Abstract
Objective
To determine the impact of obesity on complications in laparoscopic simple or radical nephrectomy.
Patients and Methods
The medical files of 215 patients who underwent laparoscopic simple or radical nephrectomy in our center between 2004 and 2014 were reviewed. A body mass index of 30 kg/m² was used to divide the patients into obese and non-obese groups. Pre-operative data and intra- and post-operative complications were compared between the 2 groups.
Results
There were respectively 163 and 52 patients in the non-obese and obese groups, which were comparable in terms of age, sex, and history of surgery. In the obese group, operative specimens were significantly heavier (772 vs. 534 g in the non-obese group; p = 0.005) and durations of surgery was significantly longer (244 vs. 216 minutes; p = 0.003). However no significant differences were found between the 2 groups for duration of hospitalization, surgical conversion, estimated blood loss, or intra- or post-operative complications.
Conclusion
Laparoscopic simple or radical nephrectomy is technically feasible in obese patients but the surgery may take more time, notably due to dissection difficulties. Our results showed that the risk of intra- and post-operative complications is not higher in obese patients compared to non-obese patients, except for a possible, but statistically undemonstrated, higher risk of abdominal wall complications, and that the laparoscopic approach should be the preferred technique in patients with high body mass index.
Key Words: Obesity, Complications, Nephrectomy
Introduction
Obesity is a growing, major public health problem in industrialized countries. A 2012 epidemiological survey revealed that in France, 32.3% of adults were overweight [body mass index (BMI) ≥ 25 and < 30 kg/m2] and 15% obese (BMI ≥ 30 kg/m2) [1]. Obese patients run a greater risk of comorbidities [2,3] such as diabetes, high blood pressure, atheroma, dyslipidemia, and breathing disorders. This association of risk factors is directly correlated with an increase in intra- and post-operative complications [4,5].
Additionally, different studies have shown that obese patients have a greater risk of developing certain cancers, among which is renal cell carcinoma [6,7]. Therefore, the number of obese patients needing kidney cancer surgery will inevitably grow.
The first case of laparoscopic nephrectomy was reported by Clayman et al. [8] in 1991 and the technique rapidly developed thereafter throughout the 1990s. Compared to open nephrectomy, the laparoscopic technique reduces pain and shortens post-operative recuperation with equivalent oncological results [9,10]. In its early days, the laparoscopic approach was contraindicated in obese patients where the technique was more difficult to perform and caused more complications [11]. However, with its evolution, more recent studies have demonstrated that although laparoscopic nephrectomy remains more difficult in obese patients, it is nonetheless safe [12,13,14,15] and provides clear benefits for them over open surgery [16,17].
Currently there is a focus on optimizing the surgical management of kidney cancer when simple or radical nephrectomy is indicated through the development of increasingly mini-invasive techniques, such as laparoscopic single-site surgery or vaginal extraction of the operative specimen, in order to limit abdominal wall complications [18,19,20].
The purpose of the present study was to assess the impact of obesity on intra- and post-operative complications in patients treated by laparoscopic simple or radical nephrectomy.
Patients and Methods
A list of all patients who underwent kidney surgery at the Lyon Sud Hospital Center (Lyon, France) between January 2004 and January 2014 was obtained from the center's anatomic pathology department. Of the 235 patients thus identified, 215 had undergone laparoscopic simple or radical nephrectomy and their patient files were retrospectively analyzed. All partial nephrectomies, open nephrectomies, and nephroureterectomies were excluded as were all files that did not provide the BMI, intra- and post-operative data, and anatomopathological data.
All laparoscopic nephrectomies were performed via an intraperitoneal approach with 3 to 4 trocars as needed. All basic surgical principles were respected [21]. For overweight or obese patients, the trocars were positioned slightly more exteriorly in comparison to non-obese patients (fig. 1). The optical trocar was placed closer to the costal edge than to the umbilicus. The incision for operative specimen extraction was chosen as a function of the anatomy of the patient and the size of the kidney: choices included iliac, subcostal, suprapubic (Pfannenstiel), or vaginal extraction routes.
Fig. 1.
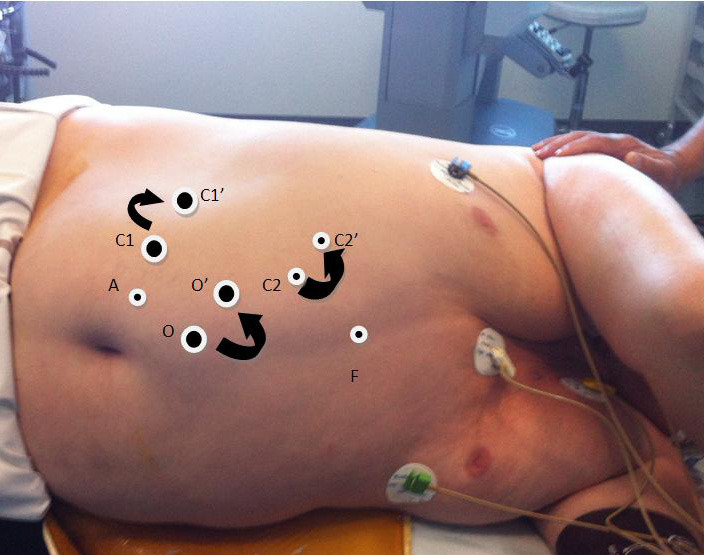
Changes in port placement in a patient with a BMI of 39 kg/m2 for right nephrectomy. O: optical trocar, C1: operator trocar for the right hand, A: trocar for the assistant, F: trocar for the liver. C1′, C2′ and O' are the modified locations for an obese patient.
BMI was calculated in the standard fashion as the weight of the patient in kilograms divided by the square of his/her height in meters. In accordance with current World Health Organization indications, patients with a BMI ≥ 30 kg/m2 were considered obese and thus placed in the obese group whereas those under this threshold were placed in the non-obese group.
Aspects compared between the 2 groups included sex, age, American Society of Anesthesiologists (ASA) score, comorbidities, indication for nephrectomy, side of nephrectomy, duration of surgery, extraction incision site, intra-operative blood loss, estimated hemoglobin loss, weight and nature of the operative specimen, mean length of hospital stay (MHS), intra- and post-operative complications, and duration of post-operative follow-up.
Early post-operative complications were defined as those occurring within post-operative days 0 to 30 and were grouped according to the Clavien-Dindo classification system. Late postoperative complications were defined as those occurring after post-operative day 30 and corresponded to hernia at the extraction incision.
Assessed comorbidities included type 2 or type 1 diabetes, arterial hypertension, history of abdominal surgery, arteriopathies (e.g., coronary heart disease, stroke), breathing disorders (i.e., chronic obstructive pulmonary disease, sleep apnea syndrome, current smoking, use of anticoagulants or antiplatelet drugs, chronic renal failure or dialysis, and immunodepression (including HIV, use of immunosuppressants for transplants, or corticosteroids).
Qualitative variables were analyzed using the chi-squared test and quantitative variables using the Student's t test. Associations between the occurrence of complications and the different patient parameters were evaluated by multi-parameter logistic regression. Statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS Statistics 17 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Patient Characteristics
There were 163 patients in the non-obese group (BMI < 30 kg/m2) and 52 in the obese group (BMI ≥ 30 kg/m2). Patient characteristics are summarized in the first half of table 1. There were no statistically significant differences except for the ASA score (p < 0.006).
Table 1.
Comparison of the characteristics and preoperative data of non-obese and obese patients
| Non-obese patients BMI < 30 (kg/m2) | Obese patients BMI ≥ 30 (kg/m2) | p | |
|---|---|---|---|
| Number of patients | 163 | 52 | |
| BMI, kg/m | 23.6 ± 3.2 | 35.4 ± 5.8 | < 0.001 |
| Age, years | 58.6 ±16.3 | 60.4 ± 12.1 | 0.378 |
| Ratio male/female, n | 81/82 | 25/27 | 0.839 |
| Ratio left/right | 84/79 | 25/27 | 0.664 |
| ASA score | 2.0 ± 0.8 | 2.3 ± 0.6 | 0.006 |
| Serum creatinine mg/dL | 0.99 ± 0.24 | 0.97 ± 0.24 | 0.606 |
| MDRD GFR mL/min/1.73m | 76.2 ± 16.6 | 76.4 ± 16.2 | 0.963 |
| Indication: | 0.142 | ||
| Tumor, n (%) | 118 (73%) | 44 (84%) | |
| Non-functional kidney, n (%) | 25 (15%) | 6 (12%) | |
| PKD, n (%) | 20 (12%) | 2 (4%) | |
| Comorbidities, n (%) | 133 (83%) | 48 (92%) | 0.089 |
| Type 2/Type 1 diabetes, n (%) | 9 (5.5%) | 13 (25%) | < 0.001 |
| HA, n (%) | 80 (49%) | 34 (65%) | 0.049 |
| History of abdominal surgery, n (%) | 99 (61%) | 31 (60%) | 0.809 |
| Arteriopathy, n (%) | 17 (10%) | 13 (25%) | 0.009 |
| COPD/SAS, n (%) | 15 (9%) | 13 (25%) | 0.004 |
| Anticoagulant/Antiplatelet use, n (%) | 24 (15%) | 14 (27%) | 0.049 |
| Smoking, n (%) | 28 (17%) | 8 (15%) | 0.737 |
| Chronic renal failure/Dialysis, n (%) | 37 (23%) | 5 (10%) | 0.035 |
| Immunodepression: transplant, corticosteroids, HIV, n (%) | 11 (7%) | 4 (8%) | 0.833 |
Comorbidities and pre-operative data are summarized in the second half of table 1. In the non-obese and obese groups respectively, 133 (83%) and 48 (92%) of patients had at least one comorbidity. When considered in total, there was no statistically significant difference for comorbidities (p = 0.09). When considered individually however, the obese group had statistically significant higher rates of diabetes (p < 0.001), arterial hypertension (p = 0.049), arteriopathy (p = 0.009), chronic breathing disorders (p = 0.004), and anticoagulants or antiplatelet drug use (p = 0.049), and the non-obese group a significantly higher rate of chronic renal failure or dialysis (p = 0.035).
Operative and Post-Operative Follow-Up Data
Operative data are summarized in table 2. There were 8 (4.9%) cases of conversion to open surgery in the non-obese group and 2 (3.8%) in the obese group. Reasons for conversion were, in the non-obese group: difficulties with dissection due to adhesions associated with previous surgery, polycystic kidney, or recurrent pyelonephritis in the operated kidney (n = 5), toxic megacolon (n = 1), uncontrolled venous bleeding (n = 1) and an uncontrolled splenic lesion necessitating splenectomy (n = 1); and in the obese group: arterial bleeding from a renal polar artery lesion (n = 2). There were 3 intraoperative complications that did not require conversion, i.e., 2 splenic lesions and 1 pancreatic lesion, all in the non-obese group.
Table 2.
Comparison of operative data of non-obese and obese patients
| Non-obese patients BMI < 30 (kg/m2) | Obese patients BMI > 30 (kg/m2) | p | |
|---|---|---|---|
| Duration of surgery, min | 216 ± 62 | 244 ± 54 | 0.003 |
| Conversion to open surgery n, (%) | 8 (4.9%) | 2 (3.8%) | 0.752 |
| Extraction incision site, n (%) | 0.873 | ||
| Iliac fossa | 123 (75%) | 41 (79%) | |
| Subcostal | 24 (15%) | 7 (13%) | |
| Pfannenstiel | 7 (4%) | 1 (2%) | |
| Vaginal | 9 (6%) | 3 (6%) | |
| Blood loss, mL | 101 ± 171 | 105 ± 111 | 0.896 |
| Hemoglobin loss, g/dL | 1.4 ± 1.1 | 1.2 ± 0.7 | 0.463 |
| Intraoperative transfusion, n (%) | 11 (6.7%) | 0 | 0.054 |
| Intraoperative complication, n (%) | 3 (1.8%) | 0 | 0.478 |
| 2 splenic lesions | |||
| 1 pancreatic lesion | |||
| Weight of the operative specimen, g | 534 ± 465 | 772 ± 524 | 0.005 |
| Anatomopathologically-confirmed cancer for renal tumor, n (%) | 98 (86%) | 44 (100%) | 0.012 |
The duration of surgery and the weight of the operative specimen were significantly higher in the obese group, respectively p = 0.003 and p= 0.005.
Data for postoperative complications are summarized in table 3. MHS was 5.6 ± 3.4 days (0–28 days) in the non-obese group versus 6.0 ± 3.0 days (2–18 days) in the obese group (p = 0.437). There were 47 (29%) intra or post-operative complications in the non-obese group versus 16 (31%) in the obese group (p = 0.790). No statistically significant differences were found between the 2 groups concerning late post-operative complications (incisional hernia).
Table 3.
Comparison of postoperative complications and follow-up data of non-obese and obese patients
| Non-obese patients BMI < 30 (kg/m2) | Obese patients BMI ≥ 30 (kg/m2) | p | |
|---|---|---|---|
| Mean length of hospital stay, days | 5.6 ± 3.4 | 6.0 ± 3.0 | 0.437 |
| Serum creatinine mg/dL | 1.33 ± 0.33 | 1.32 ± 0.43 | 0.870 |
| MDRD GFR mL/min/1.73m | 55.2 ± 13.7 | 55.5 ± 15.7 | 0.908 |
| GFR loss mL/min/1.73m | 21.0 ± 11.8 | 20.8 ± 9.0 | 0.941 |
| Number of intra or postoperative complications, n (%) | 47 (29%) | 16 (31%) | 0.790 |
| Number of patients with early postoperative complications, n (%) | 35 (21%) | 10 (19%) | 0.739 |
| Clavien-Dindo I and II | total: 30 | total: 8 | |
| transfusion: 7 | parietal abscess: 3 | ||
| parietal hematoma: 6 | parietal hematoma: 1 | ||
| vaginal incision bleeding: 1 | ileus: 1 | ||
| pneumopathy: 5 | pneumopathy: 1 | ||
| ileus: 4 | prostatitis: 1 | ||
| parietal abscess: 3 | fever: 1 | ||
| acute pancreatitis: 2 | |||
| confusion: 1 | 0.860 | ||
| cystitis: 1 | |||
| Clavien-Dindo III to V | total: 5 | total: 2 | |
| splenectomy: 1 | parietal cellulitis 1 | ||
| evisceration + splenectomy: 1 | symptomatic pleural effusion: 1 | ||
| hematoma of the perirenal space: 1 | |||
| pancreatic fistula: 1 | |||
| septic shock/death: 1 | |||
| Duration of postoperative follow-up, months | 23 ± 23 | 20 ± 23 | 0.459 |
| Number of patients with late postoperative complications, n (%) | 9 incisional hernias (5.8%) including 5 patients with no early complications | 5 incisional hernias (10%) including 4 patients with no early complications | 0.297 |
Risk Factor Analysis
Table 4 summarizes risk factors for complications. After univariate and multivariate analyses, significant risk factors for complications included: age from 50 to 69 years or ≥ 70 years [p = 0.005, respective odds ratios (OR) of 3.0 (95% confidence interval (CI): 1.2–7.1) and 3.7 (95% CI: 1.5–9.2)], ASA scores 2 and 3–4 [p ≤ 0.001, respective OR of 3.0 (95% CI: 1.1–7.9) and 5.5 (95% CI: 2.0–5.3)], and weight of the operative specimen ≥ 450 g [p = 0.004; OR of 2.4 (95% CI: 1.3–4.5)]. The comorbidities found to be significant risk factors for complications were: arterial hypertension (p = 0.04, OR of 1. 9 (95% CI: 1.0–3. 5), history of abdominal surgery (p = 0.03, OR of 2.1 (95% CI: 1.1–3.9), and chronic obstructive pulmonary disease / sleep apnea syndrome (p = 0.03, OR of 2.4 (95% CI: 1.1–5.4). The analysis of the obesity risk factor adjusted for the other risk factors of postoperative complication found OR values statistically similar to the reference value, i.e., an OR of 1.1 (95% CI: 0.6–2.2) (p = 0.79).
Table 4.
Analysis of complication risk factors and adjusted obesity
| Factor | Factor analysis without adjustment |
Obesity adjusted for each factor |
|||
|---|---|---|---|---|---|
| Complications n/N (%) | OR (95% CI) | p | OR (95% CI) | p | |
| Sex | 1.4 (0.8–2.6) | 0.38 | 1.10 (0.6–2.2) | 0.78 | |
| Female | 28/109 (26%) | ||||
| Male | 35/106 (33%) | ||||
| Age | 0.005 | 1.0 (0.5–2.0) | 0.98 | ||
| < 50 years | 8/57 (14%) | Ref | |||
| 50–69 years | 32/97 (33%) | 3.0 (1.2–7.1) | |||
| ≥ 70 years | 23/61 (38%) | 3.7 (1.5–9.2) | |||
| Obesity | 0.79 | ≠ | |||
| BMI < 30 kg/m | 47/163 | 1.1 (0.6–2.2) | |||
| BMI ≥ 30 kg/m | 16/52 | ||||
| ASA score | <0.001 | 0.99 (0.5–2.1) | 0.98 | ||
| 1 | 6/48 (13%) | Ref | |||
| 2 | 27/90 (30%) | 3.0 (1.1–7.9) | |||
| 3–4 | 23/52 (44%) | 5.5 (2.0–5.3) | |||
| Kidney weight | 2.4 (1.3–4.5) | 0.004 | 0.8 (0.4–1.7) | 0.64 | |
| < 450 g | 21/101 (21%) | ||||
| > 450 g | 42/108 (39%) | ||||
| Extraction | 0.21 | 1.1 (0.6–2.2) | 0.76 | ||
| Iliac fossa | 45/164 (27%) | 1.4 (06–3.1) | |||
| Subcostal | 6/22 (27%) | 1.1 (0.3–2.9) | |||
| Pfannenstiel | 2/8 (25%) | 0.9 (0.2–4.8) | |||
| Vaginal | 1/12 (8%) | 0.24 (0–1.9) | |||
| Duration of surgery | 1.8 (1.0–3.5) | 0.06 | 0.94 (0.4–1.9) | 0.86 | |
| < 200 min | 18/83 (22%) | ||||
| > 200 min | 42/125 (34%) | ||||
| Type 2/1 diabetes | 1.5 (0.6–3.6) | 0.43 | 1.0 (0.51–2.1) | 0.93 | |
| Absent | 54/191 (28%) | ||||
| Present | 8/22 (36%) | ||||
| HA: | 1.9 (1.0–3.5) | 0.04 | 1.01 (0.5–2.0) | 0.98 | |
| Absent | 22/99 (22%) | ||||
| Present | 40/114 (35%) | ||||
| History of ab. surgery | 2.1 (1.1–3.9) | 0.03 | 1.1 (0.6–2.2) | 0.73 | |
| Absent | 17/83 (20%) | ||||
| Present | 45/130 (35%) | ||||
| Arteriopathy | 2.1 (0.9–4.6) | 0.06 | 0.99 (0.5–2.0) | 0.98 | |
| Absent | 49/183 (27%) | ||||
| Present | 13/30 (43%) | ||||
| COPD/SAS | 2.4 (1.1–5.4) | 0.03 | 0.96 (0.5–1.9) | 0.90 | |
| Absent | 49/185 (26%) | ||||
| Present | 13/28 (46%) | ||||
| Anticoagulant/antipiatelet | 1.3 (0.6–2.8) | 0.44 | 1.14 (0.5–2.1) | 0.84 | |
| Absent | 49/175 (28%) | ||||
| Present | 13/38 (34%) | ||||
| Smoking | 0.7 (0.3–1.5) | 0.32 | 1.12 (0.6–2.2) | 0.78 | |
| Absent | 54/177 (31%) | ||||
| Present | 8/36 (22%) | ||||
| Chronic renal failure/dialysis | 1.5 (0.7–3.0) | 0.39 | 1.18 (0.6–2.3) | 0.32 | |
| Absent | 47/171 (27%) | ||||
| Present | 15/42 (36%) | ||||
| Immunodepression | 0.6 (0.2–2.1) | 0.42 | 1.12 (0.6–2.2) | 0.75 | |
| Absent | 59/198 (30%) | ||||
| 3/15 (20%) | |||||
Discussion
The prevalence of obesity is constantly increasing in industrialized countries. In France obesity increased from 8.5% in 1997 to 15% in 2012 in people aged greater than 18 years [1]. In our series, 25% of simple/radical laparoscopic nephrectomies involved obese patients.
Obesity is directly correlated with an increased risk of cardiovascular disease, high blood pressure, type 2 diabetes, and restrictive lung disease [2,3]. Numerous studies have confirmed a relationship between obesity and altered immune response via the production of pro-inflammatory cytokines by the visceral fat cells, thus provoking an infiltration of macrophages in the adipose tissue. The resulting chronic inflammation of the tissue plays a predominant role in the metabolic syndrome, associated with lipid disorders, insulin resistance, and cardiovascular diseases [22,23]. These observations oblige a cautious approach to the surgical management of obese patients.
Several teams have compared laparoscopic nephrectomy in obese and non-obese patients. Anast et al. [13] found that obese patients had not only longer durations of surgery but also greater blood loss and higher rates of transfusion than non-obese patients. Gong et al. [14] however, in their study on 239 patients who underwent radical or partial nephrectomy, did not find significant differences for blood loss between obese and non-obese patients, or significant differences for length of stay, surgical conversion, or post-operative complications. Heimbach et al. [24] compared living donor laparoscopic nephrectomy in 172 obese and 381 non-obese patients. They found that obesity was associated with increased duration of surgery and abdominal wall complication rates, but was not a risk factor for major complications. Kurzer et al. [25] showed that BMI was a risk factor for intra-operative complications in renal laparoscopic surgery in their study involving 134 patients. Hagiwara et al. [26] suggested that the area of visceral fat measured by CT scan, which is increased in metabolic syndrome, should be taken into consideration, finding that its ability to predict technical difficulties during radical laparoscopic nephrectomy was better than that of BMI. In our study, obesity did not appear to be a significant risk factor for intra and post-operative complications. Even when obesity was adjusted for the other risk factors considered in this study, our obese patients did not appear to be at greater risk of complications.
However, we did find that laparoscopy in obese patients presented technical difficulties provoking longer durations for surgery and a need to reposition trocars according to the patient's anatomy. Also, operative specimens were larger in the obese patients, necessitating a larger extraction incision and thus potentially increasing the risk of parietal complications. Indeed, 10% of our obese patients presented with late incisional hernia, compared to 5.8% of our non-obese patients, although this difference was not statistically significant in our study. In Bird et al's [20] study on 175 patients, the risk of incisional hernia at paramedian extraction sites was increased in patients with high BMI. In a prospective study on general surgeries ranging from hernia repair to pancreaticoduodenectomy and involving 6,336 patients, of whom 1,616 were obese, Dindo et al. [27] found that obesity was not a risk factor for post-operative complications, with the exception of parietal infections in open surgery, which prompts a preference for a mini-invasive approach in obese patients.
Thus, to limit abdominal wall complications, techniques such as single-port laparoscopy and vaginal extraction of the operative specimen have been developed [19,28]. In our series, 12 patients, including three who were obese, benefited from vaginal extraction. The results show promise, with a MHS of 2.2 days (0–4 days, including one ambulatory surgery patient). The only complication among the vaginal extraction patients was 1 case of limited bleeding from the vaginal incision. In a study involving 1,076 patients, Kaouk et al. [18] demonstrated the feasibility of single-port surgery, which permitted a reduced skin incision length.
The main limitation of our study is its retrospective nature. We do note, however, that all of our medical files are computerized and include a prospective collection of information. This permits a systematic approach to the exploitation of data. Finally, another aspect of our study that should be considered is that some of the cases of renal tumor in our series date to 10 years ago. Had these cases been analyzed today, some would have been assigned to partial nephrectomy.
Conclusion
Obese patients undergoing laparoscopic simple or radical nephrectomy do not encounter an increased number of intra- or post-operative complications. However, our results may suggest that obese patients run a greater risk of parietal complications, although we could not demonstrate this with statistical significance in our study. The development of single-port laparoscopy and vaginal extraction of the operative specimen show interest for and should be explored in obese patients to reduce the importance of skin incisions.
Conflict of Interest
Supported by a grant from the Bibliothèque scientifique de l'Internat de Lyon and les Hospices Civils de Lyon.
References
- 1.Enquête OBEPI 2012. http://www.roche.fr/home/recherche/domaines_therapeutiques/cardio_metabolisme/enquete_nationale_obepi_2012.html.
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors,2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99:462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua X, Ying-Ying C, Zu-Jun F, Gang X, Zu-Quan X, Qiang D, Hao-Wen J. Obesity, hypertension and diabetes mellitus affect complication rate of different nephrectomy techniques. Actas Urol Esp. 2014;38:640–646. doi: 10.1016/j.acuro.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Cantürk Z, Cantürk NZ, Cetinarslan B, Utkan NZ, Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11:769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 6.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Meretyk S, Darcy MD, Roemer FD, Pingleton ED, Thomson PG, Long SR. Laparoscopic nephrectomy: initial case report. J Urol. 1991;146:278–282. doi: 10.1016/s0022-5347(17)37770-4. [DOI] [PubMed] [Google Scholar]
- 9.Dunn MD, Portis AJ, Shalhav AL, Elbahnasy AM, Heidorn C, McDougall EM, Clayman RV. Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol. 2000;164:1153–1159. [PubMed] [Google Scholar]
- 10.Shuford MD, McDougall EM, Chang SS, LaFleur BJ, Smith JA, Jr, Cookson MS. Complications of contemporary radical nephrectomy: comparison of open vs. laparoscopic approach. Urol Oncol. 2004;22:121–126. doi: 10.1016/S1078-1439(03)00137-6. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza D, Newman RC, Albala D, Cohen MS, Tewari A, Lingeman J, Wong M, Kavoussi L, Adams J, Moore R, Winfield H, Glascock JM, Das S, Munch L, Grasso M, Dickinson M, Clayman R, Nakada S, McDougall EM, Wolf IS, Hulbert J, Leveillee RJ, Houshair A, Carson C. Laparoscopic complications in markedly obese urologic patients (a multi-institutional review) Urology. 1996;48:562–567. doi: 10.1016/S0090-4295(96)00231-2. [DOI] [PubMed] [Google Scholar]
- 12.Fugita OE, Chan DY, Roberts WW, Kavoussi LR, Jarrett TW. Laparoscopic radical nephrectomy in obese patients: outcomes and technical considerations. Urology. 2004;63:247–252. doi: 10.1016/j.urology.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 13.Anast JW, Stoller ML, Meng MV, Master VA, Mitchell JA, Bassett WW, Kane CJ. Differences in complications and outcomes for obese patients undergoing laparoscopic radical, partial or simple nephrectomy. J Urol. 2004;172:2287–2291. doi: 10.1097/01.ju.0000143820.56649.a4. [DOI] [PubMed] [Google Scholar]
- 14.Gong EM, Orvieto MA, Lyon MB, Lucioni A, Gerber GS, Shalhav AL. Analysis of impact of body mass index on outcomes of laparoscopic renal surgery. Urology. 2007;69:38–43. doi: 10.1016/j.urology.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Feder MT, Patel MB, Melman A, Ghavamian R, Hoenig DM. Comparison of open and laparoscopic nephrectomy in obese and non obese patients: outcomes stratified by body mass index. J Urol. 2008;180:79–83. doi: 10.1016/j.juro.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Klingler HC, Remzi M, Janetschek G, Marberger M. Benefits of laparoscopic renal surgery are more pronounced in patients with a high body mass index. Eur Urol. 2003;43:522–527. doi: 10.1016/s0302-2838(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 17.Romero FR, Rais-Bahrami S, Muntener M, Brito FA, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy in obese and non-obese patients: comparison with open surgery. Urology. 2008;71:806–809. doi: 10.1016/j.urology.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 18.Kaouk JH, Autorino R, Kim FJ, Han DH, Lee SW, Yinghao S, Cadeddu JA, Derweesh IH, Richstone L, Cindolo L, Branco A, Greco F, Allaf M, Sotelo R, Liatsikos E, Stolzenburg JU, Rane A, White WM, Han WK, Haber GP, White MA, Molina WR, Jeong BC, Lee JY, Linhui W, Best S, Stroup SP, Rais-Bahrami S, Schips L, Fornara P, Pierorazio P, Giedelman C, Lee JW, Stein RJ, Rha KH. Laparoendoscopic single-site surgery in urology: worldwide multi-institution alanalysis of 1076 cases. Eur Urol. 2011;60:998–1005. doi: 10.1016/j.eururo.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Adam E, Golfier F, Lunel Potencier A, Ruffion A, Paparel P. Laparoscopic nephrectomy with vaginal extraction in obese or overweight patients: the end of wound complications? Prog Urol. 2013;23:444–449. doi: 10.1016/j.purol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Bird VG, Au JK, Sandman Y, De Los Santos R, Ayyathurai R, Shields JM. Comparison of different extraction sites used during laparoscopic radical nephrectomy. J Urol. 2009;181:1565–1570. doi: 10.1016/j.juro.2008.11.113. [DOI] [PubMed] [Google Scholar]
- 21.Mucksavage P, McDougall EM, Clayman RV. Laparoscopic transperitoneal nephrectomy for renal cancer: the University of California, Irvine, technique. J Endourol. 2011;25:195–200. doi: 10.1089/end.2010.0549. [DOI] [PubMed] [Google Scholar]
- 22.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 23.Federico A, D'Aiuto E, Borriello F, Barra G, Gravina AG, Romano M, De Palma R. Fat: a matter of disturbance for the immune system. World J Gastroenterol. 2010;16:4762–4772. doi: 10.3748/wjg.v16.i38.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimbach JK, Taler SJ, Prieto M, Cosio FG, Textor SC, Kudva YC, Chow GK, Ishitani MB, Larson TS, Stegall MD. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant. 2005;5:1057–1064. doi: 10.1111/j.1600-6143.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 25.Kurzer E, Leveillee R, Bird V. Obesity as a risk factor for complications during laparoscopic surgery for renal cancer: multivariate analysis. J Endourol. 2006;20:794. doi: 10.1089/end.2006.20.794. [DOI] [PubMed] [Google Scholar]
- 26.Hagiwara M, Miyajima A, Hasegawa M, Jinzaki M, Kikuchi E, Nakagawa K, Oya M. Visceral obesity is a strong predictor of perioperative outcome in patients undergoing laparoscopic radical nephrectomy. BJU Int. 2012;110:980–984. doi: 10.1111/j.1464-410X.2012.11274.x. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 28.Gill IS, Cherullo EE, Meraney AM, Borsuk F, Murphy DP, Falcone T. Vaginal extraction of the intact specimen following laparoscopic radical nephrectomy. J Urol. 2002;167:238–241. [PubMed] [Google Scholar]


