Abstract
There is conflicting evidence about the benefit of using corticosteroid in periarticular injections for pain relief after total knee arthroplasty (TKA). We carried out a double-blinded, randomised controlled trial to assess the efficacy of using corticosteroid in a periarticular injection to control pain after TKA.
A total of 77 patients, 67 women and ten men, with a mean age of 74 years (47 to 88) who were about to undergo unilateral TKA were randomly assigned to have a periarticular injection with or without corticosteroid. The primary outcome was post-operative pain at rest during the first 24 hours after surgery, measured every two hours using a visual analogue pain scale score. The cumulative pain score was quantified using the area under the curve.
The corticosteroid group had a significantly lower cumulative pain score than the no-corticosteroid group during the first 24 hours after surgery (mean area under the curve 139, 0 to 560, and 264, 0 to 1460; p = 0.024). The rate of complications, including surgical site infection, was not significantly different between the two groups up to one year post-operatively.
The addition of corticosteroid to the periarticular injection significantly decreased early post-operative pain. Further studies are needed to confirm the safety of corticosteroid in periarticular injection.
Take home message: The use of corticosteroid in periarticular injection offered better pain relief during the initial 24 hours after TKA.
Cite this article: Bone Joint J 2016;98-B:194–200.
Keywords: knee; pain; arthroplasty; local infiltration; rehabilitation
Pain after total knee arthroplasty (TKA) may be severe.1 The use of periarticular injection to control the pain of TKA has gained wide acceptance.2-5 The agents used in modern periarticular injections typically include corticosteroid, local anaesthetic, opioid and a non-steroidal anti-inflammatory drug (NSAID).1,4
Corticosteroid is believed to be a key component because of its local anti-inflammatory effects and its ability to reduce the local stress response to surgery.1 However, there is conflicting evidence about its benefits.6-11 Some studies have shown that post-operative pain is improved with corticosteroid,6-9 whereas others have shown no benefit.10,11 Christensen et al10 conducted a randomised controlled trial (RCT) which compared patients who had a periarticular injection with or without corticosteroid, and concluded that the corticosteroid had no impact on post-operative pain. Chia et al11 compared patients who had a periarticular injection containing high- or low-dose corticosteroid and found no improvement in the level of post-operative pain.
We conducted a double-blinded RCT to investigate the clinical effectiveness of corticosteroid in a periarticular injection for pain control after TKA. Our hypothesis was that the post-operative pain score would be lower in patients who had a periarticular injection which included corticosteroid.
Patients and Methods
Study design
This prospective, two-arm, parallel-group, double-blinded, RCT was conducted at Nekoyama Miyao Hospital which specialises in knee and hip surgery.
The study was approved by the institutional review board. All patients provided written informed consent. The study was registered with the University Hospital Medical Information Network (registration number UMIN000012257) as a RCT with the title “Impact of corticosteroid on periarticular injection for pain control following total knee arthroplasty: a double-blind randomised trial” before the onset of participant enrolment.
The study focused on post-operative pain during the first 24 hours after TKA.
Participants
Participants were recruited between November 2013 and April 2014. Eligible patients were at least 18 years of age and scheduled for unilateral TKA. Exclusion criteria were poorly controlled diabetic mellitus, defined as haemoglobin A1c level was over 7.0%;12 spinal anaesthesia contraindicated; allergy or intolerance to one of the study drugs; regular opioid use; renal insufficiency, and a prolonged QT interval on electrocardiography.
Participants were informed that we were testing the efficacy of corticosteroid for pain control after TKA and that they would be randomly assigned to receive a periarticular injection of a mixture with or without corticosteroid.
Randomisation and blinding
Randomised numbers from zero to 99 were generated using computer software (Excel 2010, Microsoft, Redmond, Washington). These randomised numbers were then put into an opaque envelope. Just before surgery, a sealed envelope was selected in the pharmacy department by one of two unblinded allocating staff who did not take part in surgery or the assessment of outcome. The selected number was confirmed by the two allocating staff. They were instructed not to discuss the products used with the patient. Patients with even numbers were allocated to the group who were to have the periarticular injection which included corticosteroid, and those with odd numbers were allocated to the group without corticosteroid. A member of the pharmacy department prepared and delivered the periarticular injection solution to the operating theatre. We ensured that the patients, care providers, and outcome assessors were blinded to the group assignment during the study period. They were all unaware of the randomisation given by the allocating staff.
Interventions
We used a control solution for periarticular injection which contained ropivacaine, morphine, epinephrine, and ketoprofen without corticosteroid, and compared its effect with that of a periarticular injection solution which contained the same agents but with corticosteroid.
The corticosteroid solution consisted of methylprednisolone 40 mg (Sol Mercort, Fuji, Toyama, Japan) (1 mL), 7.5 mg/mL ropivacaine (Anapeine, AstraZeneca, Osaka, Japan) (40 mL), 10 mg/mL morphine hydrochloride hydrate (Takeda, Osaka, Japan) (0.8 mL), 1.0 mg/mL epinephrine (Bosmin, Daiichi-Sankyo, Tokyo, Japan) (0.3 mL), 50 mg of ketoprofen (Capisten, Kissei, Matsumoto, Japan) (2.5 mL), and normal saline (15.4 mL). Just before implantation of the prosthesis, 20 mL of the mixture was injected into the posterior capsule and the posteromedial and posterolateral structures.2 Small volumes of the solution were injected into multiple sites with the knee in flexion.13 After implantation, the remaining 40 mL of mixture was injected into the extensor mechanism, synovium, anterior capsule, pes anserius, retinaculum, periosteum, iliotibial band, and collateral ligaments.2
The procedure for periarticular injection without corticosteroid was identical to that for periarticular injection with corticosteroid except that the injectable solution contained an equivalent volume of normal saline.
Pre- and post-operative medication
These were identical in both treatment groups except for the use of corticosteroid. For all patients included in the study, NSAID (50 mg of flurbiprofen axetil, Ropion, Kaken, Tokyo, Japan) was given intravenously four hours after spinal anaesthesia had complete resolution. From the day after surgery, an NSAID (60 mg of loxoprofen, Surinofen, Aska, Tokyo, Japan) was given orally three times a day. No parenteral narcotics were used. For rescue analgesia, a 25 mg or 50 mg diclofenac sodium suppository (Adefuronic zupo, Teva, Nagoya, Japan) was used.
An intravenous first-generation cephalosporin (cefamezin, Cefazolin, Astellas, Tokyo, Japan) was given peri-operatively and every eight hours for the first 48 hours after surgery.
We gave 1 g of tranexamic acid intravenously (Transamin, Daiichi-Sankyo, Tokyo, Japan) just before skin incision and again six hours after the first dose.
For thromboprophylaxis we injected 1.5 mg or 2.5 mg of fondaparinux (Arixtra, GlaxoSmithKline, Tokyo, Japan) subcutaneously once every evening for ten days, starting from the first post-operative day.
Surgery and rehabilitation
All patients had a spinal anaesthetic using 2.0 mL to 2.8 mL of 0.5% bupivacaine (Marcaine, AstraZeneca, Osaka, Japan).
All operations were carried out by one of two surgeons (ST and MW). Neither pneumatic tourniquet nor drain was used during the study period. A subvastus approach was used in every case. All patients had a cemented, posterior stabilised prosthesis (Scorpio NRG, Stryker Orthopaedics, Mahwah, New Jersey) implanted.
The post-operative rehabilitation regimen was the same for both groups, and started the day after surgery.
Outcome measurements
Primary outcome
Staff who were unaware of the treatment group to which each patient had been assigned conducted all assessments.
The primary outcome was pain at rest during the first 24 hours after surgery. Intensity of pain was rated using a 100 mm horizontal visual analogue scale (VAS) in 10 mm increments, in which 0 mm represented no pain and 100 mm represented extreme pain. Before surgery, all patients were instructed in use of the VAS. Time zero was defined as the time at which spinal anaesthesia had complete resolution.14 The VAS score at rest was recorded every two hours from four to 24 hours from time zero, when the patients were awake.
After this 24-hour time period, the VAS score was recorded every eight hours for a further 48 hours, that is until 72 hours had elapsed from time zero.
Secondary outcomes
The post-operative level of pain during activity was estimated on a VAS score once a day until the third post-operative day. The strongest pain experienced during physiotherapy on a particular day was recorded as the VAS score during activity.
Range of movement was measured by the physiotherapist. The data were collected during the hospital stay (from days one to 14 after surgery) and during regularly scheduled post-operative visits (at one, two, three, six, and 12 months after surgery). The number of diclofenac sodium suppositories used as rescue analgesia was recorded.
Any complications that occurred during the course of the trial were recorded: particular emphasis was placed on wound complications, surgical site infections, and opioid-related side effects. Diagnosis of surgical site infections was performed using the Centres for Diseases Control standardised criteria.15
Sample size
Based on previous studies, we considered a 20-point decrease in the VAS score as clinically meaningful when comparing different regimens of pain control after TKA.4,16-18 We calculated that with a sample of 74 patients (37 patients per treatment group), the study would have 80% power to detect a 20-point mean decrease in the VAS score, with a type I error of 5%. For power analysis, we used a standard deviation of 30 in the VAS score for the data of a previous study in post-operative unilateral TKA patients managed under spinal anaesthesia and periarticular injection.16
Statistical analyses
We used the area under the curve of the VAS scores at rest to assess primary outcome. The area under the curve represents the cumulative VAS score over the period of observation.19 A line graph for each patient was made with VAS score on the vertical (y) axis and time after surgery on the horizontal (x) axis. The area under the curve in the line graph was divided into a series of trapezoids, and determined by calculating the sum of the areas of each of the individual trapezoids. A lower area under the curve represents better pain relief during the period of observation. The area under the curve for the first 24-hour post-operative period was compared between groups with Student’s t-test after confirming normality with the Kolmogorov-Smirnov test. The area under the curve for the first 24-hour post-operative period was also compared using the Mann–Whitney U test.
For missing primary outcome data, we replaced VAS scores either by linear interpolation in cases in which the missing scores fell between two valid scores or by the median scores for the other patients at the same time point in the same treatment group.
Comparisons between the study groups were undertaken using the chi-squared test for categorical variables and Student’s t-test for continuous variables. All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Patients
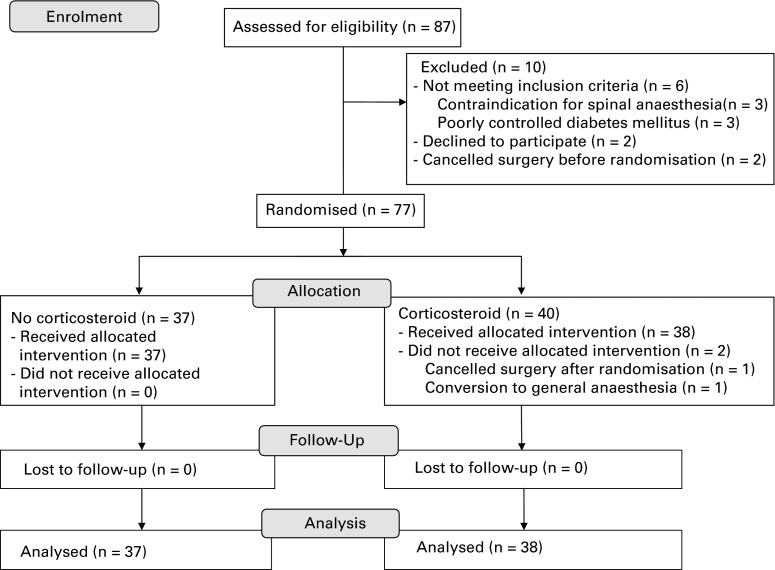
Figure 1 is a flow chart which outlines the progress of the trial. Of 87 applicants screened for eligibility, 77 were randomly assigned to receive a periarticular injection with or without corticosteroid (n = 40 and n = 37, respectively). After randomisation, we excluded two patients in the corticosteroid group: one patient cancelled the operation and one patient had to have a general anaesthetic because spinal anaesthesia failed.
Fig. 1.
Flow chart showing patient recruitment and progression of trial.
Table I summarises the demographics of the patients in the two groups. The baseline characteristics were similar in both groups.
Table I.
Patient demographics and baseline clinical characteristics (data are presented as means with ranges)
| Steroid (n = 40) | No steroid (n = 37) | p-value | |
|---|---|---|---|
| Age (yrs) | 75 (58 to 88) | 72 (47 to 88) | 0.14* |
| Sex (female/male) | 35/5 | 32/5 | 0.89† |
| Side (right/left) | 16/24 | 22/15 | 0.088† |
| Height (cm) | 149 (134 to 166) | 150 (131 to 167) | 0.50* |
| Weight (kg) | 59 (39 to 80) | 62 (40 to 108) | 0.29* |
| Body mass index (kg/m2) | 26.7 (20.5 to 38.6) | 27.3 (18.4 to 40.6) | 0.51* |
| Pre-operative diagnosis (OA/RA/AVN) | 37/2/1 | 34/1/2 | 0.71† |
| History of Diabetes Mellitus (yes/no) | 2/38 | 6/31 | 0.11† |
| Pre-operative VAS at rest (mm) | 26 (0 to 90) | 18 (0 to 60) | 0.16* |
| Pre-operative VAS during activity (mm) | 56 (0 to 100) | 55 (0 to 100) | 0.91* |
| Pre-operative flexion angle (°) | 127 (60 to 160) | 129 (95 to 160) | 0.62* |
| Pre-operative extension angle (°) | -8 (-25 to 0) | -11 (-30 to 0) | 0.13* |
| Intra-operative blood loss (mL) | 181 (43 to 490) | 219 (37 to 628) | 0.17* |
| Duration of operation (min) | 84 (70 to 101) | 88 (69 to 116) | 0.079* |
* p-values were determined with Student’s t-test † p-values were determined with the chi-squared test VAS, visual analogue scale; OA, osteoarthritis; RA, rheumatoid arthritis; AVN, avascular necrosis
Outcomes
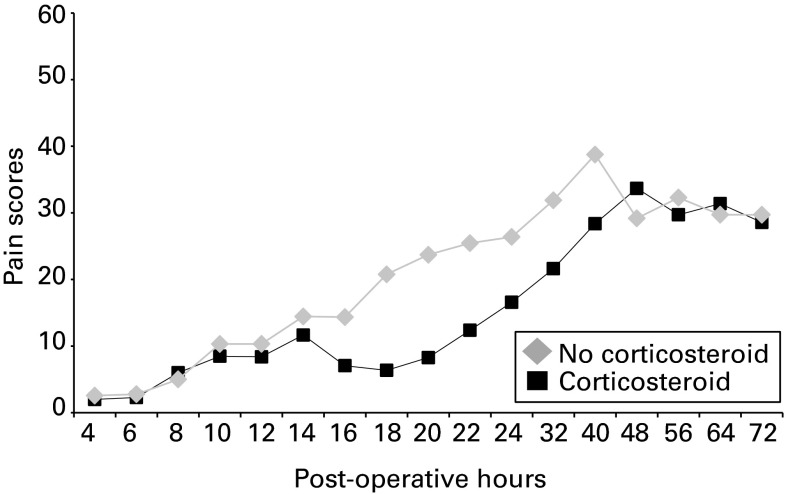
The VAS scores at rest are shown in Figure 2 and Table II. In both groups, the hypotheses of normality were not rejected according to Kolmogorov-Smirnov test (p = 0.092 in the corticosteroid group, and p = 0.17 in the no-corticosteroid group). The corticosteroid group had a significantly lower area under the curve post-operatively at four to 24 hours compared with the no-corticosteroid group (mean 139 mm × day (0 to 560) vs 264 mm × day (0 to 1460), p = 0.024, Student’s t-test). The significance of difference was also confirmed with non-parametric statistics (median 75 mm × day vs 240 mm × day, p = 0.033, Mann–Whitney U test).
Fig. 2.
Graph showing visual analogue scale scores for pain at rest after total knee arthroplasty. The area under the curve was significantly lower in the corticosteroid group at four hours to 24 hours post-operatively (p = 0.024).
Table II.
Visual analogue scale score for post-operative pain at rest (data are presented as means with ranges)
| Duration after surgery (hrs) | Steroid (n = 38) | No steroid (n = 37) | p-value |
|---|---|---|---|
| 4 | 2 (0 to 40) (n = 35) | 2 (0 to 80) (n = 35) | 0.83 |
| 6 | 2 (0 to 20) (n = 35) | 3 (0 to 50) (n = 36) | 0.80 |
| 8 | 6 (0 to 60) (n = 35) | 5 (0 to 60) (n = 36) | 0.76 |
| 10 | 8 (0 to 50) (n = 33) | 10 (0 to 60) (n = 32) | 0.67 |
| 12 | 8 (0 to 50) (n = 25) | 10 (0 to 70) (n = 31) | 0.69 |
| 14 | 12 (0 to 50) (n = 24) | 14 (0 to 60) (n = 18) | 0.63 |
| 16 | 7 (0 to 40) (n = 17) | 14 (0 to 80) (n =23) | 0.22 |
| 18 | 6 (0 to 40) (n = 33) | 21 (0 to 70) (n = 26) | 0.0019 |
| 20 | 8 (0 to 40) (n = 29) | 24 (0 to 80) (n = 35) | 0.0033 |
| 22 | 12 (0 to 50) (n = 21) | 25 (0 to 90) (n = 22) | 0.046 |
| 24 | 17 (0 to 70) (n = 38) | 26 (0 to 80) (n = 36) | 0.057 |
| 32 | 22 (0 to 80) (n = 37) | 32 (0 to 100) (n = 37) | 0.070 |
| 40 | 28 (0 to 90) (n = 37) | 39 (0 to 100) (n = 36) | 0.081 |
| 48 | 34 (0 to 90) (n = 38) | 29 (0 to 70) (n = 37) | 0.17 |
| 56 | 30 (0 to 70) (n = 35) | 32 (0 to 80) (n = 35) | 0.62 |
| 64 | 31 (0 to 70) (n = 36) | 30 (0 to 80) (n = 36) | 0.73 |
| 72 | 29 (0 to 60) (n = 35) | 30 (0 to 80) (n = 35) | 0.81 |
Visual analogue scale score was rated using a 100 mm horizontal scale in 10 mm increments All p-values were determined with Student’s t-test
In the point-by-point evaluation, the corticosteroid group had significantly lower mean VAS scores at 18, 20, and 22 hours than the no-corticosteroid group (6 mm (0 to 40) vs 21 mm (0 to 70), p = 0.0019, 8 mm (0 to 40) vs 24 mm (0 to 80), p = 0.0033, and 12 mm (0 to 50) vs 25 mm (0 to 90), p = 0.046, respectively). There was no difference in VAS score at rest between the two groups 24 hours after surgery.
The mean VAS score during activity was significantly lower in the corticosteroid group than in the no-corticosteroid group one day after surgery (34 mm (0 to 80) vs 49 mm (0 to 80), p = 0.013) (Table III).
Table III.
Visual analogue scale for post-operative pain during activity (data presented as means with ranges)
| Duration after surgery (days) | Steroid (n = 38) | No steroid (n = 37) | p-value |
|---|---|---|---|
| 1 | 34 (0 to 80) | 49 (0 to 80) | 0.013 |
| 2 | 46 (20 to 80) | 51 (0 to 100) | 0.27 |
| 3 | 40 (0 to 70) | 42 (10 to 80) | 0.82 |
Visual analogue scale score was rated using a 100 mm horizontal scale in 10 mm increments All p-values were determined with Student’s t-test
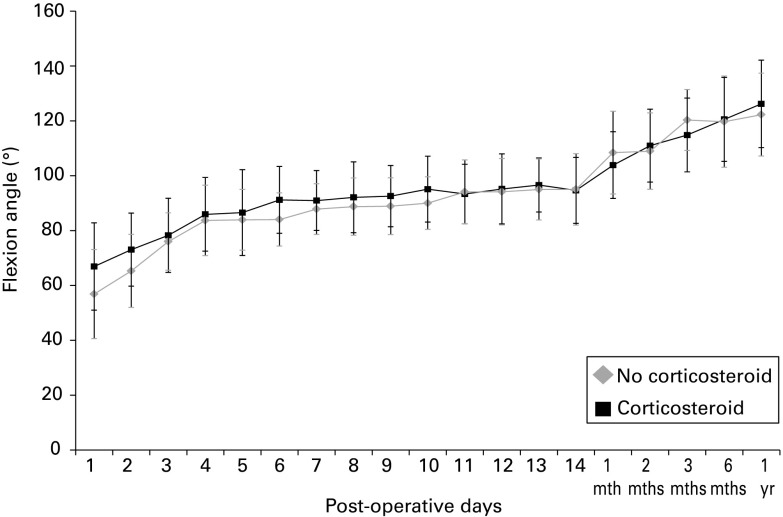
In terms of flexion angle, the corticosteroid group had a significantly better mean range of movement at one, two, and six days after surgery (mean 67° (40° to 95°) vs 57° (30° to 95°), p = 0.014, 73° (40° to 100°) vs 65° (35° to 100°), p = 0.017, and 91° (65° to 115°) vs 84° (60° to 100°), p = 0.021, respectively) (Fig. 3, Table IV). The corticosteroid group had a significantly better mean extension angle at one, two, three, and ten days after surgery (-6° (-10° to 0°) vs -9° (-20° to 0°), p = 0.0046, -7°(-15° to 0°) vs -10° (-25° to 0°), p = 0.0060 and -4° (-10° to 0°) vs -7° (-20° to 0°) p = 0.022, respectively) (Table IV).
Fig. 3.
Graph showing the mean flexion angle of the knee (with standard deviation) after total knee arthroplasty. The corticosteroid group had a significantly better mean flexion angle on post-operative days one and two (p = 0.014 and p = 0.017, respectively).
Table IV.
Range of movement after total knee arthroplasty (data are presented as means with ranges)
| Post-operative day | Steroid (n = 38) | No steroid (n = 37) | p-value |
|---|---|---|---|
| Flexion angle (°) | Flexion angle (°) | ||
| 1 | 67 (40 to 95) | 57 (30 to 95) | 0.014 |
| 2 | 73 (40 to 100) | 65 (35 to 100) | 0.017 |
| 3 | 78 (40 to 105) | 76 (60 to 105) | 0.47 |
| 4 | 86 (50 to 110) | 84 (65 to 115) | 0.54 |
| 5 | 87 (50 to 115) | 84 (55 to 110) | 0.50 |
| 6 | 91 (65 to 115) | 84 (60 to 100) | 0.021 |
| 7 | 91 (65 to 110) | 88 (70 to 110) | 0.19 |
| 8 | 92 (60 to 115) | 89 (60 to 110) | 0.23 |
| 9 | 93 (60 to 115) | 89 (70 to 110) | 0.76 |
| 10 | 95 (65 to 115) | 90 (70 to 110) | 0.14 |
| 11 | 93 (65 to 115) | 94 (70 to 120) | 0.80 |
| 12 | 95 (65 to 115) | 94 (65 to 120) | 0.77 |
| 13 | 97 (75 to 112) | 95 (80 to 120) | 0.59 |
| 14 | 95 (65 to 120) | 95 (70 to 130) | 0.90 |
| 1 mth | 104 (80 to 125) | 108 (75 to 135) | 0.19 |
| 2 mths | 111 (85 to 135) | 109 (90 to 145) | 0.58 |
| 3 mths | 115 (85 to 140) | 120 (95 to 135) | 0.13 |
| 6 mths | 121 (70 to 150) | 120 (75 to 150) | 0.83 |
| 12 mths | 126 (80 to 155) | 122 (90 to 145) | 0.39 |
| Post-operative day | Extension angle (°) | Extension angle (°) | p-value |
| 1 | -6 (-10 to 0) | -9 (-20 to 0) | 0.0046 |
| 2 | -7 (-15 to 0) | -10 (-25 to 0) | 0.0060 |
| 3 | -6 (-10 to 0) | -9 (-25 to 0) | 0.0080 |
| 4 | -7 (-15 to 0) | -8 (-20 to 0) | 0.13 |
| 5 | -6 (-10 to 0) | -8 (-20 to 0) | 0.056 |
| 6 | -6 (-10 to 0) | -7 (-15 to 0) | 0.20 |
| 7 | -6 (-10 to 0) | -7 (-20 to 0) | 0.15 |
| 8 | -5 (-10 to 0) | -7 (-20 to 0) | 0.056 |
| 9 | -5 (-15 to 0) | -7 (-20 to 0) | 0.069 |
| 10 | -4 (-10 to 0) | -7 (-20 to 0) | 0.022 |
| 11 | -4 (-15 to 0) | -6 (-15 to 0) | 0.36 |
| 12 | -5 (-20 to 0) | -7 (-15 to 0) | 0.14 |
| 13 | -4 (-15 to 0) | -5 (-10 to 0) | 0.23 |
| 14 | -4 (-15 to 0) | -6 (-15 to 0) | 0.091 |
| 1 mth | -5 (-25 to 0) | -6 (-20 to 0) | 0.41 |
| 2 mths | -5 (-25 to 0) | -6 (-20 to 0) | 0.51 |
| 3 mths | -5 (-15 to 0) | -4 (-20 to 0) | 0.85 |
| 6 mths | -3 (-20 to 0) | -4 (-20 to 0) | 0.64 |
| 12 mths | -1 (-10 to 0) | -3 (-15 to 0) | 0.18 |
All p-values were determined with Student’st-test
Table V shows the number of diclofenac sodium suppositories used as rescue analgesia: this was significantly lower in the corticosteroid group than in the no-corticosteroid group at one day after surgery (0, 0 to 2 vs 1, 0 to 2; p = 0.021).
Table V.
The mean number of suppositories used as rescue analgesia
| Steroid (n = 38) | No steroid (n = 37) | p-value | |
|---|---|---|---|
| On the night of surgery | 0 (0 to 2) | 0 (0 to 1) | 0.77 |
| Post-operative day 1 | 0 (0 to 2) | 1 (0 to 2) | 0.021 |
| Post-operative day 2 | 0 (0 to 2) | 0 (0 to 2) | 0.62 |
| Post-operative day 3 | 0 (0 to 2) | 0 (0 to 1) | 0.57 |
All p-values were determined with Student’s t-test
There were no significant differences in the rate of complications, including wound complications and surgical site infections, between the two groups (Table VI). Five patients developed a peroneal nerve palsy as a result of the injection of local anaesthetic into the area: these all recovered completely within 24 hours.
Table VI.
Complications
| Steroid (n = 38, %) | No steroid (n = 37, %) | p-value | |
|---|---|---|---|
| Nausea on the night of surgery | 2 (5.3) | 1 (2.7) | 0.57 |
| Nausea on post-operative day 1 | 1 (2.6) | 2 (5.4) | 0.54 |
| Pruritus | 1 (2.6) | 0 (0) | 0.31 |
| Respiratory depression | 0 (0) | 0 (0) | N/A |
| Wound complication | 1 (2.6) | 1 (2.6) | 0.98 |
| Surgical site infection | 0 (0) | 0 (0) | N/A |
| Transient peroneal nerve palsy | 3 (7.9) | 2 (5.4) | 0.67 |
All p-values were determined with chi squared test N/A, not applicable
Discussion
We found that the level of post-operative pain in the first 24 hours was less in the corticosteroid group than in the no-corticosteroid group. The corticosteroid group also had better results in terms of post-operative pain during activity, range of movement, and the use of rescue analgesia in the early post-operative period. The rate of complications did not differ between groups.
There have been two RCTs which do not support the efficacy of corticosteroid in periarticular injection10,11 and four RCTs which do.6-9 These conflicting results may be owing to differences in the periarticular injection solutions used in the various studies. Long-acting6,8 or intermediate-acting7,9-11 corticosteroids were used in these studies. In our study, we chose to use methylprednisolone, an intermediate-acting corticosteroid which is five-times more potent than hydrocortisone,20 one of the most frequently used corticosteroids for periarticular injection.1,4,5,10,15 Although several RCTs have compared the efficacy of different corticosteroids in intra-articular injection of the knee joint,21,22 there have been no studies which directly compare the efficacy of periarticular injection for pain control after TKA.
Surgical technique, including the surgical approach and the use of a pneumatic tourniquet could have an effect on early post-operative pain.23,24 Differences in surgical technique may also have been responsible for the conflicting results of previous studies.6-11
The usefulness of a multimodal approach to pain control after TKA has been reported.1,3,25 Although the periarticular injection of a combination of agents is the most important component of the multimodal approach,1 peripheral nerve blockade is also a key component.26,27 We deliberately did not use peripheral nerve blockade so that we would be able to study the impact of corticosteroid on the periarticular injection.
There is controversy about whether the analgesic solution should be injected into the extensor mechanism. Although we did so in the same way as other studies,1,4,16 Chia et al11 has advised against injecting the extensor mechanism because of the risk of delayed tendon rupture.
A transient peroneal nerve palsy occurred in five patients, because of infiltration into the area of the common peroneal nerve.16 Surgeons should avoid excessive infiltration in this area when injecting the posterior aspect of the capsule.2
The strengths of our study include its double-blinded, randomised, controlled design, the use of the area under the curve for the primary outcome and more meticulous measurement than in previous studies. In contrast with measurement only at a specific time point, the area under the curve represents total pain relief during the observation period.19
This study had several limitations. First, the allocation using randomised numbered, opaque, sealed envelopes could be considered suboptimal compared with other remote methods of randomisation, such as a dedicated telephone randomisation service. When a trial has the potential to subvert randomisation, the results may differ from trials in which there is no such possibility.28 Second, as this study was conducted in a single centre and only two surgeons were involved, care should be taken when generalising the findings to community settings. Third, although spinal anaesthesia is recommended for TKA owing to the low risk of complications,29 it could mask the pain scale in the early post-operative period. Fourth, we did not evaluate post-operative swelling: that was expected to be better in the corticosteroid group. Finally, although this study showed a significant difference between groups in terms of primary outcome, the sample size was underpowered to make definitive conclusions about the ratio of complications, including surgical site infection and wound complication, owing to their low frequency.
Although no previous studies have shown that including corticosteroid in a periarticular injection significantly influences the rate of surgical site infection,6-11,30 several studies have reported patients developing a surgical site infection after periarticular injection which contained corticosteroid.10,16 To analyse the impact of corticosteroid on surgical site infection, a larger sample size is needed. As even a recently conducted meta-analysis was underpowered to determine the safety of corticosteroid in periarticular injection,30 further studies are needed to answer this question unequivocally.
References
- 1.Parvataneni HK, Shah VP, Howard H, et al. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty 2007;22(suppl 2):33–38. [DOI] [PubMed] [Google Scholar]
- 2.Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg [Am] 2006;88-A:959–963. [DOI] [PubMed] [Google Scholar]
- 3.Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg [Am] 2006;88-A:282–289. [DOI] [PubMed] [Google Scholar]
- 4.Tsukada S, Wakui M, Hoshino A. Pain control after simultaneous bilateral total knee arthroplasty: a randomized controlled trial comparing periarticular injection and epidural analgesia. J Bone Joint Surg [Am] 2015;97-A:367–373. [DOI] [PubMed] [Google Scholar]
- 5.Yadeau JT, Goytizolo EA, Padgett DE, et al. Analgesia after total knee replacement: local infiltration versus epidural combined with a femoral nerve blockade: a prospective, randomised pragmatic trial. Bone Joint J 2013;95-B:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeuchi M, Kamimoto Y, Izumi M, et al. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2014;22:1638–1643. [DOI] [PubMed] [Google Scholar]
- 7.Sean VW, Chin PL, Chia SL, et al. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J 2011;52:19–23. [PubMed] [Google Scholar]
- 8.Yue DB, Wang BL, Liu KP, Guo WS. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chin Med J (Engl) 2013;126:3851–3855. [PubMed] [Google Scholar]
- 9.Kwon SK, Yang IH, Bai SJ, Han CD. Periarticular injection with corticosteroid has an additional pain management effect in total knee arthroplasty. Yonsei Med J 2014;55:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty. A double-blind randomized trial. J Bone Joint Surg [Am] 2009;91-A:2550–2555. [DOI] [PubMed] [Google Scholar]
- 11.Chia SK, Wernecke GC, Harris IA, et al. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty 2013;28:620–623. [DOI] [PubMed] [Google Scholar]
- 12.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care 2007;30:2233–2235. [DOI] [PubMed] [Google Scholar]
- 13.Dalury DF. Periarticular Injection Technique to Enhance Pain Relief After Knee Arthroplasty. JBJS Essential Surg Tech 2014;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marino J, Russo J, Kenny M, et al. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg [Am] 2009;91-A:29–37. [DOI] [PubMed] [Google Scholar]
- 15.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–608. [PubMed] [Google Scholar]
- 16.Tsukada S, Wakui M, Hoshino A. Postoperative epidural analgesia compared with intraoperative periarticular injection for pain control following total knee arthroplasty under spinal anesthesia: a randomized controlled trial. J Bone Joint Surg [Am] 2014;96-A:1433–1438. [DOI] [PubMed] [Google Scholar]
- 17.Tammachote N, Kanitnate S, Manuwong S, Yakumpor T, Panichkul P. Is pain after TKA better with periarticular injection or intrathecal morphine? Clin Orthop Relat Res 2013;471:1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H. High-volume infiltration analgesia in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand 2008;52:1331–1335. [DOI] [PubMed] [Google Scholar]
- 19.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician 1998;58:443–450. [PubMed] [Google Scholar]
- 21.Bird HA, Ring EF, Bacon PA. A thermographic and clinical comparison of three intra-articular steroid preparations in rheumatoid arthritis. Ann Rheum Dis 1979;38:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol 2004;23:116–120. [DOI] [PubMed] [Google Scholar]
- 23.Matsueda M, Gustilo RB. Subvastus and medial parapatellar approaches in total knee arthroplasty. Clin Orthop Relat Res 2000;371:161–168. [DOI] [PubMed] [Google Scholar]
- 24.Ejaz A, Laursen AC, Kappel A, et al. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop 2014;85:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berend ME, Berend KR, Lombardi AV Jr. Advances in pain management: game changers in knee arthroplasty. Bone Joint J 2014;96-B(11 Supple A):7–9. [DOI] [PubMed] [Google Scholar]
- 26.Mahadevan D, Walter RP, Minto G, et al. Combined femoral and sciatic nerve block vs combined femoral and periarticular infiltration in total knee arthroplasty: a randomized controlled trial. J Arthroplasty 2012;27:1806–1811. [DOI] [PubMed] [Google Scholar]
- 27.Sakai N, Inoue T, Kunugiza Y, Tomita T, Mashimo T. Continuous femoral versus epidural block for attainment of 120° knee flexion after total knee arthroplasty: a randomized controlled trial. J Arthroplasty 2013;28:807–814. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–412. [DOI] [PubMed] [Google Scholar]
- 29.Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg [Am] 2013;95-A:193–199. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Qin J, Tan Y, et al. Efficacy of steroid addition to multimodal cocktail periarticular injection in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 2015;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]