Supplemental digital content is available in the text.
Key Words: Arthroplasty, Replacement, Knee, Electromyography, Muscle Fatigue, Resistance Training
ABSTRACT
Objective
The objective of this study was to investigate voluntary activation of the quadriceps muscle during one set of knee extensions performed until contraction failure in patients shortly after total knee arthroplasty.
Design
This was a cross-sectional study of 24 patients with total knee arthroplasty. One set of knee extensions was performed until contraction failure, using a predetermined 10 repetition maximum loading. In the operated leg, electromyographic (EMG) activity of the lateral and medial vastus, semitendinosus, and biceps femoris muscles was recorded during the set. Muscle activity (%EMGmax) and median power frequency of the EMG power spectrum were calculated for each repetition decile (10%–100% contraction failure).
Results
Muscle activity increased significantly over contractions from a mean of 90.0 and 93.6 %EMGmax (lateral vastus and medial vastus, respectively) at 10% contraction failure to 99.3 and 105.5 %EMGmax at 100% contraction failure (P = 0.009 and 0.004). Median power frequency decreased significantly over contractions from a mean of 66.8 and 64.2 Hz (lateral vastus and medial vastus, respectively) at 10% contraction failure to 59.9 and 60.1 Hz at 100% contraction failure (P = 0.0006 and 0.0187).
Conclusion
In patients shortly after total knee arthroplasty, 10 repetition maximum–loaded knee extensions performed in one set until contraction failure increases voluntary activation of the quadriceps muscle during the set.
Clinical Trials
Gov-identifier: NCT01713140 to the abstract to increase trial transparency.
Although patients enter a fast-track or enhanced recovery program after a total knee arthroplasty (TKA),1 the loss of knee-extension strength is pronounced shortly after surgery.2–4 As knee-extension strength is related to functional performance after TKA,5 regaining knee-extension strength soon after surgery is likely imperative for an enhanced recovery of functional performance.6 The main cause of the early loss of knee-extension strength after TKA is failure of the central nervous system to activate the quadriceps muscle,7 known as arthrogenic quadriceps muscle inhibition.8 Hence, reducing quadriceps muscle inhibition after TKA by exercise modalities known to increase voluntary activation of the contracting muscle is indicated clinically.
In healthy subjects, strength training is known to increase voluntary activation of the contracting muscle during a set to contraction failure,9 that is, failure to maintain the required or expected force.10 Increased voluntary activation is commonly indicated by increased surface electromyography (EMG) amplitude and reduced median power frequency of the EMG power spectrum.11–13 This has been coupled with increased excitatory drive to the alpha-motoneuron pool of the contracting muscle as fatigue develops.12 Ultimately, these myoelectrical manifestations of muscle fatigue are thought to reflect altered motor unit recruitment in the contracting muscle—particularly recruitment of additional high-threshold motor units.14
After TKA, a great number of motor units in the quadriceps motor unit pool is likely inhibited owing to the surgical trauma and associated postoperative swelling, pain, inflammation, and joint receptor damage. Fatiguing contractions of the quadriceps muscle may theoretically counter this arthrogenic quadriceps muscle inhibition. It requires, however, that the well-known myoelectric manifestations of fatigue seen in healthy subjects are also present for voluntary fatiguing contractions with the quadriceps muscle after TKA, that is, increased EMG amplitude (muscle activity) and decreased median power frequency.
The objective of the study was to investigate voluntary activation of the quadriceps muscle during a single set of knee extensions performed until contraction failure in patients shortly after TKA. It was hypothesized that voluntary activation of the quadriceps muscle would increase during the set, manifested by increased muscle activity and decreased median power frequency of the EMG power spectrum.
METHODS
Ethics Statement
All patients in the present study received verbal and written information about the study procedures, which conformed to the Declaration of Helsinki, and gave their written informed consent to participate in the study. The Committee on Biomedical Research Ethics for the Capital Region of Denmark approved the study (H-1-2011-027), and the study was registered at ClinicalTrials.gov (NCT01713140).
Design
In a cross-sectional study design, patients, who had received a TKA 4–8 wks earlier, performed one single set of standardized knee extensions to contraction failure, using the operated leg. A relative load of 10 repetition maximum (RM) was used and EMG activity of the medial and lateral vastii of the quadriceps muscle as well as the semitendinosus and biceps femoris muscles was recorded simultaneously (Fig. 1). The absolute load (kilograms) corresponding to 10 RM was determined individually during a familiarization session no less than 3 days before the experimental session. The reporting of the present study follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, using the checklist for cross-sectional studies.15
FIGURE 1.

Simultaneous recordings of knee joint range of motion and raw electromyographic activity of the vastus medialis, vastus lateralis, semitendinosus, and biceps femoris muscles, during one single set of standardized knee extensions to contraction failure.
Participants
By consecutive sampling, 24 patients were recruited from four municipal rehabilitation centers (Brøndby, Hvidovre, Vanløse, and Vesterbro/Kgs. Enghave/Valby) in the Copenhagen area during the period August 2012 to February 2013. The inclusion criteria were as follows: primary unilateral TKA 4–8 wks earlier, 18–80 yrs, and ability to understand and speak Danish. The exclusion criteria were as follows: a total active range of motion of less than 70 degrees of flexion in the operated knee joint, abuse of alcohol or other drugs, not willing to participate, and musculoskeletal or neurologic disorder that required specialized rehabilitation.
Experimental Arrangement
The patients were placed in a unilateral knee-extension machine (Technogym, Silver Line, Gambettola, Italy) based upon weight stacks (Fig. 2). They sat with 90 degrees flexion in the hip and the machine roller in front of the lower shinbone, 5 cm above the lateral malleolus. The patients were instructed to hold on to the machine handlebars while performing contractions, so that the back thigh was kept in contact with the seat throughout the movement.
FIGURE 2.
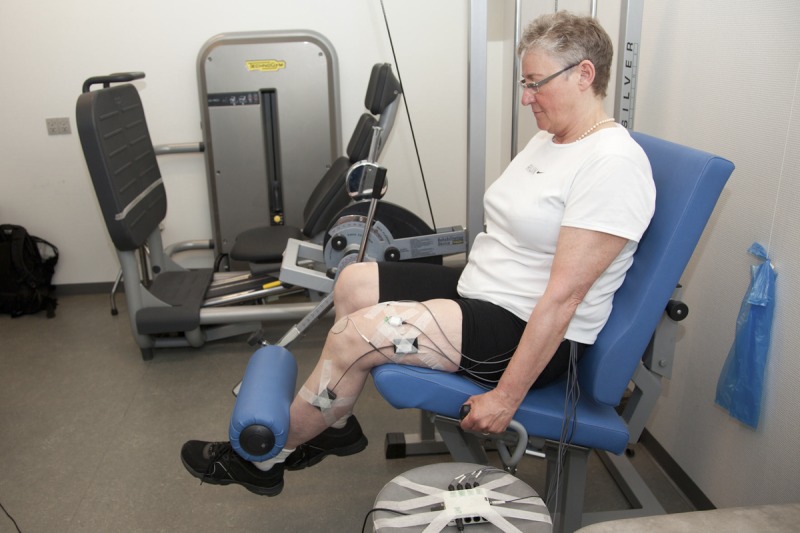
Picture of a patient performing knee extensions in the knee-extension machine.
Experimental Procedures
Familiarization Session
After a 3-min submaximal workout on a stepping machine at a self-selected intensity, the patients performed controlled knee extensions with the operated leg to establish the absolute load (kilograms) corresponding to 10 RM. The 10 RM load was determined individually, according to the researcher’s best estimate and experience with the TKA population. Kilograms were added or removed initially, based on a few repetitions with the load, to roughly estimate the 10 RM load. Having done this, contractions were performed to near failure or failure judged close to 10 RM. The load was then typically adjusted a few times after a short break until the load constituting 10 RM was found. To control for time under tension, a prerecorded audio file that allowed 3, 2, and 3 secs for the concentric, isometric, and eccentric phases, respectively, was used. Each repetition cycle of knee extensions was separated by a 2-sec isometric hold. No pauses were allowed between contraction phases, and contractions were continued until contraction failure, that is, until the load could not be lifted in the required range of motion within the defined time using proper technique. Every contraction had to be performed in a predefined minimum range of motion of 60 degrees (from 80 to 20 degrees of knee flexion), which was visually determined.
Experimental Session
After the standardized warm-up, the patients performed unilateral maximal voluntary isometric knee extensions and flexions at a knee joint angle of 60 degrees to establish reference values for the subsequent normalization of the EMG amplitude. The highest value of three maximal isometric contractions in each movement direction (knee flexion and extension) was used as the data points. The maximal contractions were performed using the knee-extension machine described above, but the weight stack was fixated and could not move. A handheld dynamometer (Power Track II Commander, JTECH Medical, Salt Lake City, UT) was placed between the shin and the resistance pad of the machine, 10 cm above the center of the lateral malleolus, to quantify knee-extension strength (please see “Secondary Outcomes”) (Fig. 3). The patients were instructed to contract as forceful as possible and to gradually increase contraction force for approximately 5 secs. Three maximal contractions were performed for each movement direction, separated by 2-min pauses. Having completed this, the patients performed one single set of unilateral knee extensions with the operated leg until contraction failure, using the experimental protocol and load described for the familiarization session. Five minutes after the set to contraction failure, another three maximal isometric knee extensions were performed to quantify knee-extension strength. Strong and standardized verbal encouragement was provided during both the maximal contractions and the knee extensions to contraction failure.
FIGURE 3.
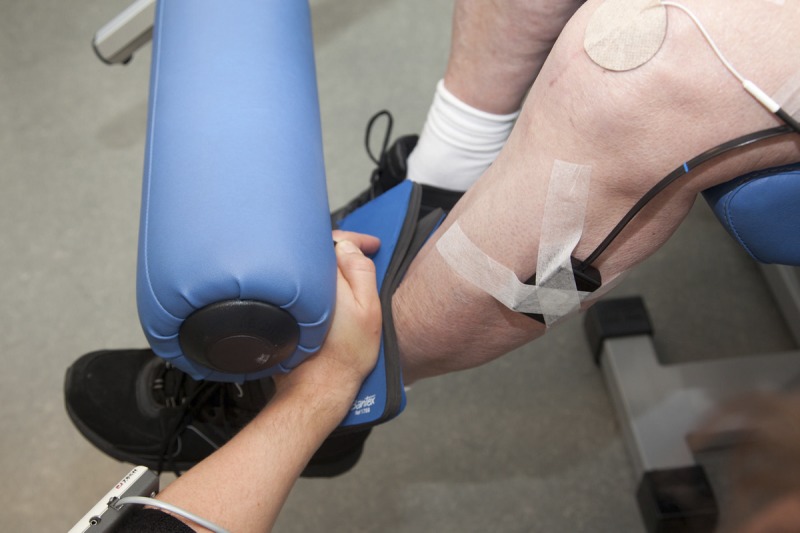
Picture of the experimental setup for the maximal isometric knee-extension strength measure using fixated handheld dynamometry.
Data Collection and Analysis
Primary Outcomes
After standard skin preparation, EMG recordings were obtained from the medial and lateral vastus of the contracting agonist (the quadriceps muscle) and two antagonists: the biceps femoris and semitendinosus muscles. Rectangular, nondisposable differential surface electrodes (DE-2.1, Delsys, Boston, MA) were placed on the skin overlying the muscles of interest, according to the recommendations of Perotto et al.,16 using electrode gel and medical grade adhesive. The size of each electrode was 41 × 20 × 5 mm, with 10-mm spacing between the two 10- × 1-mm parallel bars. A large nondisposable reference electrode was placed over the patella. The electrode wires were fixated with tape to the skin to reduce movement artifacts. The EMG signal was lead through wires via built-in preamplifiers and through a 5-ft shielded wire to a main amplifier (Bagnoli-16, Delsys). The main amplifier unit had a band-pass filter of 15–450 Hz and a common-mode rejection ratio of 92 dB. All signals were sampled at 1 kHz with a 16-bit A/D converter (6036E, National Instruments, Austin, TX). Signal quality was assessed by visual inspection of raw signals during contractions.
Offline EMG analysis was then conducted (EMGworks 3.7 Analysis, Delsys), in which both root-mean-square (RMS) filtering and Fourier transformation were applied to the raw data. Maximal muscle activity (EMGmax) was calculated as RMS values, with a window length of 1 sec and a window overlap of 0.999 secs for each of the investigated muscles, using data from the maximal voluntary knee extensions and flexions. The highest RMS value was identified for each maximal contraction, and the maximal contraction with the highest RMS value was used as the EMGmax for each muscle. Muscle activity for each repetition of the set to contraction failure for each muscle was calculated as RMS values with a window length of 1 sec and a window overlap of 0.999 secs. The highest RMS value was identified for each repetition for each muscle and expressed as percentage of the EMGmax using the the corresponding muscle’s identified EMGmax (%EMGmax). For the Fourier transformation, Fourier series of equations were applied to transform data from the time to the frequency domain, to calculate the average median power frequency of the EMG power spectrum for each investigated muscle, for each repetition in the set to contraction failure. Data for the set to contraction failure was reduced to deciles, with time points corresponding to 10%–100% contraction failure, and used as data points.
Secondary Outcomes
Knee pain in the operated leg at rest was scored on a visual analog scale (VAS) ranging from 0 mm (“no pain”) to 100 mm (“worst pain imaginable”) before and after the set to contraction failure and used as data points. Knee pain during the set to contraction failure was scored immediately after the set, as a recall of pain during the set, and used as the data point for knee pain during the set to contraction failure.
Level of perceived exertion during contractions was scored on a Borg RPE (ratings of perceived exertion) scale17 ranging from 6 to 20 points, immediately after finishing the set to contraction failure, and used as the data point.
Knee-extension strength was measured by fixated handheld dynamometry during the maximal knee extensions used for EMG normalization before the set to contraction failure. The maximal contractions were performed using the knee-extension machine, according to the procedures described above. The maximal knee extensions were repeated 5 mins after the set to contraction failure, to indicate acute changes in voluntary activation of the quadriceps muscle in response to the set to contraction failure. The peak value was identified for the strength assessments before and after the set to contraction failure and used as data points.
Statistical Analyses
A sample size estimation was initially performed to determine the number of patients required to show a 20-Hz decrease in median power frequency and a 20 %EMGmax (muscle activity) increase from 10% to 100% contraction failure for the quadriceps muscle, using 80% power, an α level of 5% (P < 0.05), and a common standard deviation of 30.13 It showed that at least 20 patients were needed for the study. To allow for dropouts of patients or data points, 24 patients were included. General linear models with repeated-measures, one-way analysis of variance were used to analyze main effects over contractions for muscle activity and median power frequency for each muscle and expressed as least square means with standard error (1 SE). Baseline data (age, height, and weight) and secondary outcomes (knee-extension strength and ratings of perceived exertion) were expressed as means with standard deviations (1 SD). Knee pain at rest and during knee extensions to contraction failure was expressed as median with interquartile range. Paired sample t tests were used to analyze changes in knee-extension strength. Wilcoxon signed rank test was used to analyze changes in knee pain at rest.
RESULTS
Participants
Figure 4 shows the flow of patients. Of the 24 patients who completed the test, data from four patients were subsequently excluded, as the data collection was erroneously terminated before contraction failure was reached. This decision was made before analyzing the data, during offline visual inspection of EMG data quality. Baseline characteristics of the remaining 20 patients who had complete data sets are shown in Table 1.
FIGURE 4.

Patient flow diagram.
TABLE 1.
Patient characteristics (N = 20)

Primary Outcomes
Quadriceps muscle activity increased significantly over contractions from 93.6 (5.7) %EMGmax at 10% contraction failure to 105.5 (4.0) %EMGmax at 100% contraction failure in the vastus medialis (P = 0.004, Fig. 5A) and from 90.0 (4.6) %EMGmax at 10% contraction failure to 99.3 (3.3) %EMGmax at 100% contraction failure in the vastus lateralis (P = 0.009, Fig. 5A). No changes over contractions was observed for the two hamstring muscles (P > 0.58, Fig. 5B).
FIGURE 5.

Muscle activity represented by normalized electromyographic (%EMGmax) amplitude (A, B) and median power frequency (Hz) (C, D) for the vastus medialis, vastus lateralis, semitendinosus, and biceps femoris muscles, during a single set of standardized knee extensions to contraction failure.
Quadriceps median power frequency decreased significantly over contractions from 64.2 (2.9) Hz at 10% contraction failure to 60.1 (2.0) Hz at 100% contraction failure in the vastus medialis (P = 0.0187, Fig. 5C) and from 66.8 (2.7) Hz at 10% contraction failure to 59.9 (1.9) Hz at 100% contraction failure in the vastus lateralis (P = 0.0006, Fig. 5C). Likewise, hamstring median power frequency decreased significantly over contractions from 66.2 (2.1) Hz at 10% contraction failure to 56.0 (1.5) Hz at 100% contraction failure in the semitendinosus (P = 0.0001, Fig. 5D) and from 75.1 (3.0) Hz at 10% contraction failure to 63.3 (2.1) Hz at 100% contraction failure in the biceps femoris (P = 0.0001, Fig. 5D).
Secondary Outcomes
The set performed to contraction failure had no acute effect on knee pain at rest, as knee pain recorded immediately after the final repetition (median, 0; interquartile range, 0–0 VAS-mm) was not significantly different from that recorded before the set (median, 0; interquartile range, 0–0 VAS-mm, P = 0.50). Knee pain during knee extensions to contraction failure was mild (median, 6; interquartile range, 0–26 VAS-mm). The rating of perceived exertion during the knee extensions to contraction failure was 15 (2) points.
The set performed to contraction failure tended to reduce knee-extension strength acutely, as knee-extension strength measured 5 mins after the final repetition (0.68 [0.2] Nm/kg) tended to be significantly different from that recorded before the set (0.71 [0.2] Nm/kg, P = 0.05).
DISCUSSION
Confirming the hypothesis, knee extensions performed in a single set until contraction failure increased voluntary activation of the quadriceps muscle during the set shortly after TKA. This was indicated by a significant increase in muscle activity and a significant decrease in median power frequency of the EMG recordings.
Increased Activation of the Quadriceps Muscle with Fatigue
The myoelectrical manifestations of fatigue seen in the present study are in general agreement with that observed in healthy subjects.18 That is, the muscle activity increased and the median power frequency decreased in the contracting muscles, while performing the same work in each repetition (10 RM load). This is also seen in healthy subjects during voluntary fatiguing contractions with the quadriceps muscle.18 It suggests increased recruitment of inactive motor units10,19 or increased synchronization or firing frequency of already active motor units.10,20,21 Taken together, the electrical manifestations of fatigue observed during the set to contraction failure in the present study is interpreted to indicate increased recruitment of inactive high-threshold motor units combined with increased synchronization of already active motor units.
Both antagonists were coactivated during the set to contraction failure—likely contributing to joint stability—but it was most pronounced for the biceps femoris muscle with average values of around 45 %EMG max. This between-hamstring muscle difference in antagonist activation during maximal knee extensions has also been reported in healthy subjects and thought to reflect a protective mechanism against internal tibial rotation and anterior ligament strain, caused by the quadriceps muscle at extended knee joint angles.22 Although the antagonist coactivation did not increase over contractions for any of the two hamstring muscles, the median power frequency decreased significantly. This could be taken to indicate an activation strategy after surgery with some common drive to the agonist and antagonist motoneuron pool, although not to a degree that was reflected in an increased antagonist muscle activity. Others have recently found increased antagonist (hamstring coactivation) during gait shortly after TKA, indicating that this strategy also presents during functional tasks.23 Although the strategy could be regarded as protective, it will also limit the force-generating capability of the quadriceps muscle.
Clinical Implications
Decreased mechanical muscle force,2,7 which is primarily caused by voluntary activation deficits7 and to a much lesser extent by muscular atrophy,7 is well known shortly after TKA. Although the sources of anomalous joint afferent discharge are thought to include swelling, pain, inflammation, and structural damage, the associated changes in the excitability of spinal and supraspinal pathways that limit quadriceps muscle activation are not fully understood after TKA but may involve different spinal reflex pathways.8 Ultimately, however, motor units within the spinal motor neuron pool innervating the quadriceps muscle are inhibited and cannot voluntarily be activated. This is likely, primarily for the high-threshold motor units, which are generally recruited at higher force levels.24 Based on the interpretation of the main findings in the present study, contractions performed until failure may work therapeutically, as a higher level of muscular activity is required when fatigue develops. Therefore, when approaching fatigue, inactive high-threshold motor units are likely recruited and motor units that were initially recruited will fatigue, producing less force.
It requires, however, that this exercise modality is tolerated shortly after TKA and that it does not seem to exacerbate postoperative symptoms. Indeed, there have been concerns that open-chain strength training exercise, such as knee extensions to contraction failure, would increase knee pain early after TKA.25 This does not seem to be the case shortly after TKA when assessed in a cross-sectional acute study design26 or after TKA when assessed prospectively in a randomized controlled study design.4 The seated knee-extension exercise was chosen because this exercise targets the quadriceps muscle specifically, unlike, for example, the leg-press exercise, to address the knee-extension strength loss early after TKA.2
Strength training as a rehabilitation modality has been emphasized in recent years,6 as well as in the latest systematic review on physical exercise after knee arthroplasty.27 Based on the findings from the present study, it is suggested to include strength training sets that are performed until contraction failure to counter the well-known voluntary activation deficits after TKA. Pertaining to this notion, strength training sets even with low loading (30% of 1 RM) seem capable of increasing quadriceps muscle mass substantially in healthy subjects, as long as the sets are performed until contraction failure.28 Thus, in a rehabilitation context, performing strength training sets until contraction failure will likely induce physiologic stimuli to both muscular hypertrophy and increased voluntary activation of the quadriceps muscle after TKA.
Limitations
Some precautions need to be taken when intepreting the present results. A cross-sectional, acute-study design was used and, therefore, the data pertain to this point in time. Hence, there is no knowing if a single set of knee extensions performed until contraction failure has an affect on voluntary activation beyond the set. This will have to be investigated in a future prospective study, using a regime of progressive strength training with repetitions to contraction failure. No acute improvement in knee-extension strength was indicated—in fact, it tended to be reduced when measured 5 mins after the set to contraction failure—likely because of persisting fatigue. However, the reliability of the dynamometer methodology used in the present study is unknown, and the study was not powered for this secondary outcome. Moreover, a recent randomized controlled study found no superior effect of exercise therapy including progressive strength training with repetitions to contraction failure compared with exercise therapy without progressive strength training on functional performance (primary outcome) or knee-extension strength (secondary outcome) shortly after TKA.4
The authors used no control for comparison; however, the myoelectrical manifestations of fatigue during fatiguing contractions are well known in healthy subjects. The challenge, however, is the interpretation of the changes in EMG amplitude or median power frequency, as they do not necessarily reflect increased motor unit recruitment. They may arise from a combination of recruitment of additional motor units, changes in motor unit firing frequency of already active units, and/or increased synchronization of already active motor units. Also, the EMG normalization procedure requires some thought. Although the EMG from an isometric maximal voluntary contraction has been endorsed as a normalization reference value,29 it may be associated with limitations. Theoretically, changing muscle length and pennation angle during dynamic contractions can influence both amplitude and frequency content of the EMG signal. Furthermore, a higher shortening velocity is associated with lower force in spite of a high normalized EMG amplitude.30 Nevertheless, during low to moderate velocity-controlled dynamic muscle contractions, there is a strong linear relation between normalized EMG and external resistance, for example, during dynamic resistance exercises with elastic bands or dumbbells.31 Because velocity of contraction was controlled in the present experiment and equal between subjects and repetitions, the potential bias from the aforementioned factors are likely small.
Finally, the increase in muscle activity and decrease in median power frequency were less than the 20% used to power the study. It is difficult to interpret if this is an effect too small to be considered clinically relevant, as it is hard to interpret what an increase in amplitude of 10 %EMGmax reflects in terms of motor unit recruitment.
CONCLUSION
In patients shortly after a TKA, 10 RM-loaded knee extensions performed in a single set until contraction failure increases voluntary activation of the quadriceps muscle during the set. This was indicated by increased muscle activity and decreased median power frequency of the EMG recordings from the contracting quadriceps muscle. Knee extensions until contraction failure may be a simple way to reduce quadriceps muscle inhibition after TKA, although it requires experimental verification in a prospective study design.
Supplementary Checklist
STROBE Checklist: http://links.lww.com/PHM/A151
Footnotes
The results from this study have not been presented at any congress, journal, or conference.
Supported by the Lundbeck Foundation Centre for Fast-track Hip and Knee Arthroplasty, the Danish Foundation for Research in Physiotherapy, and the Research Foundation, Copenhagen University Hospital, Hvidovre, Copenhagen, Denmark.
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on them or on any organization with which the authors are associated.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
REFERENCES
- 1. Kehlet H: Fast-track hip and knee arthroplasty. Lancet 2013; 381: 1600– 2 [DOI] [PubMed] [Google Scholar]
- 2. Holm B, Kristensen MT, Bencke J, et al. Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch Phys Med Rehabil 2010; 91: 1770– 6 [DOI] [PubMed] [Google Scholar]
- 3. Meier W, Mizner RL, Marcus RL, et al. Total knee arthroplasty: Muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther 2008; 38: 246– 56 [DOI] [PubMed] [Google Scholar]
- 4. Jakobsen TL, Kehlet H, Husted H, et al. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: A randomized controlled trial. Arthritis Care Res (Hoboken) 2014; 66: 1856– 66 [DOI] [PubMed] [Google Scholar]
- 5. Aalund PK, Larsen K, Hansen TB, et al. Normalized knee-extension strength or leg-press power after fast-track total knee arthroplasty: Which measure is most closely associated with performance-based and self-reported function? Arch Phys Med Rehabil 2013; 94: 384– 90 [DOI] [PubMed] [Google Scholar]
- 6. Bandholm T, Kehlet H: Physiotherapy exercise after fast-track total hip and knee arthroplasty: Time for reconsideration? Arch Phys Med Rehabil 2012; 93: 1292– 4 [DOI] [PubMed] [Google Scholar]
- 7. Mizner RL, Petterson SC, Stevens JE, et al. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am 2005; 87: 1047– 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice DA, McNair PJ: Quadriceps arthrogenic muscle inhibition: Neural mechanisms and treatment perspectives. Semin Arthritis Rheum 2010; 40: 250– 66 [DOI] [PubMed] [Google Scholar]
- 9. Fleck S, Kraemer W: Designing Resistance Training Programs 3rd ed. Champaign, IL: Human Kinetics, 2004 [Google Scholar]
- 10. Edwards R: Human muscle function and fatigue. Ciba Found Symp 1981; 82: 1– 18 [DOI] [PubMed] [Google Scholar]
- 11. Pincivero DM, Gandhi V, Timmons MK, et al. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J Biomech 2006; 39: 246– 54 [DOI] [PubMed] [Google Scholar]
- 12. Loscher WN, Cresswell AG, Thorstensson A: Excitatory drive to the alpha-motoneuron pool during a fatiguing submaximal contraction in man. J Physiol 1996; 491(Pt 1): 271– 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sundstrup E, Jakobsen MD, Andersen CH, et al. Muscle activation strategies during strength training with heavy loading vs. repetitions to failure. J Strength Cond Res 2012; 26: 1897– 903 [DOI] [PubMed] [Google Scholar]
- 14. Bigland-Ritchie B, Cafarelli E, Vollestad NK: Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 1986; 556: 137– 48 [PubMed] [Google Scholar]
- 15. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perotto A, Delagi E, Iazetti J, et al. Anatomical Guide for the Electromyographer: The Limbs and Trunk Springfield, IL: Charles C. Thomas, 2011 [Google Scholar]
- 17. Borg G: Borg’s Perceived Exertion and Pain Scales Champaign, IL: Human Kinetics, 1998 [Google Scholar]
- 18. Masuda K, Masuda T, Sadoyama T, et al. Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol 1999; 9: 39– 46 [DOI] [PubMed] [Google Scholar]
- 19. Newham DJ, Mills KR, Quigley BM, et al. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983; 64: 55– 62 [DOI] [PubMed] [Google Scholar]
- 20. Merletti R, Roy S: Myoelectric and mechanical manifestations of muscle fatigue in voluntary contractions. J Orthop Sports Phys Ther 1996; 24: 342– 53 [DOI] [PubMed] [Google Scholar]
- 21. Biedermann HJ, Shanks GL, Forrest WJ, et al. Power spectrum analyses of electromyographic activity. Discriminators in the differential assessment of patients with chronic low-back pain. Spine (Phila Pa 1976) 1991; 16: 1179– 84 [PubMed] [Google Scholar]
- 22. Aagaard P, Simonsen EB, Andersen JL, et al. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports 2000; 10: 58– 67 [DOI] [PubMed] [Google Scholar]
- 23. Thomas AC, Judd DL, Davidson BS, et al. Quadriceps/hamstrings co-activation increases early after total knee arthroplasty. Knee 2014; 21: 1115– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henneman E, Somjen G, Carpenter DO: Functional significance of cell size in spinal motorneurons. J Neurophysiol 1965; 28: 560– 80 [DOI] [PubMed] [Google Scholar]
- 25. Lin CW, March L, Crosbie J, et al. Maximum recovery after knee replacement—The MARKER study rationale and protocol. BMC Musculoskelet Disord 2009; 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bandholm T, Thorborg K, Lunn TH, et al. Knee pain during strength training shortly following fast-track total knee arthroplasty: A cross-sectional study. PLoS One 2014; 9: e91107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozzi F, Snyder-Mackler L, Zeni J: Physical exercise after knee arthroplasty: A systematic review of controlled trials. Eur J Phys Rehabil Med 2013; 49: 877– 92 [PMC free article] [PubMed] [Google Scholar]
- 28. Mitchell CJ, Churchward-Venne TA, West DW, et al. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985) 2012; 113: 71– 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burden A: How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J Electromyogr Kinesiol 2010; 20: 1023– 35 [DOI] [PubMed] [Google Scholar]
- 30. Andersen LL, Andersen JL, Magnusson SP, et al. Neuromuscular adaptations to detraining following resistance training in previously untrained subjects. Eur J Appl Physiol 2005; 93: 511– 8 [DOI] [PubMed] [Google Scholar]
- 31. Andersen LL, Andersen CH, Mortensen OS, et al. Muscle activation and perceived loading during rehabilitation exercises: Comparison of dumbbells and elastic resistance. Phys Ther 2010; 90: 538– 49 [DOI] [PubMed] [Google Scholar]


