Abstract
The surge in computing power and mobile connectivity have fashioned a foundation for mobile health (mHealth) technologies that can transform the mode and quality of clinical research and health care on a global scale. Unimpeded by geographical boundaries, smartphone-linked wearable sensors, point-of-need diagnostic devices, and medical-grade imaging, all built around real-time data streams and supported by automated clinical decision–support tools, will enable care and enhance our understanding of physiological variability. However, the path to mHealth incorporation into clinical care is fraught with challenges. We currently lack high-quality evidence that supports the adoption of many new technologies and have financial, regulatory, and security hurdles to overcome. Fortunately, sweeping efforts are under way to establish the true capabilities and value of the evolving mHealth field.
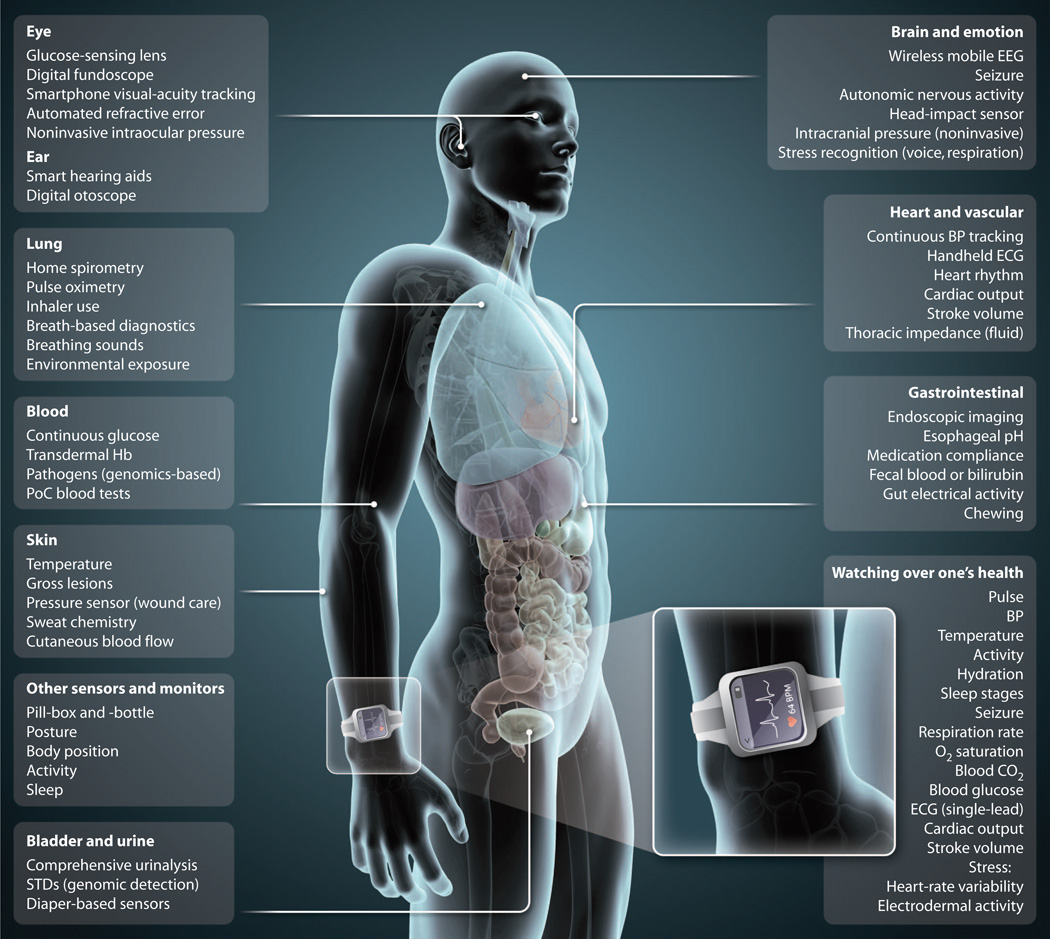
Today, a device that fits in the palm of one’s hand wields computational power that, several decades ago, would have cost tens of millions of dollars and required instruments that filled an entire room (1). Likewise, the artificial intelligence necessary to beat a chess master required massive hardware and processing support just over 15 years ago but now is downloadable as an app for any smartphone. Mirroring this unprecedented growth in personal computing power is an equally remarkable expansion in mobile connectivity: Unique mobile subscribers constitute 64% of the population worldwide (2). In the United States alone, 91% of the adult population owns a mobile phone, with most (61%) of these individuals having a smartphone (3). The use of smartphones has become so much a part of our routine lives that we now, on average, spend more time every day looking at our smartphone screen than at our TV screen (4). These extraordinary advancements in mobile computer technology and connectivity have already transformed nearly every aspect of our lives: finance, travel, entertainment, education, and, of course, communications. However, only now are mobile health (mHealth) technologies making initial inroads into health care and, in so doing, are providing the foundation to radically transform the practice and reach of medical research and care. Through progressively miniaturized and increasingly powerful mobile computing capabilities, individuals are becoming increasingly capable of monitoring, tracking, and transmitting health metrics continuously and in real time. This metamorphosis has provided the potential for acute disease diagnosis and chronic condition management to take place outside the standard doctor’s office or hospital (Fig. 1).
Fig. 1.
Sensing a shift in health care. Shown are bodywide measurements by mHealth technologies that are available to health care providers and patients to aid in the tracking, diagnosis, or management of various physiological processes and disease conditions. (Inset) Watching over one’s health. Multiple developers have reported that the listed physiological parameters are measurable with sensors in a wrist-worn device. BP, blood pressure; Hb, hemoglobin; STDs, sexually transmitted diseases.
WEARABLE SENSORS
Engineers have designed and developed a wide variety of wearable biometric sensors, such as bracelets, watches, skin patches, headbands, earphones, and clothing. Although these sensors have many forms and functions, their unifying design focus is to allow for unobtrusive, passive, and, when appropriate, continuous monitoring. Another key characteristic is their ability to seamlessly track and transfer all biometric data into an actionable and informative user interface that can be shared with health care providers, researchers, family members, or one’s social network. Whereas accelerometer-based activity trackers are the best known and most ubiquitous of the wearables, sensor technologies can go well beyond step counts to provide a wealth of medical-grade personalized information to help guide health and wellness. The potential capabilities of a wrist-worn sensor alone is exemplified by the more than one dozen biometric parameters that have been reported by different developers to be measurable in a primarily continuous fashion (Fig. 1, inset). Today, several devices are commercially available that allow individuals to determine their cardiac rhythm via a single-lead electrocardiogram (ECG), either by using their smartphone for rhythm capture whenever needed or by wearing a patch for prolonged continuous rhythm tracking (5). When a single lead is not precise enough, there are shirts that allow for 3- or even 12-lead ECG monitoring while simultaneously tracking activity levels and respiratory rate. The ability to track multiple leads increases the accuracy of arrhythmia diagnosis.
Transformations are under way in blood pressure monitoring that forego the need for an inflatable cuff and, instead, use photoplethysmography (which optically detects blood volume changes in a tissue’s microvasculature) and pulse transition time and permit more frequent, or even continuous, blood pressure tracking. Technologies such as these can bring critical care unit–like levels of monitoring to the daily lives of large human populations (6). An especially innovative family of sensors has been developed around the ability to monitor autonomic nervous system function or so-called electrodermal metrics (galvanic skin response) and/or heart rate variability, with a goal of providing objective data of individualized daily variations in levels of emotional stress and anxiety (7). The capability to quantitatively monitor emotional status might also be useful for following the efficacy of treatment strategies. Other notable examples of sensor technologies under development allow for a more personalized understanding of our daily response to the environment and include technologies that track sleep stages and disruptions (8), monitor respiratory diseases (9), and continuously track blood glucose concentrations (10).
LAB ON A CHIP
Beyond sensor capabilities, the processing power and connectivity offered by smartphones allow for a range of medical testing to be taken out of the specialized core laboratory and brought directly to the individual. A combination of microfluidics (requiring just nanoor picoliter volumes of fluid) and microelectronics allows for the “digitization” of sweat, blood, saliva, urine, tears, and breath (11). These diagnostic capabilities go well beyond improvement in the convenience of testing. Rather, they offer the possibility of entirely new diagnostic capabilities that would be accessible virtually anywhere, anytime. For example, several point-of-care (PoC) tests geared toward low-resource areas use microfluidics to allow rapid diagnosis of infectious diseases, which can accelerate individual diagnosis and treatment as well as population screening (12). In addition, smartphone-linked genetic diagnostics are being designed to enable a rapid, accurate PoC diagnosis of a range of pathogens, along with future applications for clinical measurements and fields of study (such as pharmacogenomics) in which rapid genetic diagnosis would be beneficial (13). Simulation of a dog’s powerful olfactory capabilities with development of “electronic noses,” especially those linked to smartphones, might offer truly remarkable diagnostic capabilities for a vast array of conditions, including the early detection of cancers or infectious diseases such as tuberculosis (14, 15).
IMAGING FROM AFAR
The high quality of the camera lenses and screen resolution of smartphones allows their optical systems to be used for a host of medical applications, from photometric diagnostics to medical-grade imaging (16). Taking advantage of these properties, newly developed devices permit the automated determination of refractive error merely by having an individual look through a lens attached to a smartphone (17). Another transportable imaging capability involves the enabling of remote diagnosis through the use of a smartphone case with an attached otoscope (for detecting an ear infection) (18), multimodal colposcope for cervical cancer identification (19), or optical screening tool for potentially cancerous oral lesions (20). Dermatologic diagnostics may be especially well suited for exploiting the myriad smartphone capabilities for teledermatology (21).
The technologies highlighted above can improve care simply through their ability to markedly increase the accessibility and convenience of care by bringing clinic- and hospital-quality monitoring and diagnostics to the point of need. However, their greatest potential might be in allowing for the complete redefining of “normal” physiological responses and in enhancing our understanding of the natural histories of poorly defined chronic conditions. Continuous beat-to-beat monitoring of blood pressure throughout daily activities will help to refine the catchall diagnosis of “essential hypertension” as multiple distinct phenotypes. Similarly, understanding individual variation in response to stress and, with it, calming interventions will transform the identification and treatment of a wide range of mental health disorders. These and many more similar types of individual data should prove to be critical adjuncts to developing research programs focused on individualized (that is, precision or personalized) medicine.
CHALLENGES TO TRANSLATION
Expanding the evidence base
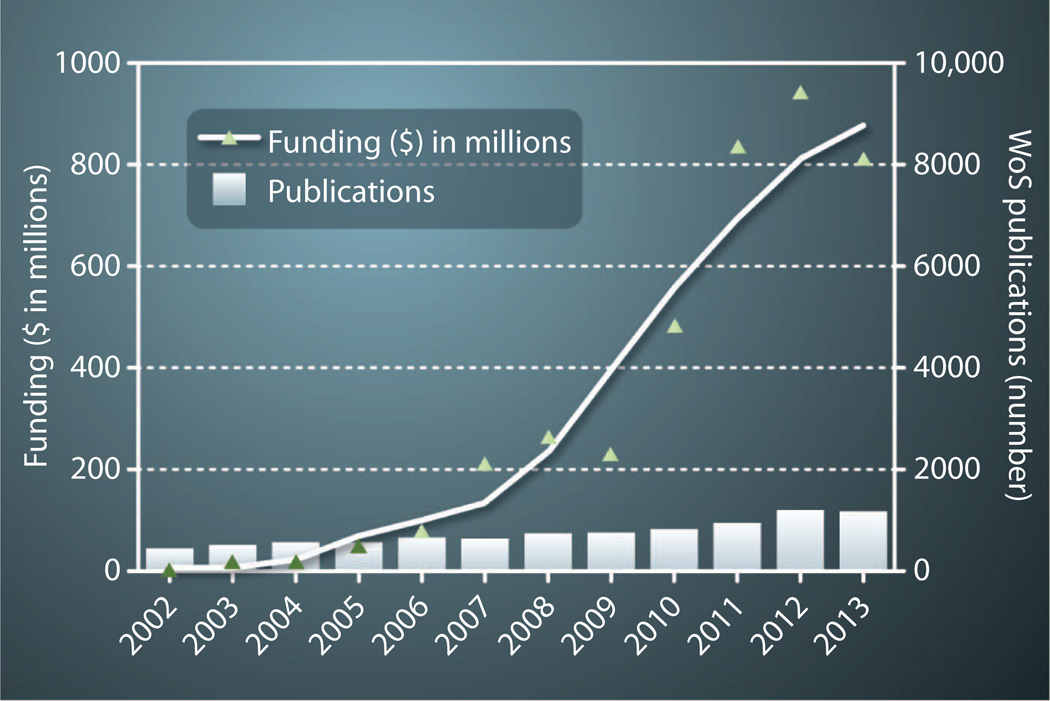
As noted by these examples, there is great potential for mHealth technologies to reengineer almost every facet of health care and, in the process, markedly improve our understanding of human physiology in health and disease. Hypotheses around mHealth’s potential are supported by predictions of financial analysts who estimate that the market for mHealth technology will grow at an annual rate of nearly 55%, from $1.5 billion globally in 2012 to $21.5 billion by 2018 (22). Buoyed by these predictions, venture capital funding for mHealth has been increasing annually (Fig. 2). However, growth of the evidence base necessary to drive transformational changes in health care has not been tracking with the expanding financial support. Indeed, although the number of mHealth-related publications is growing gradually, the majority of the published evidence in support of clinical use is limited to underpowered pilot data. For example, a recent study of mobile-enabled cardiac rehabilitation received a great deal of coverage in both the lay and medical press when it found a 40% reduction in hospital readmissions (23). However, the study compared the outcomes in only 25 individuals who used the app with 19 individuals who followed a standard program, with those 19 experiencing a historically high readmission rate. Several recent meta-analyses and systematic reviews conclude that high-quality evidence is lacking for the use of mHealth to effect behavioral changes or to manage chronic diseases, inpatient care, or health care delivery (24–26).
Fig. 2. mHealth taking center stage.
Measures are funding and number of related publications. Shown are the annual total funding for patient-facing mHealth companies and the annual number of related publications [identified with Web of Science (WoS) using search terms “telemedicine” and “mhealth*” and “digital health” and “digital medicine”]. Funding data provided by B. Dolan and A. Pai of MobiHealthNews.
The need for high-quality research in the mHealth field was reinforced in a recently released green paper from the European Commission (27). Designed to initiate broad stakeholder engagement so as to address the barriers to mHealth deployment, the authors note that more investment in research and innovation is needed to support the development of new, advanced mHealth solutions while ensuring a high degree of efficacy and reliability as well as secure data processing. The U.S. National Institutes of Health (NIH) convened an mHealth evidence workshop to address the evidence gap and drive the research “needed to assess when, where, and for whom mHealth devices, apps, and systems are efficacious” (28). Many of the challenges of mHealth research were addressed in this workshop, including the need for collection of enormous quantities of real-world, patient-generated data and the singular problems associated with the rapid development and obsolescence of the new technologies to be studied.
Obtaining the necessary data to drive change offers opportunities and challenges. On the one hand, the distinctive features of mHealth technologies and the data generated can support a reengineering of clinical trial design. Individualized trial recruitment could be enabled through mobile devices possibly using social networks, rather than the historically slow and unpredictable dependence on multiple research centers. Informed consent, data capture, and participant communication could all be handled digitally via mobile or desktop platforms. The recently announced ResearchKit program by Apple is an excellent example of how mobile technology can potentially transform research. Adaptive study designs, from both a statistical and intervention standpoint, would not only allow for a more rapid assessment of a specific intervention but also assure that the results are meaningful and scalable the moment they are published. Trials need not be anchored to a specific technology or individual device but rather can be based on a new system of care built around what the technology allows. For example, a trial to establish the clinical benefit, patient acceptance, and economic impact of hypertension management using home monitoring and wireless two-way communication could be designed to allow for transitioning technologies as they become available—perhaps moving from a wireless cuff, to an intermittent cuffless blood pressure monitor, to a continuous, wearable blood pressure device.
On the other hand, designing clinical trials to demonstrate the potentially transformative nature of mHealth requires that challenging questions be asked. Too often, studies of mHealth technology have been designed to answer the question “How can ‘these technologies’ fit into existing systems of care?” Instead, the more appropriate question is “How can systems of care be altered to best take advantage of ‘these technologies’?” Attempting to fit disruptive technologies into existing systems has historically been shown to prevent anticipated gains. Similarly, the implementation of mHealth technologies only as adjuncts to existing systems of care likely will lead to results that fall well short of demonstrating their true impact. Naturally, designing trials around nonstandard systems of care adds a layer of substantial complexity, but doing so will be important if we are to provide the health care community with a roadmap for transforming patient care.
Financial obstacles
Beyond the scarcity of clinical evidence for benefits of mHealth use, there are multiple other considerations that will drive the uptake and use of mHealth technologies. In the United States in particular, current modes of financing health care are the single greatest barrier to mHealth implantation within legacy health care systems. A fee-for-service environment that rewards reactive, sickness-based care is essentially in direct conflict with the capabilities made possible through mHealth tools. For example, hypertension is currently the singlemost common primary diagnosis for an adult ambulatory care visit in the United States, accounting for nearly 40 million visits annually. If all hypertension management were transformed to home monitoring, a large majority of these 40 million visits would be unnecessary, saving the health care system substantial costs. In addition, patients would likely find remote monitoring much more convenient than having to interrupt their routine schedules for unneeded physician appointments. Most importantly, data suggest that patients whose care incorporates self-monitoring are much more likely to achieve adequate blood pressure control compared to those using the current systems of care (29). However, within a fee-for-service reimbursement model, there is no financial incentive for practitioners to implement such a system, and with value-based care not foreseen to overtake fee-for-service until 2020, the financial environment for mHealth incorporation within existing U.S. health care systems will remain poor for years to come—all of which hamper implementation of disruptive health care solutions.
Privacy and security concerns
In order for the promise of mHealth to be fully realized, consumers, providers, and health care systems must be able to trust the reliability, privacy, and security of their data as well as the devices that collect and share it. Unfortunately, we have a long way to go to provide the needed reassurance. Although regulatory oversight is often considered to be an impediment to the rapid dissemination of innovative technologies, the existence of modern-day snake-oil scams mandates some level of oversight. A recent investigative report found that out of 1500 health apps studied, 1 of 5 claimed either to treat or to cure a wide range of medical problems by just using the light, sound, or vibrations of the phone (30). Another example of the potential hazards of inadequate regulation of health care–related apps or devices is the availability of medical diagnostic tools with the disclaimer that they are meant to be for entertainment or recreational purposes only (31). This multipurpose disclaimer allows developers to sidestep regulatory oversight or even provide any evidence on whether their app does what it purports to do. One example is a top-grossing app called Instant Blood Pressure, which promises users the ability to measure their blood pressure using just their smartphone (32). Particularly concerning are the comments on the app Web site that suggest that people are using the device to make treatment decisions about their blood pressure.
Globally, there is a great deal of uncertainty around mHealth regulation; more than 150 countries have no regulatory framework, whereas the European Union and United States are actively refining theirs. In an attempt to facilitate rather than impede innovation, the U.S. Food and Drug Administration, in consultation with the Office of the National Coordinator and the Federal Communications Commission, has chosen to use a risk-based approach to regulatory oversight, focusing only on devices or apps that would pose a risk to patient safety if they did not function as intended (33).
mHealth users are also concerned about privacy and ownership of their health data. Most countries have privacy laws that protect patient data but with a great deal of variability. In the United States, the Health Insurance Portability and Accountability Act (HIPAA) governs the privacy and security of one’s health information. However, that guidance does not extend outside the health care setting, making it possible for mHealth developers to share a substantial portion of user-generated data, often without the knowledge of the user. The U.S. Federal Trade Commission recently tested 12mHealth and fitness apps and found that consumer data from these apps were being sent to 76 different third-party companies (34). Some of the data shared include the phone’s unique device identifier as well as the owner’s running routes, dietary habits, and sleep patterns. A similar analysis of 43 fitness apps found that 40% were collecting what was classified as high-risk data—addresses, financial information, full name, health information, location, date of birth, or zip code—and more than 55% were sharing data with third-party analytical services that could potentially link those data with data from other apps (35). In the era of big data, it is critical that the terms of ownership of personal data, most especially medical data, be unambiguously stated—not buried in the universally unread and then accepted terms of use agreements—with users required to explicitly consent whenever their data are sold or transmitted to others. It is unlikely that this will occur without new laws and regulatory oversight.
One of the clear benefits of mHealth is easier accessibility to pertinent health care data, but this increased availability to both consumers and providers creates the potential for substantial security risks. Health data are already a favored target for cyber thieves, as the underground market will pay $20 for health insurance credentials compared to only $1 to $2 for credit card numbers (36). With 4.5 million lost or stolen smartphones last year alone, it is not difficult to imagine the security disaster that would ensue if each of these phones stored sensitive personal health information or, much more concerning, could potentially serve as a portal into an electronic medical record system. Cloud storage, biometrics-based security, encrypted data transfer, and remote kill switches are some of the solutions that can and, less commonly, are being used to mitigate these risks.
Avoiding data overload
A final but especially critical challenge to successful implementation of mHealth strategies is assuring the usability of the data, primarily for the consumer but also for providers. If not put in a usable context, the vast amounts of data potentially available to the consumer could easily overwhelm even the most active and knowledgeable health care consumers (just imagine all of the data mined from the wearable device shown in Fig. 2). Not every user will have the same needs, and the presentation of actionable information will need to be tailored to fit individual needs. In addition, simply providing more medical data to patients not only fails to guarantee improved outcomes but also could potentially lead to negative consequences (37). Successful implementation requires an increase in our understanding of individual variability in comfort levels with different technologies and of how to individually encourage sustained use.
Activity trackers have had poor evidence of durable use, with a conservatively estimated one-third discontinuing use by 6 months after initiation (38). A recent study of several tools to encourage medication adherence in older adults, a major area of focus of mHealth developers, found that the most common descriptors participants used to describe their experience with the devices were “frustrating” and “challenging” (39). In another study of the usage of a dietary app to promote healthy eating, investigators found that fewer than 3% used the app for at least 1 week and fewer than 10% of these individuals made positive changes in their diet (40). Users require consumer-friendly devices and apps that are self-reinforcing and enjoyable to use. These goals might be accomplished with the use of incentives, game mechanics, and social networks to promote managed competition among peers or family members.
As consumer demand for wearable sensors increases, health care providers will face the possibility of being inundated by a torrent of patient data. This tsunami will create a number of difficult challenges, including the potential requirement for 24/7 oversight, the need to summarize multiparameter, continuously collected data into a usable and clinically meaningful format, and liability challenges (41). And this scenario assumes that providers will be willing to embrace patient-generated data by putting as much trust in it as they put into data they collect. Critical to dealing with the usability of both patient and provider data will be the development of sophisticated data analytics tools and user-friendly platforms for data presentation.
PATIENTS AT THE CENTER
Within a surprisingly short period of time, the world has become intricately intertwined and completely reliant on mobile devices. Monthly digital mobile traffic now exceeds an exabyte (a quintillion bytes, or a billion gigabytes) and by 2016 is predicted to grow another 10-fold (42). Yet, the mHealth ecosystem is still in its early formative stages, in terms of both technology development and incorporation into systems of care. Leveraging Moore’s law with ever smaller and cheaper circuits, mHealth devices have the potential to decrease the cost of both clinical research and health care just as technology advancements have done in virtually all industries except health care (43). Whereas mobile medical technology has considerable promise, without rigorous testing in clinical trials, mHealth runs the risk of following the same path as the use of robotics in surgery or megavitamins—therapies that are commonly used without any clear-cut supportive data.
It is mHealth’s emphasis on the interests of the health care consumer that makes its incorporation into routine care so challenging and yet so potentially transformational. Empowering patients with accessibility to and ownership of their own medical data reverses the predominantly one-way dynamic of today’s health care system. Instead, mHealth places the consumer at the center of their health care network. And beyond mere convenience, mHealth can help to redefine what is considered normal in the context of health parameters, from a population-based perspective of comparing one individual to thousands of others, to a time-based perspective comparing an individual to him- or herself before the presence of a disease sign or symptom.
Acknowledgments
Funding: S.R.S. is supported by the U.S. NIH National Center for Advancing Translational Sciences (NCATS) grant UL1TR001114 and a grant from the Qualcomm Foundation. E.D.M. receives funding from NIH grant 8 KL2 TR000110. E.J.T. receives research funding from the Qualcomm Foundation and NIH NCATS grant UL1TR001114. Competing interests: S.R.S. is a medical advisor for Agile Edge Technologies, BridgeCrest Medical, Electrozyme, FocusMotion, and PhysIQ, and serves on the board of directors of Vantage mHealthcare. E.J.T. currently serves on the board of directors of Dexcom, is editor in chief of Medscape (WebMD), is an advisor for AT&T, and previously served on the board of directors of Sotera Wireless and Volcano.
REFERENCES AND NOTES
- 1.Markoff J. The iPad in your hand: As fast as a supercomputer of yore. [9 May 2011];New York Times. http://bits.blogs.nytimes.com/2011/05/09/the-ipad-in-your-hand-as-fast-as-a-supercomputer-of-yore/?_php=true&_type=blogs&_r=0. [Google Scholar]
- 2.Global mobile statistics 2014 Part A: Mobile subscribers; handset market share; mobile operators. [16 May 2014]; http://mobiforge.com/research-analysis/global-mobile-statistics-2014-part-a-mobile-subscribers-handset-market-share-mobile-operators. [Google Scholar]
- 3.Smith A. Smartphone ownership. [5 June 2013]; www.pewinternet.org/2013/06/05/smartphone-ownership-2013. [Google Scholar]
- 4.MillwardBrown, Ad reaction: Marketing in a multiscreen world. 2014 www.millwardbrown.com/adreaction/2014/#. [Google Scholar]
- 5.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 2014;127:95.e11–95.e17. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry I, Bernstein D, Banet M, Mulligan J, Moulton S, Grudic G, Convertino V. Body-worn, non-invasive sensor for monitoring stroke volume, cardiac output, and cardiovascular reserve. Proc. 2nd Conference on Wireless Health; La Jolla, CA. New York: Association for Computing Machinery; 2011. pp. 1–2. [Google Scholar]
- 7.Poh MZ, Swenson NC, Picard RW. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans. Biomed. Eng. 2010;57:1243–1252. doi: 10.1109/TBME.2009.2038487. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 9.McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Sys. Rev. 2011:CD007718. doi: 10.1002/14651858.CD007718.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Jiang X, Zhang L, Fan J, Wu W. Construction of near-infrared photonic crystal glucose-sensing materials for ratiometric sensing of glucose in tears. Biosens. Bioelectron. 2013;48:94–99. doi: 10.1016/j.bios.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 11.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 12.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umvilighozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 13.Stedtfeld RD, Tourlousse DM, Seyrig G, Stedtfeld TM, Kronlein M, Price S, Ahmad F, Gulari E, Tiedje JM, Hashsham SA. Gene-Z: A device for point of care genetic testing using a smartphone. Lab Chip. 2012;12:1454–1462. doi: 10.1039/c2lc21226a. [DOI] [PubMed] [Google Scholar]
- 14.Kolk AH, van Berkel JJ, Claassens MM, Walters E, Kuijper S, Dallinga JW, van Schooten FJ. Breath analysis as a potential diagnostic tool for tuberculosis. Int. J. Tuberc. Lung Dis. 2012;16:777–782. doi: 10.5588/ijtld.11.0576. [DOI] [PubMed] [Google Scholar]
- 15.Fu XA, Li M, Knipp RJ, Nantz MH, Bousamra M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014;3:174–181. doi: 10.1002/cam4.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boppart SA, Richards-Kortum R. Point-of-care and point-of-procedure optical imaging technologies for primary care and global health. Sci. Transl. Med. 2014;6:253rv2. doi: 10.1126/scitranslmed.3009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naone E. Cell phones as eye doctors. [15 October 2010];MIT Technol. Rev. www.technologyreview.com/view/421230/cell-phones-as-eye-doctors. [Google Scholar]
- 18.Hill DJ. Now your smartphone can be used to diagnose ear infections at home. [16 July 2012];Forbes. www.forbes.com/sites/singularity/2012/07/16/now-your-smartphone-can-be-used-to-diagnose-ear-infections-at-home. [Google Scholar]
- 19.Webster M, Kumar VS. Picturing cervical cancer. Clin. Chem. 2014;60:277–279. doi: 10.1373/clinchem.2013.217521. [DOI] [PubMed] [Google Scholar]
- 20.Newby K. New smartphone scans from Stanford could prevent needless oral cancer deaths. Stanford University, School of Medicine; [17 April 2012]. http://med.stanford.edu/ism/2012/april/vodaphone-0417.html. [Google Scholar]
- 21.Janda M, Loescher LJ, Soyer HP. Enhanced skin self-examination: A novel approach to skin cancer monitoring and follow-up. JAMA Dermatol. 2013;149:231–236. doi: 10.1001/jamadermatol.2013.1218. [DOI] [PubMed] [Google Scholar]
- 22.BCC Research, Mobile health (mHealth) technologies and global markets 2014. [14 March 2014]; www.bccresearch.com/market-research/healthcare/mobile-health-hlc162a.html. [Google Scholar]
- 23.Comstock J. Mayo Clinic study finds app reduces cardiac readmissions by 40 percent. [1 April 2014];MobiHealthNews. http://mobihealthnews.com/31580/mayo-clinic-study-finds-app-reduces-cardiac-readmissions-by-40-percent. [Google Scholar]
- 24.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLOS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prgomet M, Georgiou A, Westbrook JI. The impact of mobile handheld technology on hospital physicians’ work practices and patient care: A systematic review. J. Am. Med. Inform. Assoc. 2009;16:792–801. doi: 10.1197/jamia.M3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technologies to improve health care service delivery processes: A systematic review and meta-analysis. PLOS Med. 2013;10:e1001363. doi: 10.1371/journal.pmed.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Commission, editor. Digital Agenda for Europe 2014. Green paper on mobile health (“mHealth”) [Google Scholar]
- 28.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, Riley WT, Shar A, Spring B, Spruijt-Metz D, Hedeker D, Honavar V, Kravitz R, Lefebvre RC, Mohr DC, Murphy SA, Quinn C, Shusterman V, Swendeman D. Mobile health technology evaluation: The mHealth evidence workshop. Am. J. Prev. Med. 2013;45:228–236. doi: 10.1016/j.amepre.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, Schwartz C, Shackleford H, Shovelton C, Varghese J, Williams B, Hobbs FD, Gooding T, Morrey I, Fisher C, Buckley D. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: The tasmin-sr randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 30.Sharp R. Lacking regulation, many medical apps questionable at best. [18 November 2012];New England Center for Investigative Reporting. http://necir.org/2012/11/18/medical-apps. [Google Scholar]
- 31.Dolan B. The rise of the seemingly serious but ‘just for entertainment purposes’ medical app. [7 August 2014];MobiHealthNews. http://mobihealthnews.com/35444/the-rise-of-the-seemingly-serious-but-just-for-entertainment-purposes-medical-app. [Google Scholar]
- 32.Husain I. Top 10 downloaded iPhone health app can cause significant patient harm. [14 July 2014];iMedicalApps. www.imedicalapps.com/2014/07/iphone-health-app-patient-harm. [Google Scholar]
- 33.U.S. Food and Drug Administration. FDASIA Health IT Report: Proposed strategy and recommendations for a risk-based framework. [21 April 2014]; www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cdrh/cdrhreports/ucm390588.htm.
- 34.Brook C. Health and fitness apps poor at protecting privacy, FTC says. [8 May 2014];Threatpost. http://threatpost.com/health-and-fitness-apps-poor-at-protecting-privacy-ftc-says. [Google Scholar]
- 35.Privacy Rights Clearinghouse. Fact Sheet 39: Mobile health and fitness apps: What are the privacy risks? [1 December 2014]; www.privacyrights.org/mobile-health-and-fitness-apps-what-are-privacy-risks. [Google Scholar]
- 36.Clarke E. Hackers sell health insurance credentials, bank accounts, SSNs and counterfeit documents, for over $1,000 per dossier. [15 July 2013];Dell Secure Works. www.secureworks.com/resources/blog/general-hackers-sell-health-insurance-credentials-bank-accounts-ssns-and-counterfeit-documents. [Google Scholar]
- 37.O’Kane MJ, Bunting B, Copeland M, Coates VE. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): Randomised controlled trial. BMJ. 2008;336:1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledger D, McCaffrey D. Inside wearables: How the science of human behavior change offers the secret to long-term engagement. Endeavour Partners LLC; 2014. Jan, http:// endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. [Google Scholar]
- 39.Grindrod AK, Li M, Gates A. Evaluating user perceptions of mobile medication management applications with older adults: A usability study. JMIR Mhealth Uhealth. 2014;2:e11. doi: 10.2196/mhealth.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helander E, Kaipainen K, Korhonen I, Wansink B. Factors related to sustained use of a free mobile app for dietary self-monitoring with photography and peer feedback: Retrospective cohort study. J. Med. Internet Res. 2014;16:e109. doi: 10.2196/jmir.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YT, Silverman RD. Mobile health applications: The patchwork of legal and liability issues suggests strategies to improve oversight. Health Aff. 2014;33:222–227. doi: 10.1377/hlthaff.2013.0958. [DOI] [PubMed] [Google Scholar]
- 42.Peckham M. Mobile explosion: Wireless traffic could reach 10.8 exabytes a month by 2016. [14 February 2012];PC World. www.pcworld.com/article/249922/mobile_explosion_wireless_traffic_could_reach_10_8_exabytes_a_month_by_2016.html. [Google Scholar]
- 43.Skinner JS. The costly paradox of health-care technology. [5 September 2013];MIT Technol. Rev. www.technologyreview.com/news/518876/the-costly-paradox-of-health-care-technology. [Google Scholar]