Abstract
The androgen receptor (AR) mediates the majority of androgen effects on target cells. The DNA cis-regulatory elements that respond to AR share sequence similarity with cis-regulatory elements for glucocorticoid, mineralocorticoid and progesterone receptors (GR, MR and PR respectively). As a result, many of the current AR screening models are complicated by inaccurate activation of reporters by one of these receptor pathways. Identification of more selective androgen testing systems would be beneficial for clinical, pharmacological and toxicologic screening of AR activators. The present study describes the development of a selective androgen-responsive reporter cell line that expresses AR but does not express GR, MR and PR. CV1 cells were stably transduced to express human AR and an androgen-responsive gaussia luciferase gene. Clonal populations of AR expressing cells were isolated. Quantitative RT-PCR (qPCR) and western analysis confirmed stable integration of AR in the most responsive clonal line which was named ‘CV1-ARluc’. Stimulation of CV1AR-luc with androgenic ligands (testosterone and 5α-dihydrotestosterone) for 18 h caused an increase in luciferase activity in a dose-dependent manner. Other steroid hormones including aldosterone, cortisol, and progesterone did not stimulate luciferase response. The CV1-ARluc also increased luciferase activity when treated with human serum extracts. In conclusion, the CV1-ARluc cells provide a novel model system for screening of new AR agonists and antagonists and can determine the androgenic activity of human serum samples.
Keywords: Androgen activity, luciferase, stable in vitro bioassay, human serum, AR selectivity
1. INTRODUCTION
Androgens are hormones that play an essential role in the differentiation and maintenance of primary and secondary male sexual characteristics[1]. The two main human androgens are testosterone (T), which is involved in the initial virilization phases of the human male embryo, and 5α-dihydrotestosterone (DHT), which is the active hormone in most androgen target tissues [2]. T is mainly synthesized by the testicular Leydig cells, in peripheral tissues, as well as to a lesser degree in ovaries and adrenals. T is converted to DHT by 5α-reductases and also can be converted to estradiol by aromatase. DHT is the most active physiologic androgen, inducing tenfold higher androgen receptor (AR, NR3C4) bioactivity than T [3, 4]. In addition, other endogenously produced steroids exhibit various degrees of androgenic activity [5, 6]. Several synthetic androgen-related compounds (AR agonists and antagonists) have also been developed to modulate androgen signaling in therapeutic settings [7, 8].
Androgens mediate their effects through binding and activation of the AR. AR is a member of the steroid nuclear receptor superfamily [9] and acts as a ligand-dependent transcription factor [10]. Among this family, five steroid receptors are known: estrogen (ESR, NR3A1), progesterone (PR, NR2C3), androgen, mineralocorticoid (MR, NR3C2) and glucocorticoid (GR, NR3C1) receptors. AR activates a wide range of target genes that encode proteins and noncoding RNAs, including regulatory microRNA species [11].
Similar to the other steroid receptors, unbound AR is located in the cytoplasm. Upon ligand binding, AR goes through a series of conformational changes, dimerization and translocation to the nucleus, which is mediated by a nuclear localization signal. Translocated AR binds to androgen response elements (ARE). These ARE are characterized by a consensus (or near consensus) sequence 5′-TGTTCT-3′, which is located in the promoter or enhancer regions of AR gene targets. The DNA cis-regulatory elements that respond to AR share sequence similarity with cis-regulatory elements for GR, MR and PR. The similarity of the response element for AR and the other steroid receptors, and particularly the wide-spread expression of the GR, has been problematic in the development of selective receptor screening assays.
The determination of androgen levels or the discoveries of new androgenic compounds are key elements for the diagnosis of a number of diseases in children and adults. Assays that detect bioactive serum androgens in a sensitive and selective manner benefit the diagnosis and treatment of several pediatric endocrine disorders, such as precocious puberty and ambiguous genitalia. In addition, androgen bioassays provide a screening tool for androgen abuse and endocrine disruptors [12]. Over the past 10 years, several bioassays were developed using different methods [13]. One of the first assays developed relied on a chloramphenicol acetyltransferase (CAT) reporter model [14]. This system was limited by experimental variation due to the transient nature of transgene expression. A luciferase reporter bioassay, using MDA-MB453 cells, was developed by Wilson et al [15]. The major caveat of this assay was that it responds to AR as well as to GR agonists. Other androgen-reporter cell lines were developed but most of them were transiently transfected [16–18]. Transient transfection assays [19] can provide similar information with stable assays but may not reflect endogenous levels of receptor. A stable expression of AR in the cells can eliminate the need for repetitious transient transfections, reduce the variability associated with these transient assays and moreover be utilized for high-throughput studies. Until now, a selective androgen-responsive transcriptional activation assay has not been widely available.
The aim of this study was to develop a stable cell-based in vitro bioassay that expresses the human AR (hAR) gene with sensitive and selective reporter readout. For this purpose, a stable cell line was made with CV1 cells stably transduced with hAR and an MMTV promoter-driven luciferase reporter gene. The resulting model is selective for androgens and does not exhibit reporter activation by other steroid receptors. In addition the model appears useful to determine circulating androgenic bioactivity in human serum samples.
2. MATERIALS AND METHODS
2.1 Materials
T, DHT, cortisol (Cort), progesterone (Prog), aldosterone (Aldo), androstenedione (AD4), hydroxyflutamide (OHF) and the 11-keto and 11-hydroxy forms of androstenedione and T were purchased from Sigma (Missouri, USA). Coelenterazine used for the luciferase assay was purchased from Promega (Wisconsin, USA). Penicillin, streptomycin, hygromycin, geneticin (G418) and DMEM/F12 medium were purchased from Life technologies (New York, USA).
2.2 Cell line
The CV1 monkey kidney cell line was obtained from the American Type Culture Collection (ATCC). The cells were grown in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) from GE Healthcare Life Science (Utah, USA) and antibiotics including 1% penicillin/streptomycin. The cells were incubated under a humid atmosphere of 5% CO2, at 37°C, and the medium was changed every 3 days. CV1 cells were plated at a density of 20,000 cells/well (48 well dish) in growth medium and grown to 60% confluence after which they were treated for steroids activity.
CV1-ARluc cells were plated in a 48 wells culture plate in 500 μl of growth medium (10% FBS/DMEM-F12, G418 and Hygromycin). The cells were incubated under a humid atmosphere of 5% CO2, at 37°C. All treatments were performed with charcoal-stripped FBS serum to eliminate contaminating steroids.
2.3 Stable Transduction
CV1 cells (20,000 cells/well) were plated about 18 h before transduction in a 48 well-dish. The lentivirus pBM14-MMTV with the Gaussia Luciferase gene was diluted 1:10 in DMEM/F12 medium and added to the flask with 8 μg/mL of polybrene. The flask was centrifuged at 1200 rpm for 80 min and after 4 h in a humidified 5% CO2 incubator the cells were supplemented with 1 ml of DMEM/F12 containing 10% FBS without any antibiotics. After 48 h, the cells were selected in medium containing 1200 μg/ml of G418. The medium was changed three times a week. The obtained cells, named CV1-luc, were transduced with a lentivirus containing the hAR gene and the hygromycin selective gene. The stable transfection was performed as described above, using a multiplicity of infection (MOI) of 10 and 8 μg/mL of polybrene. 50 clones were obtained after 14 days of dual antibiotic (G418 and Hygromycin) selection. The clones were isolated using cloning rings (Sigma, Missouri, USA) and re-seeded and grown in a 48-well dish. After reaching 60% confluence, the cells were treated in DMEM/F12 containing 10% charcoal-stripped FBS and 10 nM of testosterone. After 24 h the treated cells were assayed for luciferase activity using the appropriate luminescence kit (Coelenterazine, Promega). The clone with the largest T induced luciferase activity was named CV1-ARluc and was used for further studies.
2.4 Isolation of RNA and qPCR analysis
The cells (25.000 cells/well) were grown for 24 h in 48 well culture. Total RNA was isolated from the cells previously plated using an RNeasy plus mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The quantity and purity were assessed by a ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). For cDNA generation, 100 ng of total RNA was reverse transcribed using the High Capacity Kit (Applied Biosystems, Foster City, CA, USA). For qPCR, 12 ng of prepared cDNA was mixed with Fast Universal PCR Master Mix (Applied Biosystems). AR and peptidylprolyn isomerase A (PPIA) primers were purchased from Integrated DNA Technologies (Coralville, IA, USA). PPIA was used as the housekeeping control gene.
2.5 Protein extraction and protein assay
Cells were lysed in 200 μL Mammalian Protein Extraction Reagent (Pierce Chemical Co., Illinois, USA), and the protein content was estimated by the bicinchoninic acid (BCA) protein assay using the BCA protocol (Thermo Scientific, Illinois, USA).
2.6 Western analysis
CV1 and CV1-ARluc cell lines were plated at a density of 75,000 cells/well (24 well-dish), in growth medium. Samples were lysed with lysis buffer (2% sodium dodecyl phosphate, 62.5 μM Tris, 0.04% bromophenol blue, 0.5 % dithiothreitol) and heated at 95 °C for 5 min. Proteins were then loaded (20 μg) on 10% Bis-Tris gel and electrophoresed for 1 h before transferring to polyvinylidene difluoride membranes. The membranes were then blocked with 5% BSA for 1 h and incubated with primary antibody (AR, polyclonal rabbit antihuman, 1:1000 BSA, Sigma) and secondary antibody (goat anti-rabbit, 1:5000, Life Technologies).The Pierce ECL Western Blotting Substrate kit (Life technologies) was then used for signal development.
2.7 AR translocation study
CV1-ARluc cells were grown on microscope slides from Globe Scientific (previously treated with 50 μg/ml of Poly D-Lysine at room temperature for 1 h) in 100-mm plates for 24 h in growth medium. Cells were treated with DHT for 18 h and subsequently fixed with methanol at −20 °C for 20 minutes and washed three times with PBS. Slides were then incubated overnight with a rabbit anti-human AR antibody (Sigma) and then with a secondary goat anti-rabbit antibody (Sigma) for 1h at room temperature. Prolong Gold mounting medium with DAPI was used to visualize the cell nucleus.
2.8 Gaussia Luciferase analysis
CV1 and CV1-ARluc cells were plated at a density of 25,000 cells/well (48 well-dish), in growth medium for 24 h and then treated for indicated time points. The treated medium was collected and 25 μL was mixed with 50 μL of coelenterazine (previously diluted 1:100 in 50 mM Tris, 150 mM NaCl and water). Luminescence was then measured by FLUOstar OPTIMA microplate reader according to the manufacturer’s instructions (Life Technologies).
2.9 Sera
Charcoal dextran stripped human serum was obtained by Equitech-Bio. In the present study, we analyzed human serum from 20 healthy adults (10 females and 10 males), age 20–35 years. All samples were collected under protocols approved by the Institutional Review Board (IRB) at the University of Michigan.
2.10 Extraction method
The indicated concentrations of steroids were prepared separately in DMEM/F12 medium with10% charcoal-stripped FBS, and stripped human serum using ethanol as a carrier solvent. Several extraction methods were tested and were found to have variable abilities to disrupt androgen regulation of reporter activity (data not shown). The method that exhibited androgenic activity most similar to unextracted medium was solid phase extraction using Sep-Pak Cartridges column from Waters (Chromatography division Millipore Corporation, MA, USA). The columns were activated with 4 ml of methanol and subsequently washed with 4 ml of deionized water. Standards made separately in DMEM/F12 with 10% charcoal stripped FBS and stripped human serum were dispensed at a volume of 600 μl in the columns, followed by another wash and elution with 2 ml of 100% methanol. Samples were evaporated at 37°C using a thin stream of nitrogen gas and the dried extract was then re-suspended in 300 μl of DMEM/F12 medium with 10% charcoal stripped FBS.
3. RESULTS
3.1. AR expression in transduced CV1 cell line
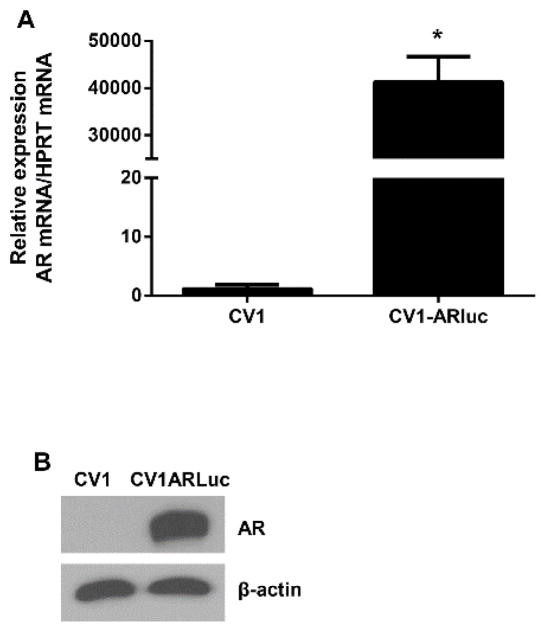
A double transduction with the lentiviral hAR and Gaussia luciferase constructs was used to obtain a CV1 cell line stably expressing the hAR and the androgen responsive gaussia luciferase gene. A total of 48 geneticin and hygromycin resistant clones were obtained. Out of the androgen responsive clones, to the most responsive to testosterone exhibited an 80-fold induction of reporter gene activity (data not shown). All experiments were conducted using this clone, CV1-ARluc. The untransduced parent CV1 and the transduced CV1-ARluc cell lines were initially tested for human AR expression by qRT-PCR analysis and western blot (Fig. 1). We demonstrate that the CV1-ARluc cell line expresses high levels of AR, whereas AR was found to be absent in the parent CV1 cell line.
Fig. 1. AR expression in transduced and wild type CV1 cells.
CV1 and CV1-ARluc cells were maintained in 10% FBS medium for 24 h and then lysed. (A) RT-PCR analysis of AR expression was tested in both cell lines. AR expression in CV1-ARluc was significantly higher than the CV1 cells. (B) Western analysis of AR was performed on 20 μg of total protein. β-actin was used as a loading control. The untransfected cell line did not show detectable AR, indicating the absence of this protein in this cell line. Figures are representative of three independent experiments with similar results.
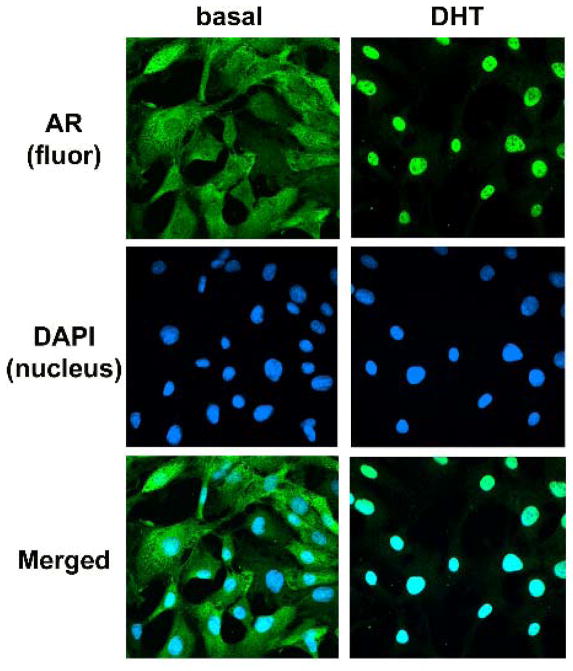
To further characterize the cell lines, we analyzed the cytoplasmic and nuclear expression of AR in CV1-ARluc by immunofluorescence (Fig. 2). Since ligand steroid receptors undergo nuclear localization, we traced the fluorescence for AR before and after treatment with DHT, a potent androgen. In the absence of the ligand, AR staining in the CV1-ARluc cells was predominantly located in the cytoplasm. Upon addition of 10 nM DHT for 18 h, 100% of the cells stained positive in the nucleus (Fig. 2). The observed translocation of AR from the cytoplasm to the nucleus in treated cells but not in untreated cells confirmed the specificity of the signal.
Fig. 2. DHT binding causes AR nuclear translocation.
Fluorescence microscopy of CV1-ARluc cells stably transfected with a hAR and treated with or without 10 nM DHT for 18 h. Green fluorescence represents AR immunoreactivity and blue fluorescence is DAPI (nucleus).
3.2 Sensitivity of CV1-ARluc cell line
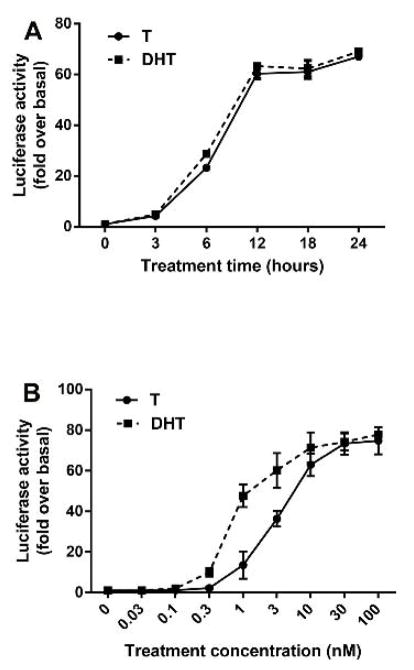
As shown in Fig. 3 A, the luciferase activity, after treatment with testosterone or DHT (10 nM) was clearly detectable at 3 h and increased progressively when the incubation with the respective ligands was continued up to 24 h. This result suggests that the assay can be used for steroids with androgenic activity even for short exposures.
Fig. 3. Time course for AR activation and CV1-ARluc response to known androgens.
(A) The cells were incubated with testosterone and DHT 10 nM for 0–24 h. The results are expressed as fold increase over basal of luciferase activity. The results represent the mean ± S.E. of similar experiments performed in triplicate. (B) CV1-ARluc cells were incubated with increasing concentrations of two known androgens: Testosterone and DHT. The values represent the mean ± S.E. of similar experiments performed in triplicate.
In response to increasing concentrations of T and DHT, there was a sigmoidal increase in reporter gene expression (Fig. 3 B). The first significant response of the reporter gene expression to the androgens was detected at 0.1 nM of DHT and 0.3 nM of T (Fig. 3 B). The lower concentrations of steroids being able to stimulate a significant increase in luciferase expression indicate the high sensitivity of the cells.
3.3. Selectivity of CV1-ARluc cell line to other steroids
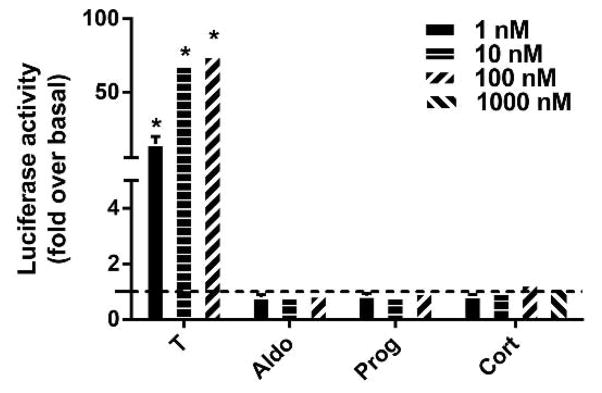
Since some members of the nuclear steroid receptors exhibit cross-talk activity via binding of other steroid hormones to their ligand binding domain, we examined the specificity of CV1-ARluc for androgens. This was done by incubating the CV1-ARluc cells with cortisol, progesterone and aldosterone at increasing concentrations. In addition, to insure that GR activity was not present in this model and to determine if high concentrations of cortisol regulate the AR, we incubated cells with concentrations of cortisol up to 1000 nM. As shown in Fig. 4, none of these steroids showed any significant response suggesting a high selectivity of the CV1-ARluc system towards androgenic steroids.
Fig. 4. Reporter gene regulation in response to androgenic and non-androgenic steroids.
CV1-ARluc cells were incubated with 1, 10 and 100 nM of aldosterone and progesterone and 1, 10, 100 and 1000 nM of cortisol. The dotted line represents basal conditions. The data represent the mean ± S.E. of three independent experiments, each performed in triplicate (*p < 0.05 versus basal).
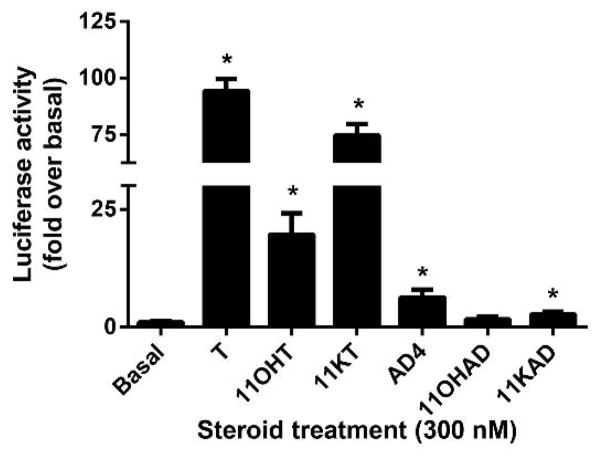
3.4. Treatment of CV1-ARluc cell line with different C19 steroids
Several studies suggested that these C19 steroids provide a pool of circulating precursors for peripheral conversion to more active androgens. The adrenal glands secrete a variety of C19 steroids including androstenedione and the 11-keto and 11-hydroxy forms of androstenedione and T. To better analyze the sensitivity of the CV1-ARluc to weaker different C19 steroids, the cell line was treated with T, 11-hydroxytestosterone (11OHT), 11-ketotestosterone (11KT), AD4, 11-hydroxyandrostenedione (11OHAD), 11-ketoandrostenedione (11KAD) at a constant dose of 300 nM. As showed in Fig. 5, the luciferase activity was increased with treatment with all of the previously mentioned steroids with the exception of 11OHAD.
Fig. 5. CV1-ARluc cell line treatment with different C19 steroids.
CV1-ARluc cells were incubated with 300 nM of T, 11OHT, 11KT, AD4, 11OHAD and 11KAD. The data represent the mean ± S.E. of three independent experiments performed in triplicate (*p < 0.05 versus basal).
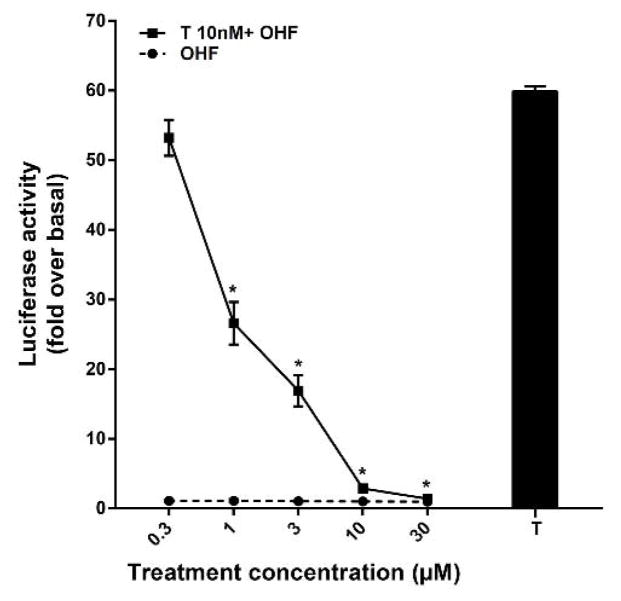
3.5. Effects of a potent anti-androgens on CV1-ARluc
The androgen specificity was also demonstrated by the ability of hydroxyflutamide (OHF), a non-steroidal anti-androgen, to suppress the activity of T (Fig. 6). The effects of OHF were tested in the CV1-ARluc cells. CV1-ARluc cells were treated with increasing concentrations of OHF, in the presence and absence of the maximally stimulating concentration of T (10 nM). As shown in Fig. 6, OHF alone did not show any significant agonistic activity. However, OHF inhibited T activity in a concentration dependent manner with a significant repression seen for 1–30 μM. The response to testosterone was completely suppressed by 30 μM OHF.
Fig. 6. Antagonistic activity of hydroxyflutamide in CV1-ARluc.
Cells were incubated with 10 nM T alone as well as increasing concentration of OHF alone or in the presence of 10 nM testosterone. The values represent the mean ± S.E. of similar experiments performed in triplicate (*p < 0.05 versus 10 nM T alone).
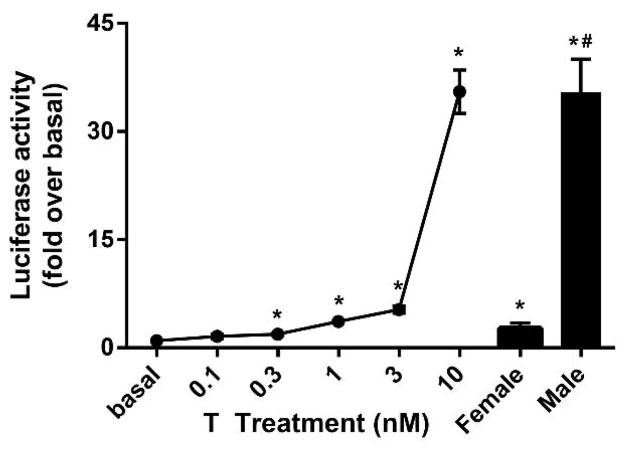
3.6. Serum androgen bioactivity
To examine the androgen activity in human serum, the experiment was performed on extracted samples processed with solid phase extraction. In Fig. 7, a dose response curve was performed using charcoal stripped human serum prepared with different doses of T. Serum androgen bioactivity was also determined from 10 male and female serum samples between ages 20–40 years. These bioassay values indicate the androgen activity in these samples. The range value of the active androgen in female and male were equivalent to 0.9 nM and 15 nM of T, respectively. These data demonstrate that the androgen activity in serum samples can be measured with CV1-ARluc cell line.
Fig. 7. Comparison of androgen bioactivity levels in human serum samples.
Testosterone (0.3–30 nM) and human serum extract (10 male and 10 female) effects on reporter activity. Cells were treated with extracted samples for 18 h. The experiment was performed in triplicate (*p < 0.05 versus basal and #p < 0.05 versus female).
4. DISCUSSION
We have developed an in vitro assay that is simple, rapid, and quantitative with potential to screen active androgenic compounds including those in human serum. The CV1-ARluc cell line expresses an androgen-responsive luciferase reporter gene and a human AR gene. The CV1-ARluc cell line combines high stability, fast growth, high selectivity, high sensitivity and rapid response to androgens.
Cell-based assays can provide several benefits compared with immunoassay, mass spectrometry and various chromatography methods [3]. Over the past several years, there has been an expansion in the use of gas chromatography-mass and liquid chromatography tandem mass spectrometry for measurements of androgens. These methods are able to analyze multiple steroid hormones but they are not useful in defining the activity of unknown androgenic steroids. Cell-based hormone receptor assays have become an important resource for drug discovery and androgen related diseases. The cells used to develop these assays should have two specific requirements: a reporter system driven by an ARE and an abundant expression of AR. A variety of reporter genes have been used in the past years including β-lactamase [20], β-galactosidase [21–26] and luciferase [15, 18, 27–32] reporter genes. To develop an androgen reporter line superior to the ones available, we chose not to use yeast cells for several reasons. Although yeast-based reporter models [22], have certain advantages (easy handling, rapid growth, inexpensive media components), they require laborious cell preparation and complex cell lysis steps. Importantly, using yeast assays to express mammalian proteins also raises concerns regarding glycosylation, phosphorylation and post translational modifications. Moreover, the estimation of androgenic bioactivity in serum with yeast-based assays has been unsuccessful. In yeast cell lines, anti-androgens have not shown any antagonistic effects, probably due the permeability problem of the cell walls.
To avoid interference with other nuclear receptors, we selected a cell line with a low background activity of C3 group nuclear receptors and with a good response to androgens when the hAR was stably introduced. We chose to use an MMTV promoter-driven luciferase gene since this approach has been shown to be successful in generation of in vitro and in vivo models for screening of estrogen compounds [33]. With the present work we show that, this approach can be successfully used to generate a selective androgen reporter cell line, CV1-ARluc. The MMTV promoter is quite selective to AR as well as to GR and PR and, also, contains a number of regulatory sites that can be targeted by other steroids. Wilson et al [34] developed a stable cell line, MDA-kb2, a derivative of a human breast cancer cell line, containing a stably integrated MMTV-luciferase reporter. This cell line strongly responds to glucocorticoids due to endogenous GR. This makes the cell line unsuitable as a selective screening tool. PC3 cells transfected by Kim et al [18], show more androgen specificity obtained by transient transfection of an MMTV promoter and a human AR with high sensitivity. Other bioassays for screening of androgen compounds in human serum have been reported using transient transfection; we have used a stable transduction to develop our cell model because stable expression of AR can eliminate the variability associated with repeated transient transfection. A different cell line, using CHO cells and a stable transfection, was developed by Paris et al [4]. The cell line was stably transfected with hAR but has retained glucocorticoid response due to GR. The T47D cell line was used by Blankvoort et al [31] to develop a new androgen cell-based assay named AR-LUX. T47D expresses an endogenous AR and was stably transfected with a luciferase reporter gene. This assay was used to estimate the levels of some anabolic steroids in the urine of cattle. However, this cell line also responds to added estrogen and progesterone, which may reduce the specificity of the assay.
There is one additional AR bioassay that is highly selective for androgens and there is no reporter response to non-androgenic steroids [20]. Wilkinson et al developed an AR bioassays using the HEK293 cell line. This model is particularly applicable for high throughput screening for AR activation. However, this model makes use of a hybrid AR receptor (GAL4DBD-ARLBD) and therefore may not have all regulatory characteristics of the full length AR.
In the present study, we have developed a new cell line, named CV1-ARluc, which stably express the human AR and an androgen-driven gaussia luciferase reporter. The cell line is able to estimate levels of androgen bioactivity in human serum samples. The androgen specificity of the assay was tested by using high concentrations of different steroids such as cortisol, progesterone and aldosterone, which did not active reporter expression. The low background activity of C3 group nuclear receptors in the CV1 cell line was supported by RT-PCR data, where no GR, ER, AR, PR and MR expression was detected. The specificity of the assay was also showed by checking the inhibitory effects of a synthetic anti-androgen receptor ligand, OHF. OHF anti-androgen effects were also seen in other AR models including CHO [35], DU-145 [36] and PALM [37]. We evaluated androgenic bioactivity in male and female serum samples. In concordance with the data previously reported using a different in vitro assay to detect androgen activity in human serum samples by Roy et al [38], our assay shows an androgen bioactivity in female serum samples from 0.8 to 2 nM and from 10 to 25 nM in normal males. It can be concluded that this assay provides a valid and practical method to analyze serum samples for androgenic activity. The assay can be used to evaluate the physiological androgen levels during androgen-related diseases.
HIGHLIGHTS.
We have developed a new cell line, named CV1-ARluc, which stably express the human AR and an androgen-driven gaussia luciferase reporter.
The androgen specificity of the assay was tested by using high concentrations of different steroids such as cortisol, progesterone and aldosterone, which did not active reporter expression.
The transactivation assay developed with the help of the cell line is highly sensitive as it detects androgen activity at 0.1 nM of DHT and 0.3 nM of T.
This assay provides a valid and practical method to analyze male and female serum samples for androgenic activity.
Acknowledgments
This work was supported by the National Institute of Health (Grant DK069950 to W.E.R).
Abbreviations
- AR
androgen receptor protein
- ARE
androgen response element
- GR
glucocorticoid receptor protein
- MR
mineralocorticoid receptor protein
- PR
progesterone receptor protein
- T
testosterone
- DHT
5α-dihydrotestosterone
- Cort
cortisol
- AD4
androstenedione
- Prog
progesterone
- 11OHT
11-hydroxytestosterone
- 11KT
11-ketotestosterone
- 11OHAD
11-hydroxyandrostenedione
- 11KAD
11-ketoandrostenedione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chemical reviews. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiener JS, Teague JL, Roth DR, Gonzales ET, Jr, Lamb DJ. Molecular biology and function of the androgen receptor in genital development. The Journal of urology. 1997;157:1377–1386. [PubMed] [Google Scholar]
- 3.Raivio T, Tapanainen JS, Kunelius P, Janne OA. Serum androgen bioactivity during 5alpha-dihydrotestosterone treatment in elderly men. Journal of andrology. 2002;23:919–921. [PubMed] [Google Scholar]
- 4.Paris F, Servant N, Terouanne B, Sultan C. Evaluation of androgenic bioactivity in human serum by recombinant cell line: preliminary results. Molecular and cellular endocrinology. 2002;198:123–129. doi: 10.1016/s0303-7207(02)00375-1. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell F. Reproductive endocrinology: Testosterone metabolite nonessential for androgen effects. Nature reviews. Endocrinology. 2012;8:256. doi: 10.1038/nrendo.2012.37. [DOI] [PubMed] [Google Scholar]
- 6.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. The Journal of clinical endocrinology and metabolism. 2013;98:1182–1188. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson R, Mongan NP, Johansson M, Shcherbina L, Abrahamsson PA, Gudas LJ, Sterner O, Persson JL. Clinical trial update and novel therapeutic approaches for metastatic prostate cancer. Current medicinal chemistry. 2011;18:4440–4453. doi: 10.2174/092986711797287539. [DOI] [PubMed] [Google Scholar]
- 8.Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chemical research in toxicology. 2003;16:1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, Fujiki R. Molecular biology of nuclear steroid receptor. Nihon rinsho. Japanese journal of clinical medicine. 2008;66:2–6. [PubMed] [Google Scholar]
- 10.Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan R, Jiang J, Gusev Y, Jones A, Kearbey JD, Miller DD, Schmittgen TD, Dalton JT. MicroRNAs are mediators of androgen action in prostate and muscle. PloS one. 2010;5:e13637. doi: 10.1371/journal.pone.0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagchi Bhattacharjee G, Paul Khurana SM. In vitro reporter assays for screening of chemicals that disrupt androgen signaling. Journal of toxicology. 2014;2014:701752. doi: 10.1155/2014/701752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campana C, Pezzi V, Rainey WE. Cell-based assays for screening androgen receptor ligands. Seminars in reproductive medicine. 2015;33:225–234. doi: 10.1055/s-0035-1552989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu LC, Liu L, Ren XM, Zhang MR, Cong N, Xu AQ, Shao JH. Evaluation of androgen receptor transcriptional activities of some pesticides in vitro. Toxicology. 2008;243:59–65. doi: 10.1016/j.tox.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Wilson VS, Bobseine K, Lambright CR, Gray LE., Jr A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicological sciences : an official journal of the Society of Toxicology. 2002;66:69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- 16.Sun H, Xu XL, Xu LC, Song L, Hong X, Chen JF, Cui LB, Wang XR. Antiandrogenic activity of pyrethroid pesticides and their metabolite in reporter gene assay. Chemosphere. 2007;66:474–479. doi: 10.1016/j.chemosphere.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 17.Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicology and applied pharmacology. 1999;155:150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Park YI, Dong MS. Comparison of prostate cancer cell lines for androgen receptor-mediated reporter gene assays. Toxicology in vitro : an international journal published in association with BIBRA. 2006;20:1159–1167. doi: 10.1016/j.tiv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 19.He B, Kemppainen JA, Wilson EM. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. The Journal of biological chemistry. 2000;275:22986–22994. doi: 10.1074/jbc.M002807200. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson JM, Hayes S, Thompson D, Whitney P, Bi K. Compound profiling using a panel of steroid hormone receptor cell-based assays. Journal of biomolecular screening. 2008;13:755–765. doi: 10.1177/1087057108322155. [DOI] [PubMed] [Google Scholar]
- 21.Purvis IJ, Chotai D, Dykes CW, Lubahn DB, French FS, Wilson EM, Hobden AN. An androgen-inducible expression system for Saccharomyces cerevisiae. Gene. 1991;106:35–42. doi: 10.1016/0378-1119(91)90563-q. [DOI] [PubMed] [Google Scholar]
- 22.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. The Journal of endocrinology. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Majumder CB, Roy P. Development of a yeast-based assay to determine the (anti)androgenic contaminants from pulp and paper mill effluents in India. Environmental toxicology and pharmacology. 2007;24:114–121. doi: 10.1016/j.etap.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicology and applied pharmacology. 1997;143:205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Lee YS, Kwon HB, Lee K. Novel yeast bioassay system for detection of androgenic and antiandrogenic compounds. Toxicology in vitro : an international journal published in association with BIBRA. 2003;17:237–244. doi: 10.1016/s0887-2333(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa J, Saito K, Goto J, Dakeyama F, Matsuo M, Nishihara T. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicology and applied pharmacology. 1999;154:76–83. doi: 10.1006/taap.1998.8557. [DOI] [PubMed] [Google Scholar]
- 27.Michelini E, Leskinen P, Virta M, Karp M, Roda A. A new recombinant cell-based bioluminescent assay for sensitive androgen-like compound detection. Biosensors & bioelectronics. 2005;20:2261–2267. doi: 10.1016/j.bios.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Leskinen P, Michelini E, Picard D, Karp M, Virta M. Bioluminescent yeast assays for detecting estrogenic and androgenic activity in different matrices. Chemosphere. 2005;61:259–266. doi: 10.1016/j.chemosphere.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 29.Eldridge ML, Sanseverino J, Layton AC, Easter JP, Schultz TW, Sayler GS. Saccharomyces cerevisiae BLYAS, a new bioluminescent bioreporter for detection of androgenic compounds. Applied and environmental microbiology. 2007;73:6012–6018. doi: 10.1128/AEM.00589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, Gray LE., Jr Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicological sciences : an official journal of the Society of Toxicology. 2002;66:82–90. doi: 10.1093/toxsci/66.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Blankvoort BM, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJ, Aarts JM. Development of an androgen reporter gene assay (AR-LUX) utilizing a human cell line with an endogenously regulated androgen receptor. Analytical biochemistry. 2001;298:93–102. doi: 10.1006/abio.2001.5352. [DOI] [PubMed] [Google Scholar]
- 32.Sedlak D, Paguio A, Bartunek P. Two panels of steroid receptor luciferase reporter cell lines for compound profiling. Combinatorial chemistry & high throughput screening. 2011;14:248–266. doi: 10.2174/138620711795222446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, Vethaak AD, van der Burg B. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicological sciences : an official journal of the Society of Toxicology. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JD, Leihy MW, Shaw G, Renfree MB. Androgen physiology: unsolved problems at the millennium. Molecular and cellular endocrinology. 2002;198:1–5. doi: 10.1016/s0303-7207(02)00362-3. [DOI] [PubMed] [Google Scholar]
- 35.Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, Huhtaniemi IT. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. The Journal of steroid biochemistry and molecular biology. 2004;88:157–166. doi: 10.1016/j.jsbmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Delta5-androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11083–11088. doi: 10.1073/pnas.95.19.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, Orio F, Jr, Balaguer P, Sultan C. A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects. Molecular and cellular endocrinology. 2000;160:39–49. doi: 10.1016/s0303-7207(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 38.Roy P, Alevizaki M, Huhtaniemi I. In vitro bioassays for androgens and their diagnostic applications. Human reproduction update. 2008;14:73–82. doi: 10.1093/humupd/dmm038. [DOI] [PubMed] [Google Scholar]