Abstract
Empathy shapes the landscape of our social lives. It motivates prosocial and caregiving behaviors, plays a role in inhibiting aggression, and facilitates cooperation between members of a similar social group. Thus, empathy is often conceived as a driving motivation of moral behavior and justice, and as such, everyone would think that it should be cultivated. However, the relationships between empathy, morality, and justice are complex. We begin by explaining what the notion of empathy encompasses and then argue how sensitivity to others’ needs has evolved in the context of parental care and group living. Next, we examine the multiple physiological, hormonal, and neural systems supporting empathy and its functions. One troubling but important corollary of this neuro-evolutionary model is that empathy produces social preferences that can conflict with fairness and justice. An understanding of the factors that mold our emotional response and caring motivation for others helps provide organizational principles and ultimately guides decision-making in medical ethics.
Keywords: empathy, morality, fairness, group biases, decision-making, justice, social neuroscience
Introduction
Empathy is everywhere in both the popular and academic arenas including medicine, law and economics. However, not everyone agrees with the idea that empathy is a good thing, from a moral point of view, or that it is something we should cultivate because it makes us better people. Recall, for instance, the visceral responses from conservative pundits when President Obama, speaking of his choice to nominate a new Justice, said: “I will seek someone who understands that justice isn’t about some abstract legal theory or footnote in a casebook; it is also about how laws affect the daily realities of people’s lives.” That kind of judge, Obama explained, will have empathy: “I view the quality of empathy, of understanding and identifying with people’s hopes and struggles as an essential ingredient for arriving at just decisions and outcomes.” Obama spoke at length about the “empathy deficit” in a January 20, 2008 campaign speech in Atlanta: “I’m talking about an inability to recognize ourselves in another; to understand our brother’s keeper; we are our sister’s keeper; that, in the words of Dr. King, we are all tied together in a single garment of destiny.” President Obama considers principles like freedom and fairness, not just for ourselves but for everyone, to be products of our care for others. This agrees with his invocations of empathy combined with concern for the less advantaged. However, legal and just do not always go together, nor does empathy systematically lead to moral decisions.
The relationships between empathy, morality, and justice are indeed complex. For instance, regardless of whether one considers that the law should be a self-referring construct of “pure geometry,” i.e., absent social or environmental context considerations, or a “social process that deals with human activity, with cause and effect, with the past and the future” (Cohen 1935), one can argue that law and morality are two distinct domains and that a system of law can rest on an immoral foundation like the Apartheid in South Africa between 1948 until 1994. Do we need judges who have the empathy to recognize what is like to be a young teenage mom or to understand what it’s like to be poor, African American, or gay? Do we need medical ethicists to have empathy when examining cost-effectiveness or resource allocation in medical care?
The purpose of this article is to examine the intersection of neuroscience and psychology on the study of empathy and moral decision-making1. Substantial progress has been made in recent years towards a comprehensive understanding of the evolutionary processes that have favored the development of complex social behaviors in humans, along with the brain architecture that supports them. In particular, research in social neuroscience, relying on multi-level integrative analysis studies (from genes to social interactions) provides a mechanistic comprehension of empathy and caring for others. Drawing from theoretical and empirical work in developmental science, social psychology, and affective neuroscience, we will argue that empathy should be regarded with caution and is not enough to serve as a central motivation in driving moral judgment and decision-making. The evidence supports a more moderate view of the role of empathy in morality. Cognitive reasoning is equally important for moral reasoning and justice. Understanding the role of empathy in morality requires a precise description of what the concept empathy embodies.
The too many meanings of empathy
One reason that the notion of empathy has become so popular in academia as well as to the lay public is that this concept is used to refer to a heterogeneous collection of related phenomena. However, careful analysis shows that they are not aspects or facets of a single thing, as one might say that an attitude has cognitive, affective, and behavioral components (Batson 2009). Empathy is such an unwieldy concept that any academic book on the topic usually includes a whole chapter to define exactly what empathy is. Keeping track of these different conceptualizations is important because they refer to distinct psychological processes that vary, sometimes widely, in their function, phenomenology, mechanisms, and effects (Coplan 2011).
Furthermore, given that empathy encompasses so many different facets, it should not come as a surprise that there is no single measure to reliably assess this disposition. All self-report questionnaires parse empathy into a number of dimensions such as personal distress, perspective taking, and empathic concern, or at least cognitive and emotional empathy. But these dispositional measures do not consistently relate to specific neural mechanisms. For instance, a developmental study with participants aged between 4 and 17 years reported that while females scored higher than males on an empathy questionnaire, a difference that increased with age, no change was detected in the pattern of the neural response measured with functional MRI when participants viewed stimuli depicting individuals being physically hurt (Michalska, Kinzler, and Decety 2013).
Despite such diverse understandings of empathy, recent research in developmental and social neuroscience has narrowed down its scope such that that it involves three dissociable components that are not completely overlapping in functions and mechanisms, but yet can interact (Decety and Jackson 2004; Decety 2011). These components include:
Affective sharing, which reflects the natural capacity to become affectively aroused by others’ emotions.
Empathic concern, which corresponds to the motivation of caring for another’s welfare.
Perspective taking, which is the ability to consciously put oneself into the mind of another individual and imagine what that person is thinking or feeling.
Each of these emotional, motivational, and cognitive facets of empathy emerges from specific neurobiological processes and reflects evolved functions that allow humans to thrive by detecting and responding to significant social events necessary for surviving, reproducing, and maintaining well-being.
Neurobiological mechanisms underlying empathy
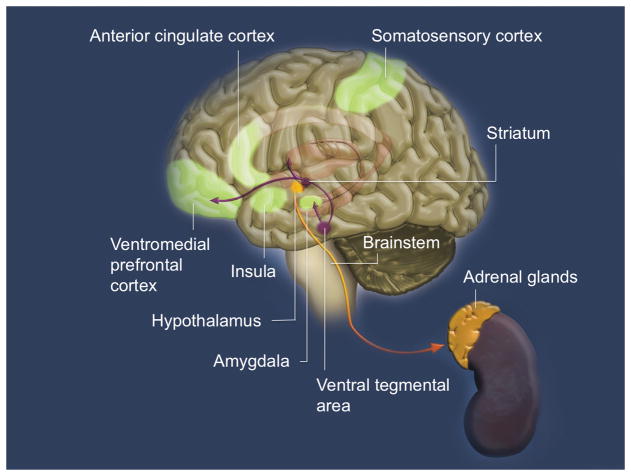
While it is important to consider the broad range of species-specific behaviors when dealing with motivated behaviors, there is a clear evolutionary continuity in parental care and the underlying physiological mechanisms across mammalian species. In humans, the evolutionary emergence of higher-level neural structures occurred without the replacement of more primitive neural systems. Rather, the human brain is organized so that the same inputs are parallel processed at multiple levels, with the responses orchestrated at lower levels of the central nervous system elaborated on and modulated by higher levels of the neuraxis (Decety, Norman, Berntson, and Cacioppo 2012). It is worth noting that the representation of function across the neuraxis does not entail that lower level structures are entirely subject to commands from higher level. In fact, a large percentage of neural processes occur without the engagement of neo-cortical structures. Indeed, higher level cortical processing may be necessary only in situations with high ambiguity and low predictability. This framework applies to affective sharing and empathic concern, which are present in non-human animals although perspective taking (or cognitive empathy) is arguably specific to our species (see Figure 1 for the multiple neural systems involved in empathy).
Figure 1.
Empathy is implemented by a complex network of distributed, often recursively connected, interacting neural regions including the brainstem, amygdala, hypothalamus, striatum, insula, anterior cingulate cortex, and orbitofrontal cortex. The experience of empathy also involves the autonomic nervous system (parasympathetic and sympathetic branches which represent antagonist and coordinated regulation of internal states), and neuroendocrine processes implicated in social behaviors and emotional states. Thus empathy and motivation to care for others emerge from the interaction of multiple areas and circuits in conjunction with the autonomic nervous system and the neuroendocrine system.
Affective sharing
One primary facet of empathy, affective or emotional sharing, is essential in generating the motivation to care for others and is relatively independent of mindreading and perspective-taking capacities. It is often viewed as the simplest form of empathy and can be observed across a multitude of species from birds to rodents and humans (Edgar, Nicol, Clark, and Paul 2012), and appears very early in ontogeny (Cheng, Chen, and Decety 2014). Often, affective sharing is synonymous with emotional contagion.
However, the latter concept has a much greater scope than the former. Specifically, emotional contagion usually refers to the tendency to automatically mimic and synchronize facial expressions, vocalizations, and postures with those of another individual to converge emotionally. Affective sharing, as used here, is not necessarily an automatic process and does not entail convergence of emotion; rather it is the detection of another’s motivational and emotional states that can elicit an adaptive response (such as caring or helping) from the observer. For instance, a mother rat who detects signals from her pup expressing hunger will experience affective sharing without feeling hungry herself, as would be implied by emotional contagion.
Non-human animals show preference towards in-group members in detection and reaction to the distress of others. For instance, rodents are discriminant in their reactions to others in distress. In one study, a female mouse moving toward a dyad member in physical pain led to a decrease in the physical symptoms of pain (less writhing) in the dyad member only when the mouse was a cage mate of the mouse in pain not when they were a stranger (Langford et al. 2010). Similarly, female mice exhibit higher fear responses when exposed to the pain of a close relative than when exposed to the pain of a more distant relative (Jeon et al. 2010). Importantly, it is not necessarily genetic affiliation that solely facilitates assistive behaviors. Rats fostered from birth with another strain have been shown to help strangers of the fostering strain but not rats of their own strain (Ben-Ami Bartal, Rodgers, Bernardez Sarria, Decety, and Mason 2014). Thus, strain familiarity, even to one’s own strain, seems required for the expression of pro-social behavior in rodents.
Studies using electroencephalography (EEG) in children and adults viewing stimuli depicting conspecifics in physical pain have documented specific event-related potentials (ERPs) components. These include an early automatic attentional salience (N2) and late positive potentials (LPP) associated with affective arousal and affective appraisal of the stimuli, respectively, which are detectable as of 3 years of age (Cheng et al. 2014; Cheng, Hung, and Decety 2012; Sheng and Han 2012). Numerous functional magnetic resonance imaging studies (fMRI) with both children (Decety and Michalska 2010) and adults (Lamm, Decety, and Singer 2011) have reliably demonstrated that when participants watch (or even imagine) another person experiencing pain, sadness or emotional distress, brain regions involved in the first-hand physical pain are activated. These regions include the ACC, the anterior insula (aINS), supplementary motor area (SMA), amygdala, somatosensory cortex, and periaqueductal gray area (PAG). Thus, observing another individual in distress induces a visceral arousal in the perceiver by eliciting neural response in regions known to be involved in the first-hand experience of pain (also known as the pain matrix), a network also implicated in salience processing that relates to interoceptive-autonomic processing (Seeley et al. 2007).
Interestingly, the neural response elicited by the perception of others in distress is either strengthened or weakened by interpersonal relationships, implicit attitudes, and group preferences. Activity in the neural network including the ACC, aINS, and PAG is significantly enhanced when individuals view their loved-ones in physical pain as compared to strangers (Cheng, Chen, Lin, Chou, and Decety 2010). A priori, implicit, value-based attitudes toward conspecifics also modulate the response. For example, study participants were significantly more sensitive to the pain of individuals who had contracted AIDS as the result of a blood transfusion as compared to individuals who had contracted AIDS as the result of their illicit drug addiction, as evidenced by higher subjective ratings of pain and greater neuro-hemodynamic activity in the ACC, aINS, and PAG, although the actual intensity of the facial expressions that they viewed was strictly similar across all videos clips (Decety, Echols, and Correll 2009). Another fMRI study found modulation of empathic neural responses by racial group membership (Xu, Zuo, Wang, and Han 2009). Notably, the response in the ACC to viewing others in pain decreased remarkably when participants viewed faces of racial out-group members relative to racial in-group members. This effect was comparable in Caucasian and Chinese subjects and suggests that modulations of empathic neural responses by racial group membership are similar in different ethnic groups. Another study demonstrated that the failures of an in-group member are painful, whereas those of a rival out-group member gives pleasure—a feeling that may motivate harming rivals (Mina Cikara, Botvinick, and Fiske 2011). In that study, participants who reported greater rival-specific aggression not only reported more pleasure, but also exhibited greater ventral striatum activity (a subcortical region involved in reward and pleasure) in response to watching rivals fail, even against a third party.
Sharing the pain of other or simply attention to salient information?
The overlap in activation in between viewing others in pain and experiencing pain oneself is often interpreted in favor of a shared neural representations between self and others, which is the fuel of resonance-based, mirroring, social cognition. The idea is deceptively simple: shared representations or resonance mechanisms (including mirror neurons) underlie our ability to read intentions and emotions in to the behavior of other people2. Unsurprisingly, shared neural representations for felt pain and perceived pain in other seem to fit perfectly with this implicit simulation interpretation. However, fine grain data analyses of fMRI data demonstrate that the activation in the ACC in the firsthand experience of pain and the perception of pain in others are neither necessarily coincident nor coextensive (Morrison and Downing 2007). In addition, vicariously instigated activations in this neural network are not necessarily specific to the emotional experience of pain. Rather they reflect more general processes such as negative stimulus evaluation, attention to noxious stimuli, somatic monitoring, and the selection of appropriate skeletomuscular defensive movements (Decety 2011). In support of this interpretation, one study reported that perceiving a hated person’s face, compared with that of a neutral person, elicited increased activity in the insula and ACC, and activity in these regions was correlated to the subjective rating of hate participants felt for the hated people (Zeki and Romaya 2008). Another fMRI study found greater activity in this pain network, including the aINS, ACC, and somatosensory cortex when Jewish participants viewed hateful (anti-Semitic) individuals compared with likable targets in pain (Fox, Sobhani, and Aziz-Zadeh 2013). Together, these studies demonstrate that increased activity in this pain network seems to be more related to increased salience and relevance of the pain-related cues rather than to increased empathy-related processing per se. Thus, activation of shared neural representations in the affective-motivational regions of the pain matrix are not specific to the sensory qualities of pain, but instead are associated with more general survival mechanisms such as attention to highly salient cues, aversion and withdrawal when exposed to danger and threat.
Empathic concern
Empathic concern refers to other-oriented emotion elicited by and congruent with the perceived welfare of a person in need. This motivation is a product of (a) perception of another as in need and (b) intrinsic valuing of that other’s welfare (Batson 2009), and has evolved with generalized parental nurturance. All mammals depend on other conspecifics for survival and reproduction. Caring for the needs of others is thus a vital product of our evolution, particularly parental care, which is necessary for infant survival and development (Decety et al. 2012). Depending on each species, the level of care varies, but the underlying neural circuitry for responding to infants (especially signals of vulnerability and need) is universally present and highly conserved across mammalian species (Numan and Insel, 2003. Animal research demonstrates that being affected by others’ emotional states, an ability integral to maintaining the social relationships important for survival, is organized by basic neural, autonomic, and neuroendocrine systems subserving attachment-related processes, which are implemented in the brainstem, preoptic area of the thalamus, basal ganglia, paralimbic areas, as well as the autonomic nervous system (Panksepp 1998).
Converging evidence from animal behavior, neuroimaging studies in healthy individuals, and lesion studies in neurological patients demonstrates that caring for others employs a large array of systems neural mechanisms, extending beyond the cortex, including the amygdala, brainstem, hypothalamus, insula, ACC, and orbitofrontal cortex (Preston 2013). It also involves the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and endocrine and hormonal systems that regulate bodily states, emotion, and social sensitivity. In particular, oxytocin, a neuropeptide with widespread targets in both the brain and periphery, has been implicated in the regulation of various social behaviors ranging from social bonding, attachment and parental care. A number of studies have found that individuals carrying a G allele for the rs53576 variant of the oxytocin receptor gene exhibit higher levels of empathic concern and prosocial behaviors (Smith, Porges, Norman, Connelly, and Decety 2014).
This motivation to care for others is deeply rooted in our biology, is very flexible, and arises early in ontogeny. Children’s capacities to respond emotionally to the joys and sorrows of others and to express empathic concern are present during the first year of life (Davidov, Zahn-Waxler, Roth-Hanania, and Knafo 2013). People can feel empathic concern for a wide range of targets when cues of vulnerability and need are highly salient, including nonhumans, and in western culture particularly domestic animals like puppies (Batson 2012). Neural regions involved in perceiving the distress of other humans are similarly recruited when witnessing the distress of domesticated animals (Franklin et al. 2013). Furthermore, the motivation of caring for others is associated with positive feelings which reinforce this behavior. Behavioral and functional neuroimaging studies demonstrate that being nice and caring for others makes us feel good by the release of dopamine through the projection of neural pathways from the brainstem to the nucleus accumbens. The fronto-mesolimbic reward network is engaged to the same extent when individuals receive monetary rewards and when they freely choose to donate money to charitable organizations (Moll et al. 2006). Another fMRI study found that participants who showed sympathetic behavior by tossing a ball to the isolated player (in a computer simulation) reported enhancement of self-positive feelings and anticipation of feeling improvements of the isolated player as well as increase activity in the striatum (Kawamichi, Tanabe, Takahashi, and Sadato 2013). Additional support for a link between positive arousal and generosity comes from an fMRI study that demonstrated that increased activity in the ventral striatum predicted increased subjects’ donations to orphans depicted in photographs (Genevsky, Västfjäll, Slovic, and Knutson 2013).
Finally, research shows that empathic concern reduces cortisol activity in stressful situations for participants who gave social support to a partner during the experiment (Smith, Loving, Crockett, and Campbell 2009). In mothers who are asked to make caregiving decisions to meet the needs of children, dispositional empathic concern is associated with ventral striatum, ventrolateral prefrontal cortex, and SMA activation (Ho, Konrath, Brown, and Swain 2014).
Overall, the subcortical and cortical circuits that developed originally in service of parental nurturance in mammalian species continued to evolve in humans, accompanied by an increase in the plasticity and flexibility provided by the prefrontal cortex, which led to a heightened capacity for learning. In this way, these circuits began to operate at the level of the social group and cultural level. Importantly, the biological mechanisms underpinning empathic concern are distinct from those involved in affective sharing.
Perspective-taking
Perspective-taking or cognitive empathy refers to the ability to consciously put oneself into the mind of another individual to understand what that person is thinking or feeling. This “putting oneself in another’s skin” is achieved through a variety of strategies, each of which are argued to relate to emotional and cognitive outcomes (Myers, Laurent, and Hodges 2013). Perspective-taking has been linked to the recognition of one’s uniqueness in the face of others, as well as the appreciation of other’s independent experiences and emotional states (Gilin, Maddux, Carpenter, and Galinsky 2013). Accordingly, the neural network recruited by affective perspective taking partly overlaps with that underlying theory of mind and comprises dorsomedial prefrontal cortex (dmPFC), the posterior superior temporal sulcus (pSTS) as well as amygdala, aINS and ACC (Schnell, Bluschke, Konradt, and Walter 2011).
Cognitive empathy has been linked to social competence and social reasoning, and a substantial body of behavioral studies has documented that affective perspective taking is a powerful way to elicit empathy and concern for others including for out group members (Batson 2012). For instance, taking the perspective of an out-group member decreases the use of explicit and implicit stereotypes for that individual, and leads to more positive evaluations of that group as a whole (Galinsky and Moskowitz 2001). Assuming the perspective of another brings about changes in the way we see the other, and these changes generalize to people similar to that other, notably members of the same social groups to which they belong (Kidd and Castano 2013). Some studies have documented long-lasting effects of such interventions. For instance, Sri Lankan Singhalese participants expressed enhanced empathy toward Tamils even a year after participating in a 4-day intergroup workshop (Malhotra and Liyanage 2005).
Adopting the perspective of another person, in particular someone from another social group, is cognitively demanding and hence requires additional attentional resources and working memory, which tax executive function abilities. Cognitive neuroscience research demonstrates that when individuals adopt the perspective of another, neural circuits common to the ones underlying first-person experiences are activated as well (Lamm, Meltzoff, and Decety 2009). However, taking the perspective of another produces increased activation in regions of the prefrontal cortex subserving executive function (working memory, attention and inhibitory control). In another fMRI study, participants viewed video clips featuring patients undergoing a painful medical intervention, and were asked to either put themselves in the shoes of the patient (imagine self perspective), or to focus on the patient’s feelings and affective expressions (imagine other perspective) (Lamm, Batson, and Decety 2007). Explicitly projecting oneself into the patient’s situation led to higher levels of personal distress, and was associated with enhanced activation in the amygdala and ACC. Imagining the other’s perspective was accompanied by higher empathic concern, lower personal distress, increased activity in the executive attention network and vmPFC, and reduced the amygdala response.
Thus, affective perspective taking simultaneously engages neural regions associated with theory of mind, executive functions and limbic areas involved in the experience of emotion. Interestingly, burgeoning work in social neuroscience provides support for a primary role of cognitive empathy (and not emotional empathy) in explaining individual differences in individuals’ concern for justice. Two neuroimaging studies, one using functional MRI (Yoder and Decety 2014b) and another one using high-density EEG (Yoder and Decety 2014a) showed that justice sensitivity not only predicted behavioral ratings of praise and blame when participants evaluate morally-laden behavior, but also modulated the online neural response and functional connectivity between the pSTS and prefrontal cortex. Justice sensitivity modulates activity across several domain-general systems, particularly in regions of the prefrontal cortex involved in intention, understanding and goal representations in service of moral decision-making, and importantly does not influence the salience network involved in affective appraisal. These findings are also supported by a study that examined the association between individual differences in different facets of empathy (affective, motivational, and cognitive), justice sensitivity, and psychopathy (Decety and Yoder 2015). Participants rated the permissibility of everyday moral conflict in situations that pit personal benefit against moral standards of justice. Contrary to common sense, affective empathy (emotional sharing) was not associated with sensitivity to justice for others. Rather, cognitive empathy and concern predicted sensitivity to justice for others as well as endorsing moral rules. It may then be more effective to focus on utilizing perspective taking to elicit empathic concern for others, rather than emphasizing emotional sharing.
Empathy can lead to parochial moral behavior
Empathy has some unfortunate features that can conflict with moral behavior. Individuals who identify and cooperate with in-group members enjoy numerous benefits, including the fulfillment of many basic psychological needs (Cikara and Van Bavel 2014). The value humans place on group membership is exemplified by the ease with which humans form groups and favor in-group members, across cultures and from a very early age. However, the functional benefits of group membership notwithstanding, group life is also a source of prejudice, biases, and of social strife.
Several aspects of empathy, such as accuracy and concern for others, as well as generosity and other-oriented behavior, are influenced by social status. Social class seems to shape not only people’s values and behavior but also their affective responses that relate to sensitivity to the welfare of others. Research shows that lower class individuals, relative to their upper-class counterparts, score higher on a measure of empathic accuracy, and judge the emotions of a stranger more accurately (Kraus, Côté, and Keltner 2010). Another set of studies indicates that relative to upper class people, lower class individuals exhibited more generosity, more support for charity, more trust behavior towards a stranger, and more helping behavior towards a person in distress (Piff, Kraus, Côté, Cheng, and Keltner 2010). Despite their reduced resources and subordinate rank, lower class individuals are more willing than their upper class counterparts to increase another’s welfare, even when doing so is costly to the self. The authors speculated that, relative to upper class individuals, lower class individuals construe themselves more in terms of their relationships to others, and this self– other overlap facilitates their sensitivity to other people’s welfare. Moreover, such acts of generosity and prosociality among lower class people play a critical role in cultivating relationships and strengthen social bonds.
Even assigning individuals to arbitrary groups readily elicits evaluative preferences for in-group relative to out-group members, and this can affect empathetic responding. In one such study, participants were assigned to artificial groups and then asked to perform pain intensity evaluations of pictures depicting bodily injuries from self, in-group, or out-group perspectives. Participants rated the stimuli as more painful when they had to adopt the perspective of an in-group member as compared to their own perspective, while the out-group perspective did not induce different responses to the painful stimuli as compared to the self perspective (Montalan, Lelard, Godefroy, and Mouras 2012). Moreover, the ratings differences between the painful and non-painful pictures were more important in the in-group perspective than in the out-group perspective. In an fMRI study, participants were scanned while viewing in-group or out-group perpetrators intentionally harming in-group or out-group members. Participants showed greatest empathic sadness and anger for an in-group victim harmed by a member of the out-group (Molenberghs, Gapp, Wang, Louis, and Decety 2014). In support of this finding, there was increased activity in the orbitofrontal cortex when viewing in-group members being harmed by out-group individuals.
In group morality, oxytocin, and children
The moral problem of group biases can be detected at the neurohormonal level as well. For instance, oxytocin, which is often naively described as the “moral” hormone, in fact promotes human ethnocentrism, i.e., the tendency to view one’s group as superior to other group, fueling prejudice and xenophobia. A series of experiments showed that oxytocin administration creates intergroup bias because it motivates in-group favoritism and, in some cases, out-group derogation. These findings provide evidence for the idea that neurobiological mechanisms in general, and oxytocinergic systems in particular, evolved to sustain and facilitate within-group coordination and cooperation (De Dreu, Greer, Van Kleef, Shalvi, and Handgraaf 2011). The authors concluded that, rather than making humans prosocial, oxytocin functions to strengthen an evolved, functional tendency to discriminate between in-group and out-group as well as to give members of one’s own group preferential treatment. Thus, again oxytocin should not be construed of as a panacea for moral behavior. Indeed, it can facilitate just the opposite behavior.
While empathic concern is one of the earliest social emotional competencies that develop (Davidov et al. 2013), children do not display empathy and concern toward all people equally. Instead they show bias towards individuals and members of groups with which they identify. For instance children at two years of age display more empathy-related behaviors toward their mother than toward unfamiliar individual. In line with the in-group hypothesis, 8-year-old children were more likely to be emotionally reactive toward their in-group members compared with members of the out-group, and dispositional empathy (as well as social anxiety) was positively correlated with group identification (Masten, Gillen-O’Neel, and Brown 2010). Moreover, children (aged 3–9 years) view social categories as marking patterns of intrinsic interpersonal obligations; that is, they view people as intrinsically obligated only to their own group members, and consider within-group harm as wrong regardless of explicit rules, but they view the wrongness of between-group as contingent on the presence of such rules (Rhodes and Chalik 2013).
Straightforward predictions between empathic concern and morality are highly influenced by context. For instance, in one study, when individuals viewed the British and African nations as two separate races, they felt greater guilt over historic transgressions and had lesser expectations of forgiveness for atrocities committed than when they viewed individual nations as part of a greater whole (Morton and Postmes 2011). Moreover, in another study, upper-class individuals were more likely to make calculated, dispassionate moral judgments in dilemmas in which utilitarian choices were at odds with visceral moral intuitions (Côté, Piff, and Willer 2013). In this way, the lowered concern of upper-class individuals ironically led them to make moral decisions that were more likely to maximize the greatest good for the greatest number.
Further evidence from studies with adults suggests that while empathic concern does not necessarily change notions of fairness (e.g., what is the just action in a certain situation), it does change the decision an individual will make. In one such study (Batson, Klein, Highberger, and Shaw 1995), college students required to assign a good and bad task to two individuals overwhelmingly endorsed random assignment (i.e., a coin flip) as the most fair means for deciding who would be assigned with the bad task. However, when asked to consider the feelings of a worker who had recently suffered hardship, students readily offered the good task to the worker, rather than using random assignment.
All these behavioral, developmental, and functional neuroimaging studies clearly demonstrate that distinct components of empathy are influenced by many aspects of social categorizations. They are by-products of living in social groups and they shape in fundamental ways how people perceive their social environment, experience empathy, and behave prosocially toward others.
Empathy and decision-making
Empathy, whether in the form of affective sharing or empathic concern elicited by cues of vulnerability, can have important consequences for decision-making. For instance, people can be moved to help identifiable others. This preference for giving to single vivid individuals over less identifiable others has been called the “identifiable victim effect.” The identifiable victim effect resists explanation by normative economic models, since identifiable stimuli add no objective value or relevant information. In one series of experiments, participants’ greater willingness to help identified victims, relative to non-identified ones, was examined by varying the singularity of the victim (single vs. a group of eight individuals), and the availability of individually identifying information (the main difference being the inclusion of a picture in the “identified” versions) (Kogut and Ritov 2005). The results support the proposal that the “identified victim effect” is largely restricted to situations with a single victim: the identified single victim elicited considerably more contributions than the non-identified single victim, while the identification of the individual group members had essentially no effect on willingness to contribute. Importantly, participants also reported experiencing empathic distress when there is a single, identified victim more than in any other condition. Hence, the empathetic reaction to the victims appears to be a major source of the effect rather than an objective decisional process.
While the precise ways in which empathy contributes to moral judgment remain debated, in addition to influencing moral evaluation, it may also play an important developmental role. This may lead to the aversion of violent actions without necessarily empathizing with the victims of such actions (Miller, Hannikainen, and Cushman 2014). One paradigm often used in psychological and some neuroscience studies of moral judgment is a thought experiment borrowed from philosophy, the Trolley Dilemma, but the utility of this paradigm in assessing everyday moral judgment has recently been subject of great debate (Rosas and Koenigs 2014). This classic thought problem, comparing impersonal and personal moral decision-making has led to a great deal of inquiry about the nature of individuals who will push the large man in front of the trolley.
Are individuals who make utilitarian judgments in personal situations more rational and calculating, or are they simply colder and less averse to harming others? Support for a link between empathy and moral reasoning is given by studies demonstrating that low levels of dispositional empathic concern predict utilitarian moral judgment in some situations (Gleichgerrcht and Young 2013). A functional neuroimaging study recently examined the neural basis of such indifference to harming while participants were engaged in moral dilemmas (Wiech et al. 2013). A tendency towards counterintuitive impersonal utilitarian judgment was associated both with ‘psychoticism’ (or psychopathy), a trait linked with a lack of empathic concern and antisocial tendencies, and with ‘need for cognition’, a trait reflecting preference for effortful cognition. Importantly, only psychoticism was also negatively correlated with activation in the vmPFC during counterintuitive utilitarian judgments. These findings suggest that when individuals reach highly counterintuitive utilitarian conclusions, it does not need to reflect greater engagement in explicit moral deliberation. It may rather reveal a lack of empathic concern, and diminished aversion to harming others. Lesions of the orbitofrontal cortex (including the vmPFC) have been associated with increased utilitarian choices in highly conflicting moral dilemmas more often than control subjects, opting to sacrifice one person’s life to save a number of other individuals (Young and Dungan 2012).
Reason provides the leash to use empathy wisely
Across both popular press and academic research, the use of empathy has become pervasive to the point of cliché, possibly because of the generally admitted idea that empathy plays a central role in smooth social interaction and moral behavior. However, a critical analysis of the work in social neuroscience calls for a more cautious understanding of the functions of empathy in moral decision-making.
Empathy does play an important function in motivating caring for others and in guiding moral judgment in various forms, but this is far from being systematic or irrespective to the social identity of the targets, interpersonal relationships, and social context. Its role in shaping people’s understanding of why harming others is wrong and in producing the relevant motivation is, however, more limited than people think. This is because social forces that unite and divide groups affect empathy, moral reasoning, and prosocial behavior. Both behavioral and social neuroscience studies have reliably demonstrated that empathy (affective sharing and concern) is experienced more readily for in-group members, and found that people show diminished neural responses when witnessing an out-group relative to an in-group member in distress and emotional pain, or out-group derogation. The other side of the coin is that empathic concern, because it evolved to favor kin and members of one own social group, can bias decision-making by valuing one single individual over a group of others, and this can frontally conflict with fairness and justice; for instance, spending millions of dollars to develop a drug for a very limited number of patients suffering from an orphan disease at the expense of a much larger group of people in need. Similarly, empathy can impair our capacity for justice and consequentialist action. Empathic concern reduces our willingness to sacrifice individuals for the good of the many, as in the classic trolley moral dilemmas, or makes us respond more to humanitarian crises when there are individuals to identify with than when they impact tens of thousands of people. Cues of vulnerability, like baby faces, are powerful motivator of empathy and have evolved to facilitate parental care. The same cues in a criminal, however, may elicit lighter sentence from jurors. It may be disturbing to recognize that sometimes psychopaths “get it right” by making consequentialist judgments, to benefit the many at the expense of the few, precisely because they are less swayed by empathy (Paytas 2014).
Clearly, empathic reactions are inherently linked to partiality. And this partiality requires a framework of justice principles to counter its biasing effects and keep social allocation behaviors in check (Blader & Tyler, 2002). This idea is not new. John Stuart Mill (1875) warned us about people who may be amiable to those with whom they sympathize, and grossly unjust and unfeeling to the rest of the world.3
These parochial tendencies need to be rationally regulated and guided. This is especially important when one strives to uphold general principles of justice and fairness and it obviously has clear implications in social policy and law. For instance, in Sweden, a closely patrolled pro-immigration consensus has sustained extraordinarily liberal policies while placing a virtual taboo on questions about the social and economic costs. In neighboring Norway, however, a strong tradition of free speech and efficient administration has produced a hard-nose approach about which and how many refugees take in. The Norwegian Foreign Ministry has imposed a much stricter policy than Sweden’s, due to careful calculations of all the social, health, housing and welfare benefits mandated by the state. Those calculations indicated that supporting a single refuge would cost $120,000, which would be enough to support 26 Syrians in a Jordanian refugee camp (Eakin 2014). Therefore the Norwegian government chose to send money to support the Jordanian refugee camps rather than accepting immigrants.
Skeptics may argue that reason cannot lead us in directions that are good, just, or moral. It can deliver a roadmap to peace if the peace is the goal, but it can also provide a path to conflict if conflict is desired. Reason may help to convince the most selfish and skeptical of us to make sound decisions for himself as well as for the many. Everyone cares about his or her well-being, yet because we live in a global community we can interact and comprehend each other’s perspectives. For instance, if we ensure a sustainable system of energy and health care, providing a basic standard of living for everyone, then even in times of hardship, we will never be too badly off. If we guarantee that all people are educated, then that will lead to increased reasoning, science, and art, ultimately resulting in more chances to benefit from the harvest of the educated, well-fed minds we have helped to cultivate.
When reason and empathy work together
It has been argued that human rights, in the aftermath of the Second World War, were created as a social response to human suffering. While the concept of human rights can be explained by the need to protect vulnerable human beings, vulnerability is a condition, not the ground of human rights. Vulnerability only became relevant for human rights after it was assumed that every human being has intrinsic dignity. Thus, human rights result as a confluence of both factors: one normative (the recognition of intrinsic worthiness of every individual), and one factual (the observation that human beings are vulnerable, fragile, and exposed to suffering) (Andorno and Baffone 2014). We are indeed both capable of great empathy and generosity for the distress of members of both our own species and other animals, and indifferent or callous toward suffering of others. Empathy alone is powerless in the face of rationalization and denial. But reasoning and empathy can achieve great things.
Finally, acknowledging our evolved tendencies and biases for caring or intervene to help our relatives and less so for strangers does not mean that we should see ourselves as marionettes dancing on the strings of evolution, nor that what is found in nature is good4. Yes certain aspects of our behavior may be genetically guided, instilled by natural selection in our savanna-dwelling ancestors. But genes aren’t destiny. Genetic does not mean unchangeable. All sorts of environmental factors can affect the expression of genes. Likewise, we can use reason to curtail our inclination to categorize ourselves and others in terms of social group membership, whether this is based on race, tribe, SES, or nationality.
Thus empathy influences many facets of our social relations with others and is clearly an essential input into decision-making, but not necessarily for the best.
Acknowledgments
The writing of this article was supported by grants from the John Templeton Foundation (Wisdom Research at the University of Chicago) and from NIH (R01 MH087525; R01 MH084934).
Footnotes
Morality encompasses notions of justice, fairness, and rights, as well as maxims regarding interpersonal relations. Another theoretical view contends that morality includes the full array of psychological mechanisms that are active in the moral lives of people across cultures. Rather than stating the content of moral issues (e.g., justice and welfare), this definition specifies the function of moral systems as an interlocking sets of values, virtues, norms, practices, identities that work together to suppress or regulate selfishness and make cooperative social life possible. What seems clear is that, regardless of the definition, a central focus of morality is the judgment of the rightness or wrongness of acts or behaviors that knowingly cause harm to people.
Due to space constraint, we cannot elaborate on the validity of the resonance/mirror neurons account of social cognition and the unprecedented enthusiasm that has captivated so many scholars across disciplines. See Hickok (2014) for a debunking of all the grandiose claims that have been made on behalf of mirror neurons. Sophisticated analyses of fMRI data using multi-voxel pattern analysis show that while perception, execution, and imagination of simple actions such as grasping overlap in several cortical regions, patterns of activation within these commonly activated regions are actually distinct (Filimon et al., 2014).
Another example is John Rawls’s “veil of ignorance” as a method to defend against the motivational force of empathy for oneself or others by a procedure that minimizes the influences of one’s emotion (Rawls, 1971).
The naturalistic fallacy is the idea that what is found in nature is good. It was the basis for Social Darwinism, the belief that helping the poor and sick would get in the way of evolution. Scientists want to describe the natural world honestly, without people deriving morals about how we ought to behave -- as in: if animals engage in infanticide or cannibalism, it must be OK. There is absolutely no need to derive morals from evolution about how we ought to behave.
References
- Andorno R, Baffone C. Human rights and the moral obligation to alleviate suffering. In: Green RM, Palpant NJ, editors. Suffering and Bioethics. New York: Oxford University Press; 2014. pp. 182–200. [Google Scholar]
- Batson CD. These things called empathy: Eight related but distinct phenomena. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge: MIT Press; 2009. pp. 3–15. [Google Scholar]
- Batson CD. The empathy-altruism hypothesis: Issues and implications. In: Decety J, editor. Empathy: From Bench to Bedside. Cambridge: MIT Press; 2012. pp. 41–54. [Google Scholar]
- Batson CD, Klein TR, Highberger L, Shaw LL. Immorality from empathy-induced altruism: When compassion and justice conflict. Journal of Personality and Social Psychology. 1995;68(6):1042–1054. [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. eLife. 2014;3:e01385. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader SL, Tyler TR. Justice and empathy: What motivates people to help others? In: Ross M, Miller DT, editors. The Justice Motive in Everyday Life. Cambridge: Cambridge University Press; 2002. pp. 226–230. [Google Scholar]
- Cheng Y, Chen C, Decety J. An EEG/ERP investigation of the development of empathy in early and middle childhood. Developmental Cognitive Neuroscience. 2014;10:160–169. doi: 10.1016/j.dcn.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, Decety J. Love hurts: an fMRI study. Neuro Image. 2010;51(2):923–9. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Development and Psychopathology. 2012;24(2):623–36. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Cikara M, Botvinick MM, Fiske ST. Us versus them: social identity shapes neural responses to intergroup competition and harm. Psychological Science. 2011;22(3):306–13. doi: 10.1177/0956797610397667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Van Bavel JJ. The neuroscience of intergroup relations: An integrative review. Perspectives on Psychological Science. 2014;9(3):245–274. doi: 10.1177/1745691614527464. [DOI] [PubMed] [Google Scholar]
- Cohen FS. Transcendental nonsense and the functional approach. Columbia Law Review. 1935;35(6):809–849. [Google Scholar]
- Coplan A. Understanding empathy: Its features and effects. In: Coplan A, Goldie P, editors. Empathy - Philosophical Perspectives. New York: Oxford University Press; 2011. pp. 3–44. [Google Scholar]
- Côté S, Piff PK, Willer R. For whom do the ends justify the means? Social class and utilitarian moral judgment. Journal of Personality and Social Psychology. 2013;104(3):490–503. doi: 10.1037/a0030931. [DOI] [PubMed] [Google Scholar]
- Davidov M, Zahn-Waxler C, Roth-Hanania R, Knafo A. Concern for others in the first year of life: Theory, evidence, and avenues for research. Child Development Perspectives. 2013;7(2):126–131. [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3(1):92–108. [Google Scholar]
- Decety J, Echols S, Correll J. The Blame Game: The effect of responsibility and social stigma on empathy for pain. Journal of Cognitive Neuroscience. 2009;22(5):985–997. doi: 10.1162/jocn.2009.21266. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13(6):886–99. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Norman GJ, Berntson GG, Cacioppo JT. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Progress in Neurobiology. 2012;98(1):38–48. doi: 10.1016/j.pneurobio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Decety J, Yoder KJ. Empathy and motivation for justice: Cognitive empathy and concern, but not emotional empathy predicts sensitivity to injustice for others. Social Neuroscience. 2015 doi: 10.1080/17470919.2015.1029593. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin H. Syrian refugees, Nordic dilemma. International New York Times. 2014 Sep 20–21;:8. [Google Scholar]
- Edgar JL, Nicol CJ, Clark CCa, Paul ES. Measuring empathic responses in animals. Applied Animal Behaviour Science. 2012;138(3–4):182–193. [Google Scholar]
- Fox GR, Sobhani M, Aziz-Zadeh L. Witnessing hateful people in pain modulates brain activity in regions associated with physical pain and reward. Frontiers in Psychology. 2013;4(October):772. doi: 10.3389/fpsyg.2013.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RG, Nelson AJ, Baker M, Beeney JE, Vescio TK, Lenz-Watson A, Adams RB. Neural responses to perceiving suffering in humans and animals. Social Neuroscience. 2013;8(3):217–27. doi: 10.1080/17470919.2013.763852. [DOI] [PubMed] [Google Scholar]
- Galinsky AD, Moskowitz GB. Perspective-taking: Decreasing stereotype expression, stereotype accessibility, and in-group favoritism. Journal of Personality and Social Psychology. 2001;78(4):708–724. doi: 10.1037//0022-3514.78.4.708. [DOI] [PubMed] [Google Scholar]
- Genevsky A, Västfjäll D, Slovic P, Knutson B. Neural underpinnings of the identifiable victim effect: affect shifts preferences for giving. The Journal of Neuroscience. 2013;33(43):17188–96. doi: 10.1523/JNEUROSCI.2348-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilin D, Maddux WW, Carpenter J, Galinsky AD. When to use your head and when to use your heart: the differential value of perspective-taking versus empathy in competitive interactions. Personality & Social Psychology Bulletin. 2013;39(1):3–16. doi: 10.1177/0146167212465320. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Young L. Low levels of empathic concern predict utilitarian moral judgment. PloS One. 2013;8(4):e60418. doi: 10.1371/journal.pone.0060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Konrath S, Brown SL, Swain JE. Empathy and stress related neural responses in maternal decision making. Frontiers in Neuroscience. 2014;8(June):152. doi: 10.3389/fnins.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience. 2010;13(4):482–8. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamichi H, Tanabe HC, Takahashi HK, Sadato N. Activation of the reward system during sympathetic concern is mediated by two types of empathy in a familiarity-dependent manner. Social Neuroscience. 2013;8(1):90–100. doi: 10.1080/17470919.2012.744349. [DOI] [PubMed] [Google Scholar]
- Kidd DC, Castano E. Reading literary fiction improves theory of mind. Science. 2013;342(6156):377–80. doi: 10.1126/science.1239918. [DOI] [PubMed] [Google Scholar]
- Kogut T, Ritov I. The “identified victim” effect: an identified group, or just a single individual? Journal of Behavioral Decision Making. 2005;18(3):157–167. [Google Scholar]
- Kraus MW, Côté S, Keltner D. Social class, contextualism, and empathic accuracy. Psychological Science. 2010;21(11):1716–23. doi: 10.1177/0956797610387613. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuro Image. 2011;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2009;22(2):362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, Sternberg WF. Social approach to pain in laboratory mice. Social Neuroscience. 2010;5(2):163–70. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Liyanage S. Long-term effects of peace workshops in protracted conflicts. Journal of Conflict Resolution. 2005;49(6):908–924. [Google Scholar]
- Masten CL, Gillen-O’Neel C, Brown CS. Children’s intergroup empathic processing: the roles of novel ingroup identification, situational distress, and social anxiety. Journal of Experimental Child Psychology. 2010;106(2–3):115–28. doi: 10.1016/j.jecp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Kinzler KD, Decety J. Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Developmental Cognitive Neuroscience. 2013;3:22–32. doi: 10.1016/j.dcn.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill JS. Dissertations and Discussions. London: Longmans, Green, Reader and Dyer; 1875. [Google Scholar]
- Miller RM, Hannikainen IA, Cushman FA. Bad actions or bad outcomes? Differentiating affective contributions to the moral condemnation of harm. Emotion. 2014;14(3):573–87. doi: 10.1037/a0035361. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Gapp J, Wang B, Louis WR, Decety J. Increased moral sensitivity for outgroup perpetrators harming ingroup members. Cerebral Cortex (New York, NY: 1991) 2014:1–9. doi: 10.1093/cercor/bhu195. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;(13):14–16. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalan B, Lelard T, Godefroy O, Mouras H. Behavioral investigation of the influence of social categorization on empathy for pain: a minimal group paradigm study. Frontiers in Psychology. 2012;3(October):389. doi: 10.3389/fpsyg.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuro Image. 2007;37(2):642–51. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Morton TA, Postmes TOM. Moral duty or moral defence? The effects of perceiving shared humanity with the victims of ingroup perpetrated harm. European Journal of Social Psychology. 2011;134:127–134. [Google Scholar]
- Myers MW, Laurent SM, Hodges SD. Perspective taking instructions and self-other overlap: Different motives for helping. Motivation and Emotion. 2013;38(2):224–234. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Panksepp J. Affective neuroscience: the foundations of human and animal emotions. London: Oxford University Press; 1998. [Google Scholar]
- Paytas T. Sometimes psychopaths get it right: A utilitarian response to “the mismeasure of morals. Utilitas. 2014;26(02):178–191. [Google Scholar]
- Piff PK, Kraus MW, Côté S, Cheng BH, Keltner D. Having less, giving more: the influence of social class on prosocial behavior. Journal of Personality and Social Psychology. 2010;99(5):771–84. doi: 10.1037/a0020092. [DOI] [PubMed] [Google Scholar]
- Preston SD. The origins of altruism in offspring care. Psychological Bulletin. 2013;139(6):1305–41. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- Rhodes M, Chalik L. Social categories as markers of intrinsic interpersonal obligations. Psychological Science. 2013;24(6):999–1006. doi: 10.1177/0956797612466267. [DOI] [PubMed] [Google Scholar]
- Rawls J. A Theory of Justice. Cambridge: Harvard University Press; 1971. [Google Scholar]
- Rosas A, Koenigs M. Beyond “utilitarianism”: Maximizing the clinical impact of moral judgment research. Social Neuroscience. 2014;9(6):661–7. doi: 10.1080/17470919.2014.937506. [DOI] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage. 2011;54(2):1743–54. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage. 2012;61(4):786–97. doi: 10.1016/j.neuroimage.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Smith AM, Loving TJ, Crockett EE, Campbell L. What’s closeness got to do with it? Men’s and women’s cortisol responses when providing and receiving support. Psychosomatic Medicine. 2009;71(8):843–51. doi: 10.1097/PSY.0b013e3181b492e6. [DOI] [PubMed] [Google Scholar]
- Smith KE, Porges EC, Norman GJ, Connelly JJ, Decety J. Oxytocin receptor gene variation predicts empathic concern and autonomic arousal while perceiving harm to others. Social Neuroscience. 2014;9(1):1–9. doi: 10.1080/17470919.2013.863223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kahane G, Shackel N, Farias M, Savulescu J, Tracey I. Cold or calculating? Reduced activity in the subgenual cingulate cortex reflects decreased emotional aversion to harming in counterintuitive utilitarian judgment. Cognition. 2013;126(3):364–72. doi: 10.1016/j.cognition.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience. 2009;29(26):8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J. Spatiotemporal neural dynamics of moral judgment: a high-density ERP study. Neuropsychologia. 2014a;60:39–45. doi: 10.1016/j.neuropsychologia.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J. The Good, the bad, and the just: justice sensitivity predicts neural response during moral evaluation of actions performed by others. The Journal of Neuroscience. 2014b;34(12):4161–6. doi: 10.1523/JNEUROSCI.4648-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dungan J. Where in the brain is morality? Everywhere and maybe nowhere. Social Neuroscience. 2012;7(1):1–10. doi: 10.1080/17470919.2011.569146. [DOI] [PubMed] [Google Scholar]
- Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556. [DOI] [PMC free article] [PubMed] [Google Scholar]