemoperfusion of inoperable pancreas carcinoma and local recurrence (Article in German) Rofo. 2003;175:695–704. doi: 10.1055/s-2003-39212. [DOI] [PubMed] [Google Scholar]
- 28.Akahori T, Sho M, Tanaka T, Nishiofuku H, Kinoshita S, Nagai M, Kichikawas K, Nakajima Y. Significant efficacy of new transcatheter arterial chemoembolization technique for hepatic metastases of pancreatic neuroendocrine tumors. Anticancer Res. 2013;33:3355–3358. [PubMed] [Google Scholar]
- 29.Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival rate. Pancreas. 2011;40:1271–1275. doi: 10.1097/MPA.0b013e318220e5b9. [DOI] [PubMed] [Google Scholar]
- 30.Jarzabek M, Jargietto T, Wolski A, Poluha P, Szczerbo-Trojanowska M. Drug-eluting microspheres transarterial chemoembolization (DEM TACE) in patients with liver metastases. Pilot study. Pol J Radiol. 2001;76:26–32. [PMC free article] [PubMed] [Google Scholar]
- 31.De Baere T, Deschamps F, Teritheau C, Rao P, Conengrapht K, Schlumberger M, Leboulleux S, Baudin E, Hechelhammer L. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol. 2008;19:855–861. doi: 10.1016/j.jvir.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors – a retrospective single-center analysis. Digestion. 2003;68:94–101. doi: 10.1159/000074522. [DOI] [PubMed] [Google Scholar]
- 33.Schuster R, Lindner M, Wacker F, Krössin M, Bechrakis N, Foerster MH, Thiel E, Keilholz U, Schmittel A. Transarterial chemoembolization of liver metastases from uveal melanoma after failure of systemic therapy: toxicity and outcome. Melanoma Res. 2010;20:191–196. doi: 10.1097/CMR.0b013e328334c36e. [DOI] [PubMed] [Google Scholar]
- 34.Huppert PE, Fierlbeck G, Pereira P, Schanz S, Duda SH, Wietholtz H, Rozeik C, Claussen CD. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010;74:e38–44. doi: 10.1016/j.ejrad.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentini G, Aliberti C, Del Conte A, Tilli M, Rossi S, Ballardini P, Turrisi G, Benea G. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009;23:131–137. [PubMed] [Google Scholar]
- 36.Vogl TJ, Eichler K, Zangos S, Herzog C, Hammerstingl R, Balzer J, Gholami A. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol. 2007;133:177–184. doi: 10.1007/s00432-006-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K, Sullivan K, Berd D, Mastrangelo MJ, Shields CL, Shields JA, Sato T. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005;15:297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Peker A, Cicek O, Soydal Ç, Küçük NÖ, Bilgiç S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol. 2015;21:54–59. doi: 10.5152/dir.2014.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devcic Z, Rosenberg J, Braat AJ, Techasith T, Banerjee A, Sze DY, Lam MG. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. Nucl Med. 2014;55:1404–1410. doi: 10.2967/jnumed.113.135855. [DOI] [PubMed] [Google Scholar]
- 40.Michl M, Haug AR, Jakobs TF, Paprottka P, Hoffmann RT, Bartenstein P, Boeck S, Haas M, Laubender RP, Heinemann V. Radioembolization with Yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology. 2014;86:24–32. doi: 10.1159/000355821. [DOI] [PubMed] [Google Scholar]
- 41.Paprottka PM, Hoffmann RT, Haug A, Sommer WH, Raessler F, Trumm CG, Schmidt GP, Ashoori N, Reiser MF, Jakobs TF. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol. 2011;35:334–342. doi: 10.1007/s00270-011-0248-1. [DOI] [PubMed] [Google Scholar]