Abstract
This meta-analysis was designed to evaluate the diagnostic performance of stool DNA testing for colorectal cancer (CRC) and compare the performance between single-gene and multiple-gene tests.
MEDLINE, Cochrane, EMBASE databases were searched using keywords colorectal cancers, stool/fecal, sensitivity, specificity, DNA, and screening. Sensitivity analysis, quality assessments, and performance bias were performed for the included studies.
Fifty-three studies were included in the analysis with a total sample size of 7524 patients. The studies were heterogeneous with regard to the genes being analyzed for fecal genetic biomarkers of CRC, as well as the laboratory methods being used for each assay. The sensitivity of the different assays ranged from 2% to 100% and the specificity ranged from 81% to 100%. The meta-analysis found that the pooled sensitivities for single- and multigene assays were 48.0% and 77.8%, respectively, while the pooled specificities were 97.0% and 92.7%. Receiver operator curves and diagnostic odds ratios showed no significant difference between both tests with regard to sensitivity or specificity.
This meta-analysis revealed that using assays that evaluated multiple genes compared with single-gene assays did not increase the sensitivity or specificity of stool DNA testing in detecting CRC.
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer-related death worldwide.1 The 5-year survival rate of CRC patients with localized disease is about 90% following curative surgery and about 60% for patients with lymph node metastasis.2 Hence, early diagnosis is critical and of utmost importance in reducing CRC-related mortality.3 In fact in countries with active CRC screening programs, there has been a decrease CRC mortality.4
CRC develops from the transformation of normal bowel epithelium into a precancerous state and finally into a malignancy. The changes in the epithelium are results of complex molecular alterations, including mutations and epigenetic changes in the genomic DNA.5–7 Current CRC screening options include colonofibroscopy, barium enema, flexible sigmoidoscope, and fecal occult blood testing (FOBT).5,8 However, these methods are not ideal screening tool in the clinical setting as these screening methods are invasive, unpleasant, and nonoptimal for patients.8 More importantly, most of these tests are of poor sensitivity or specificity.8 Fecal DNA testing has the advantage of being noninvasive, technically easy, and convenient. This strategy stems from the fact that malignant cells continuously shed into the colonic lumen, creating a source for disease-specific DNA biomarkers in a patient's stool.
Numerous studies evaluated different potential fecal DNA biomarkers for screening CRC.5 These biomarkers comprised a wide variety of genetic alterations, including DNA mutations and changes in the methylation status of a gene.5 Some tests were based on detection single genetic changes while others evaluated multiple genes. The difference assays varied significantly in sensitivity and specificity, and the relative diagnostic performance among the assays is unclear. We performed a systematic review and meta-analysis to evaluate the diagnostic performance of single-gene assays compared with multiple-gene assays that utilized stool DNA for screening for CRC.
METHODS
The meta-analysis was performed in accordance with the PRISMA 2009 guidelines.9 MEDLINE, Cochrane, EMBASE databases were searched until August 7, 2015 using following keywords CRC, stool/fecal, DNA, screening, sensitivity, and specificity. Studies reporting results in treatment-naive patients with confirmed diagnosis of primary CRC were included. The included studies had a control group of normal healthy subjects. All included studies used stool DNA testing as CRC screening tool, and employed colonofibroscopic or surgical pathology examination as the reference standard. Studies involving patients with the diagnoses of secondary or metastatic instead of primary colon cancers, precancerous lesions (such as metaplasia, dysplasia, etc.), and other chronic inflammatory diseases mimicking malignancy (such as inflammatory bowel disease) were excluded. Studies with incomplete patients’ profiles, missing essential data, questionable diagnosis or disease status, trials lacking appropriate informed consent, and articles not reporting quantitative data of primary study endpoints of interest were also omitted. L667etters, commentaries, editorial, case reports, expert opinions, and articles not published in English were excluded.
Study Selection and Data Extraction
All potential studies were reviewed thoroughly by 2 independent reviewers. A third reviewer was consulted to resolve any discrepancy between reviewers. All essential data and relevant information, including the name of the first author, year of publication, study design, subject demographics, pathology and cancer stages, targeted genes, and detection method of targeted genes, were extracted from the included studies.
Quality Assessment
The Revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS 2) tool was utilized for quality assessment for the included studies.10 The QUADAS 2 tool consists of 4 key domains that cover patient selection, index tests, reference standard, and flow of patients through the study and timing of the index tests and reference standard (flow and timing). The quality assessment was also performed by the independent reviewers and a third reviewer was consulted for any uncertainties.
Statistical Analysis
The outcomes of the meta-analysis were the diagnostic performance, denoted as sensitivity, specificity, the positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio of single-gene and multigene tests. Representation of accuracy estimates from each study in a receiver operating characteristic (ROC) space and computation of Spearman correlation coefficient between the log (SEN) and log (1 − SPE) were assessed for threshold effect. A typical pattern of “shoulder arm” plot in an ROC space and a strong positive correlation would suggest a threshold effect.11,12
Heterogeneity among the studies was assessed by the Cochran Q and the I2 statistic. For the Q statistic, P < 0.10 was considered statistically significant for heterogeneity. For the I2 statistic, which indicates the percentage of the observed between-study variability due to heterogeneity rather than chance, the following ranges were used: no heterogeneity (I2 = 0%–25%), moderate heterogeneity (I2 = 25%–50%), large heterogeneity (I2 = 50%–75%), and extreme heterogeneity (I2 = 75%–100%). If a Q statistics (P < 0.1) or I2 statistic (I2 > 50%) indicated heterogeneity between studies, the random-effects model was preferred (DerSimonian–Laird method). Otherwise, the fixed-effect model (Mantel–Haenszel method) was recommended. We pooled the results of single-gene test in each study. A 2-sided P value <0.05 was considered statistically significant. The homogeneity test, pooled estimates for sensitivity, specificity, the positive likelihood ratio, negative likelihood ratio diagnostic odds ratio (DOR), and summary ROC curve were performed by using Meta-Disc version 1.4.12 Pairwise comparison of ROC curves was performed based on model of Hanley and McNeil.13 In addition, publication bias was inspected by a Deeks funnel plot of the diagnostic odds ratio against study size. Deeks funnel plot was conducted using Stata software (version 14.0, StataCorp, College Station, TX).14
RESULTS
Literature Search
Of the 363 articles initially identified, 267 were excluded for being irrelevant (Figure 1). The remaining 96 studies were fully reviewed, of which 43 were excluded for not precisely meeting all the inclusion criteria. The resultant 53 studies were included in the analysis.8,15–66
FIGURE 1.
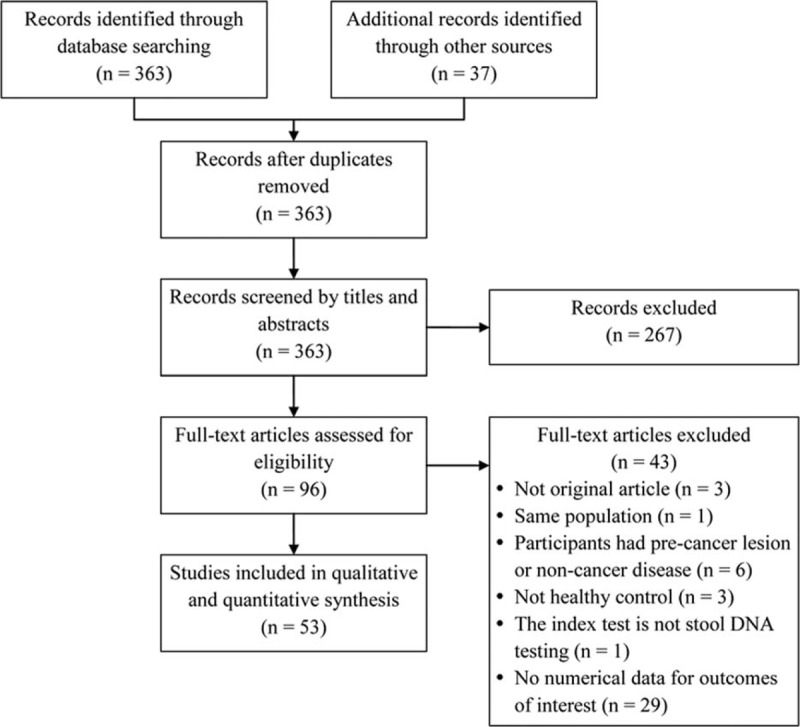
Flow diagram of study selection.
Study Characteristics
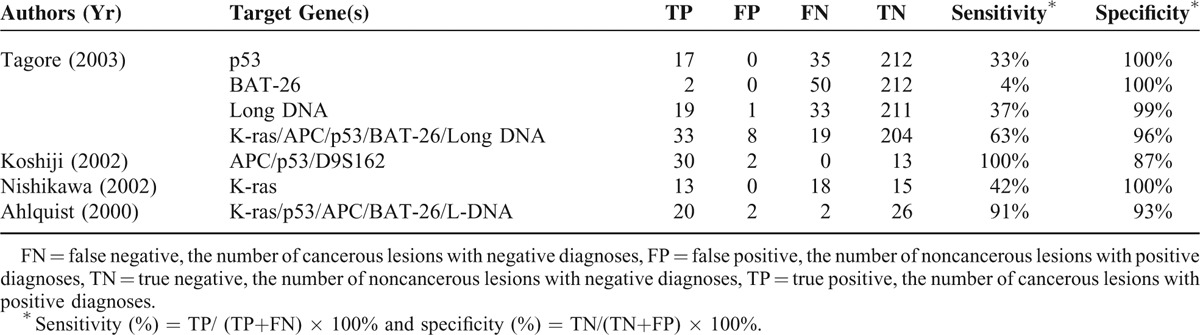
The total number of participants in the studies was 7524 (Table 1 ), with the percentage of males ranging from 35% to 83%. The numbers of patients in the CRC group and control group ranged from 14 to 116 and from 5 to 1426, respectively. The staging system applied, stages of cancer, frequency of each cancer stage in a given study, targeted genes assessed, and the analysis methods to detect the presence genetic alterations of the targets genes were heterogeneous varied significantly across the studies. Across the studies a wide type or different genes were analyzed (Table 2 ). The sensitivity and specificity of a given assay for detecting CRC in stool samples also varied across the studies; for each assay, the range for sensitivity was 2% to 100% and for specificity was 81% to 100%.
TABLE 1.
Summary of Basic Characteristics of Studies Included in Meta-Analysis
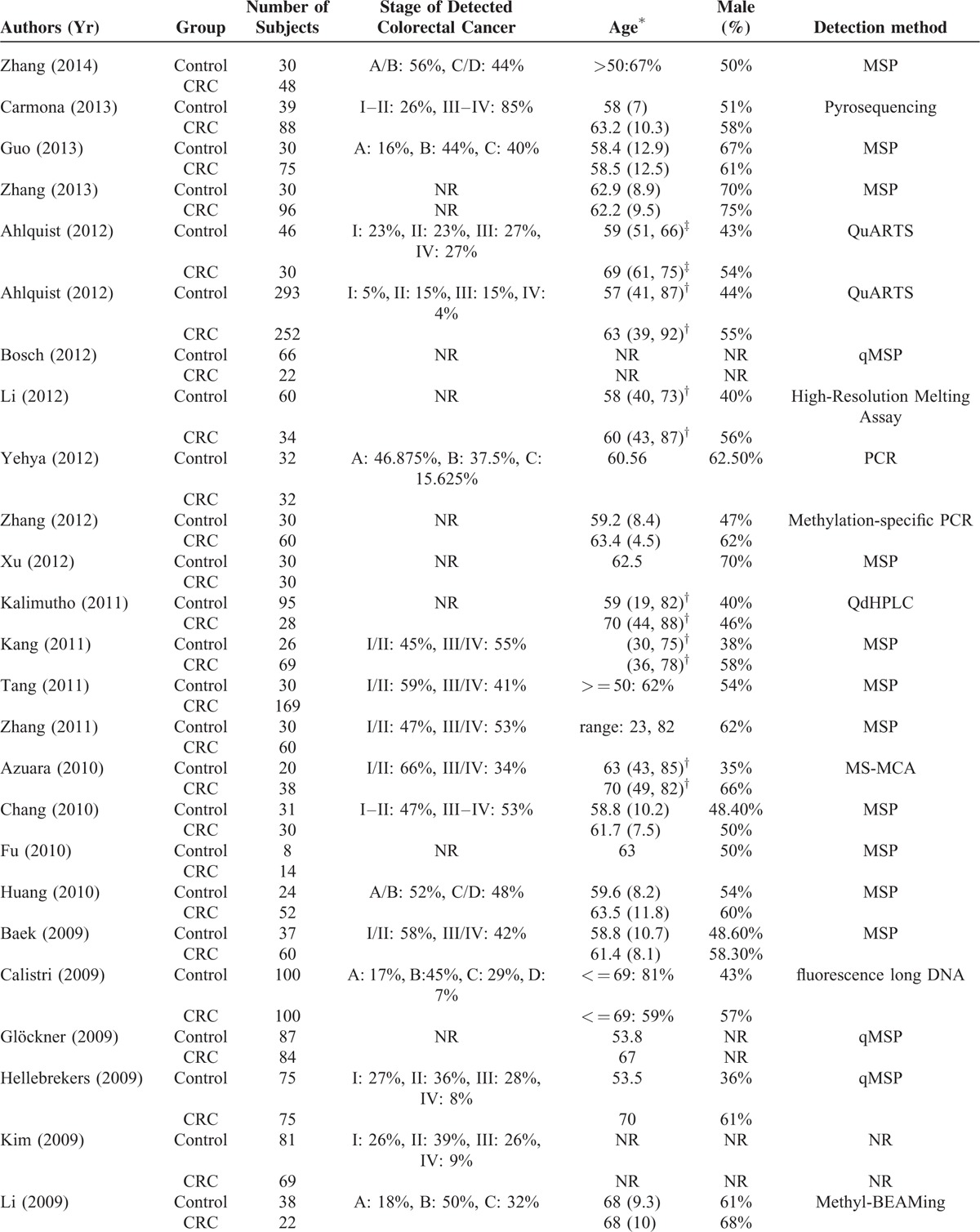
TABLE 1 (Continued).
Summary of Basic Characteristics of Studies Included in Meta-Analysis
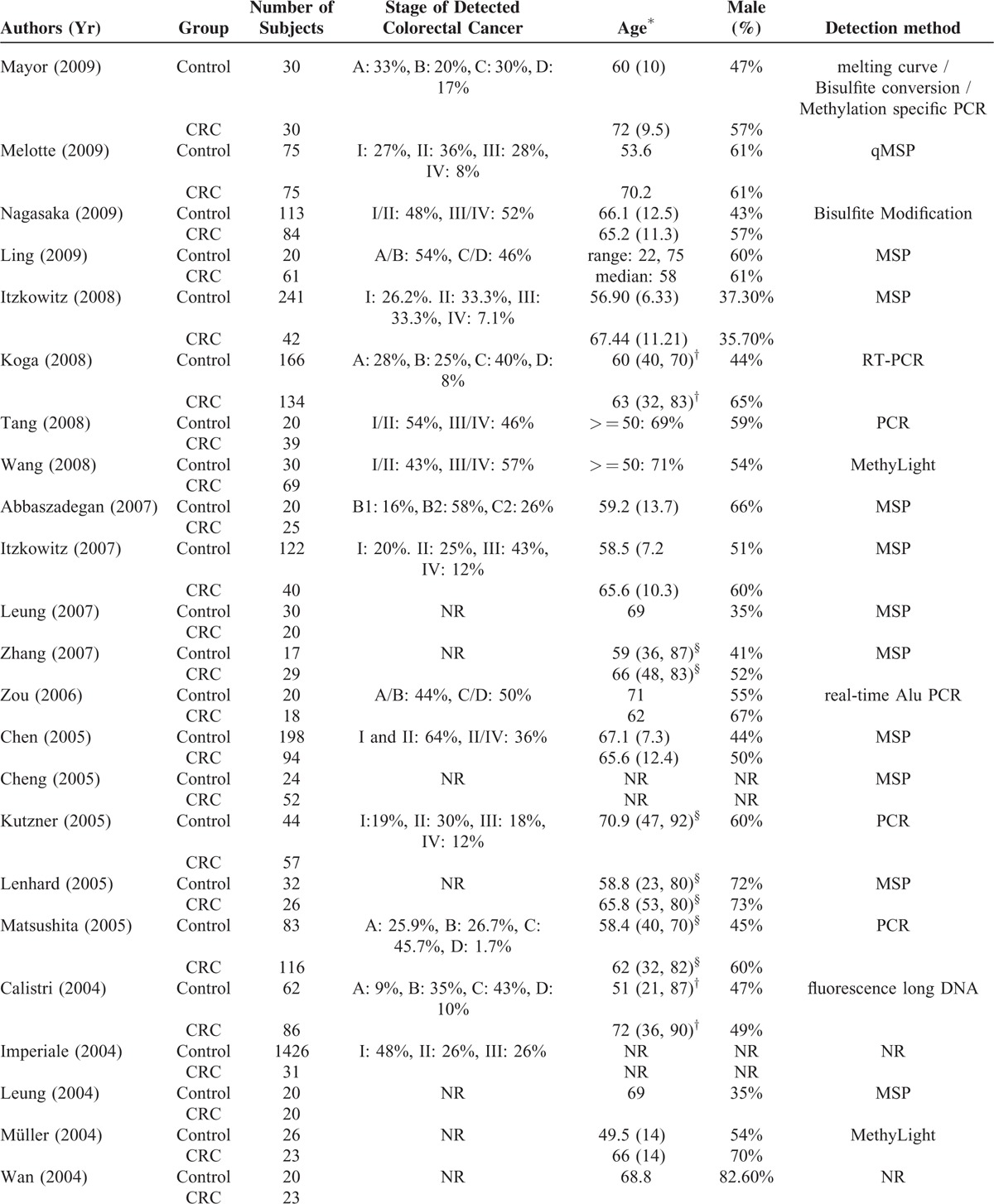
TABLE 1 (Continued).
Summary of Basic Characteristics of Studies Included in Meta-Analysis
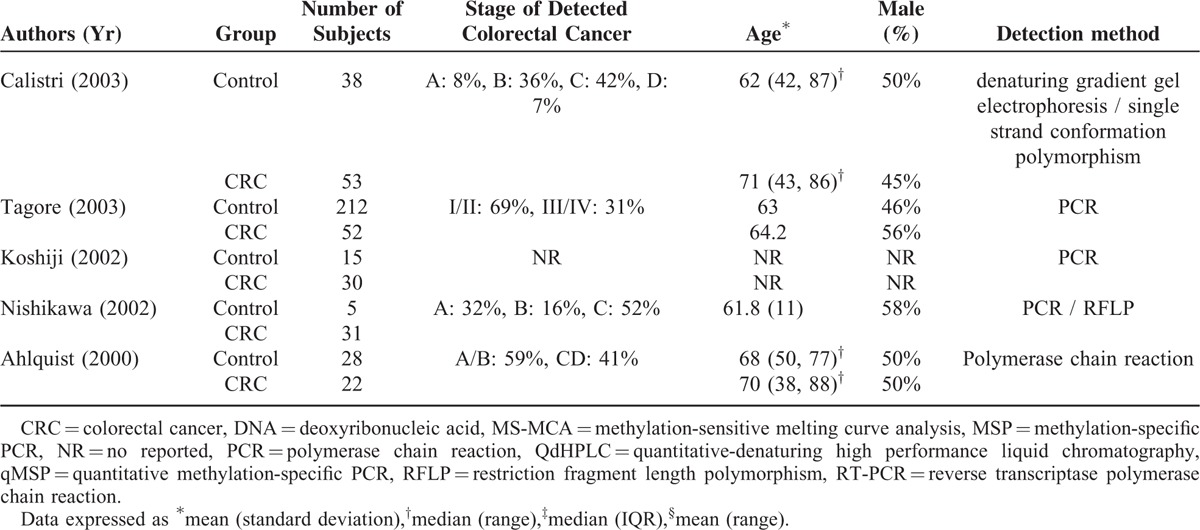
TABLE 2.
Summary of Performance of Studies Included in Meta-Analysis
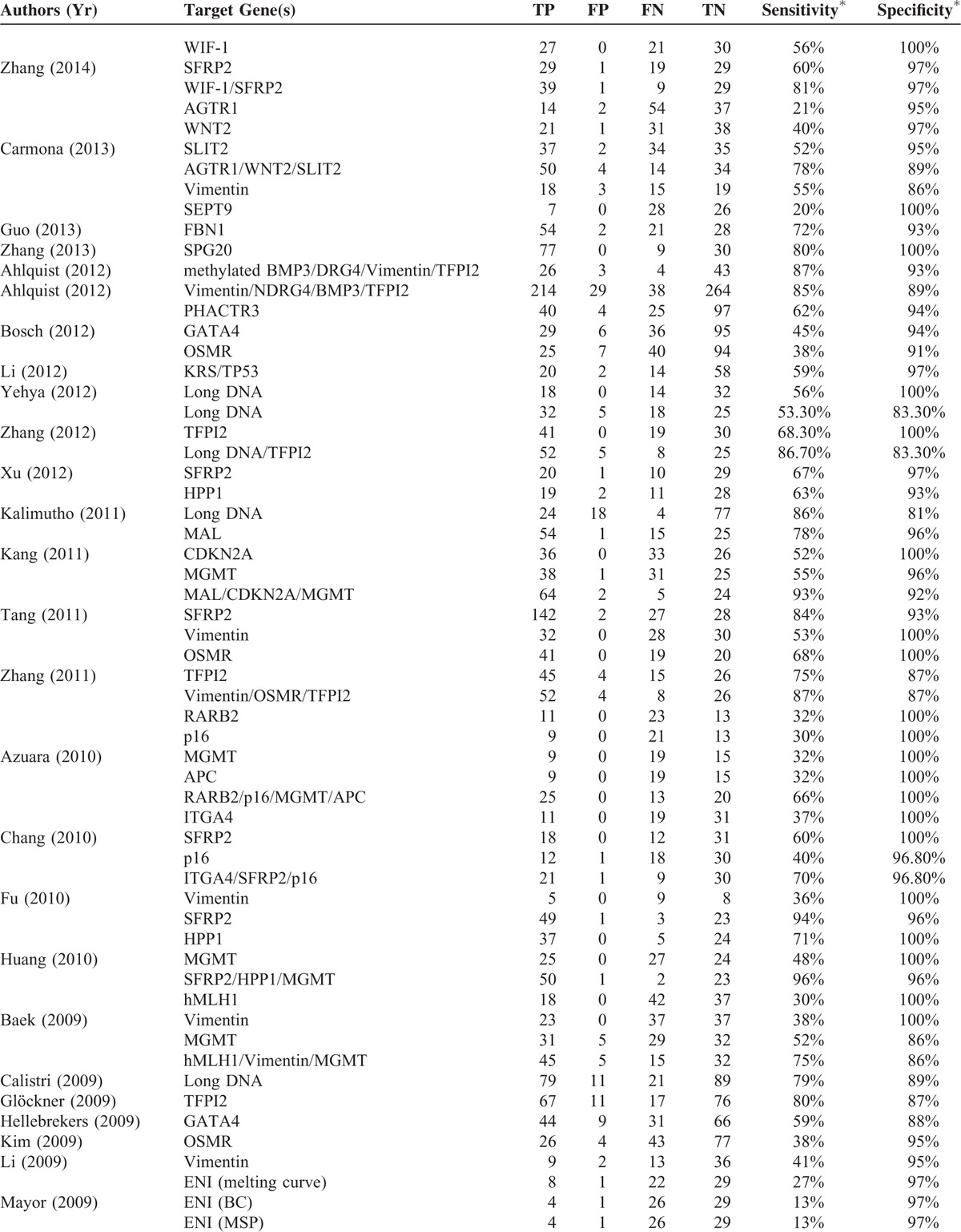
TABLE 2 (Continued).
Summary of Performance of Studies Included in Meta-Analysis
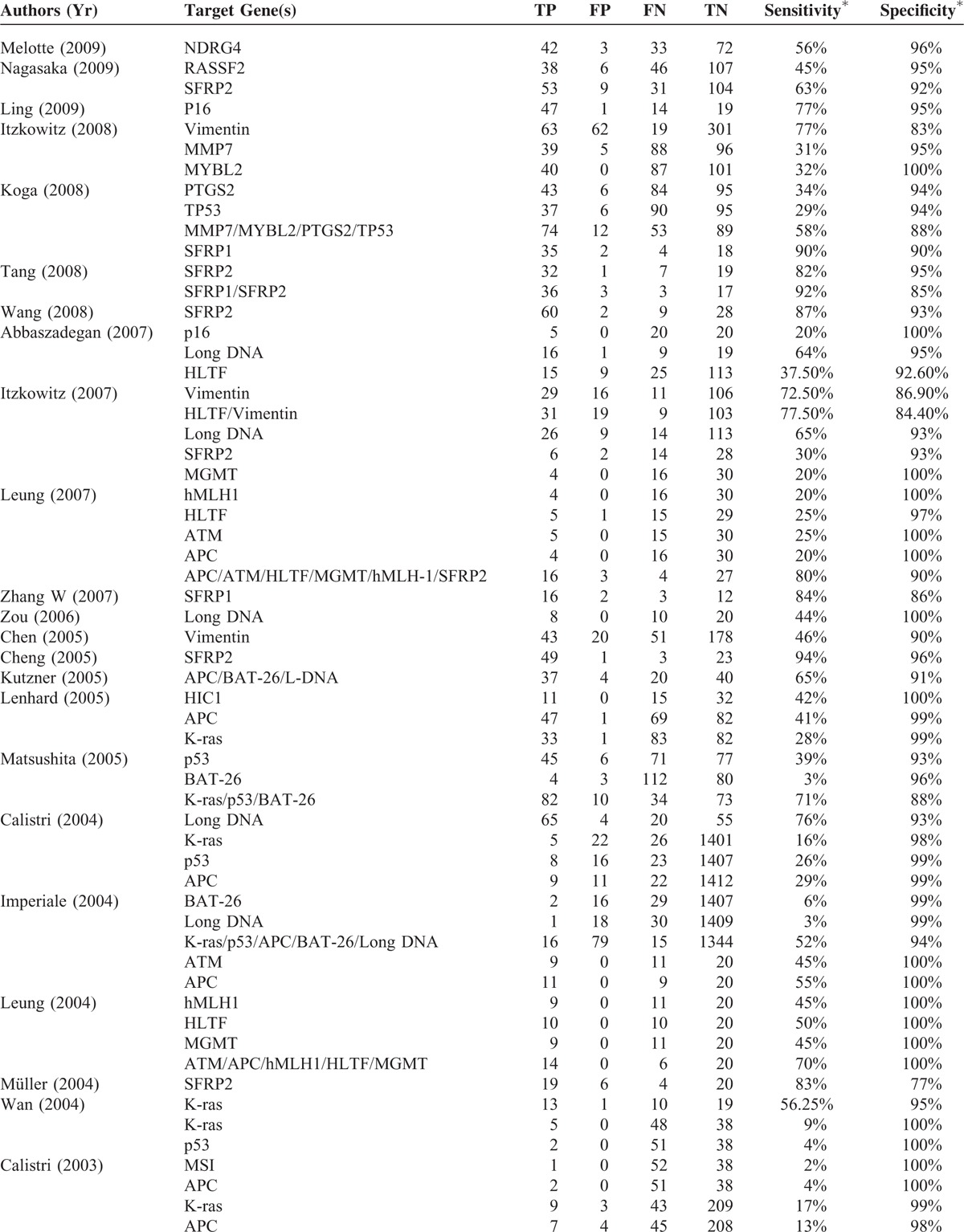
TABLE 2 (Continued).
Summary of Performance of Studies Included in Meta-Analysis
Performance of Single-Gene and Multi-Gene Test
A Spearman rank correlation was performed as a further test for threshold effect. The Spearman correlation coefficient was 0.080 (P = 0.205) for a single-gene assay and 0.257 (P = 0.126) for multigene test, The results indicate that other factors than threshold are causing variations in accuracy estimates among individual studies.
For evaluation of the sensitivity of a stool-based biomarker test using a single-gene or multiple-genes, the homogeneity tests found Q = 1244.70 (d.f. = 47, P < 0.001) and I2 = 96.2% for single-gene test; and Q = 115.35 (d.f. = 20, P < 0.001) and I2 = 82.7% for multigene test, indicating significant heterogeneity existed between these studies. Consequently, the random-effects model was used for the pooled analysis. The pooled sensitivities of single-gene and multigene tests were 48.0% and 78%, respectively (Figures 2A and 3A).
FIGURE 2.
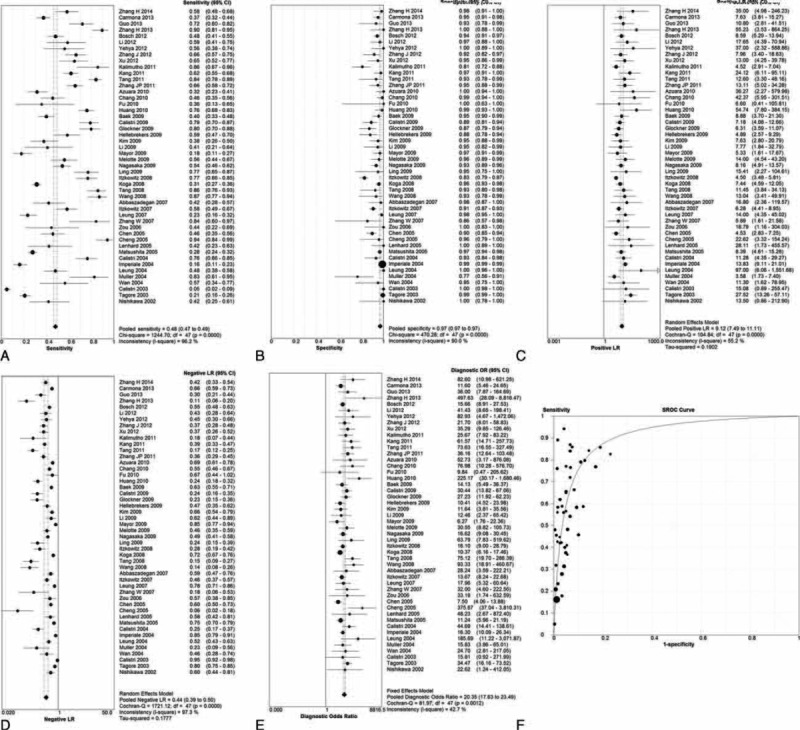
The summary of single-gene assay (A) sensitivity, (B) specificity, (C) positive likehood ratios, (D) negative lokehood ratios, (E) diagnostic odds ratio, (F) summary ROC curves.
FIGURE 3.
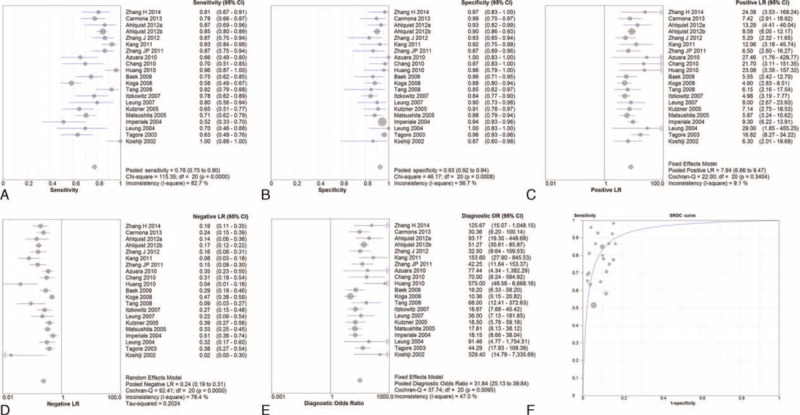
The summary of multiple-gene assays (A) sensitivity, (B) specificity, (C) positive likehood ratios, (D) negative lokehood ratios, (E) diagnostic odds ratio, (F) summary ROC curves.
The homogeneity tests for assessing the specificity of a single-gene or a multigene stool-based biomarker assay indicated Q = 470.28 (d.f. = 47, P < 0.001) and I2 = 90.0% for single-gene test; and Q = 46.17 (d.f. = 20, P = 0.001) and I2 = 56.7% for multigene test. These findings indicated the presence of significant heterogeneity among the studies; hence, the random-effects model was used for the pooled analysis. The pooled specificities of single-gene and multigene tests were 97.0% and 93%, respectively (Figures 2B and 3B).
In addition, the pooled positive likelihood ratios of single-gene and multigene tests were 9.17 and 7.94, respectively (Figures 2C and 3C), while the negative positive likelihood ratio of single-gene and multigene tests were 0.44 and 0.24, respectively (Figures 2D and 3D).
Summary ROC Curves and Diagnostic Odds Ratio
For all studies, the pooled DOR was 20.35 (95% CI: 17.63–23.49) for the single-gene assay and 31.64 (95% CI: 25.13–39.84) for multigene assay (Figures 2E and 3E). Moreover, the area under the summary ROC curves was 0.908 (standard error = 0.013) for the single-gene assay and 0.934 (standard error = 0.011) for multigene assays (Figures 2F and 3F). No significant difference was observed between the ROC curves for single-gene and multigene tests (P = 0.063). These findings suggest both types of assays have good diagnostic discrimination for the presence of absence of CRC.
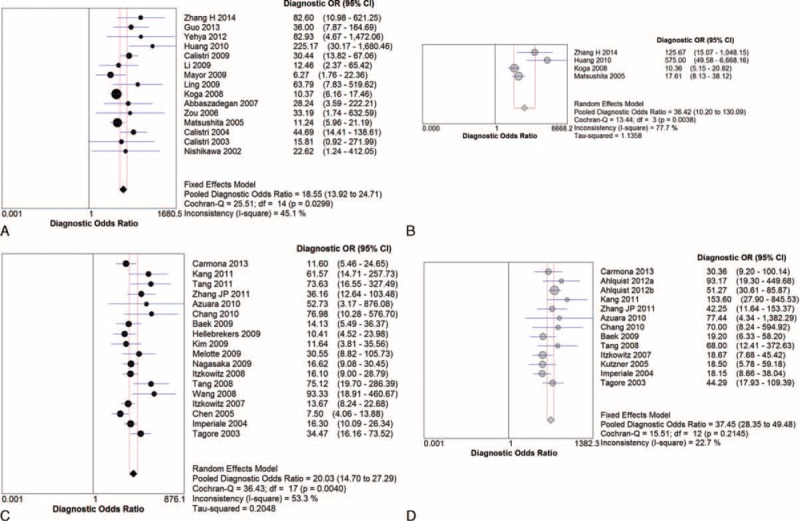
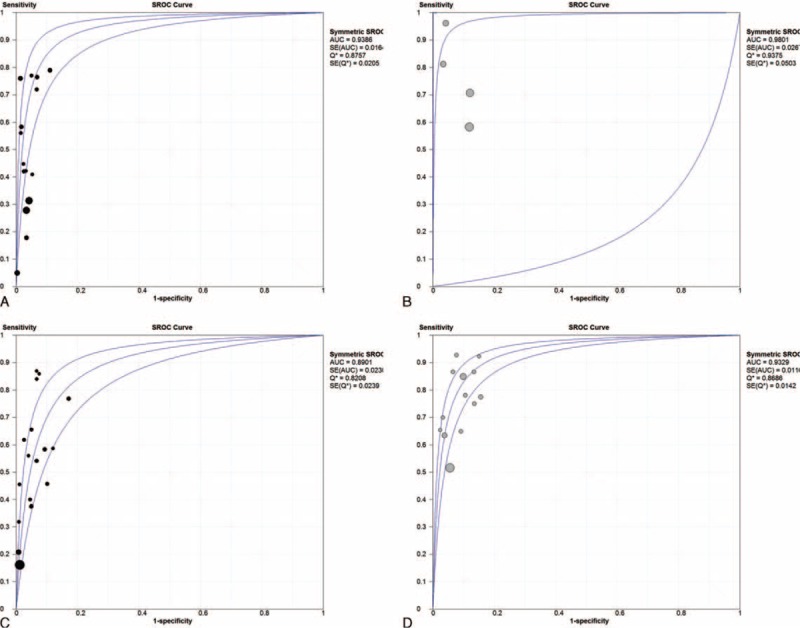
Subgroup Analyses According to Duke and AJCC Classification as Cancer Staging System
Further subgroup analyses were performed according to Duke criteria and AJCC classification of primary CRC. DOR and summary ROC curves were represented only. According to the Duke criteria, the pooled DOR was 18.55 for the single-gene assay and 36.42 for multigene assays (Figure 4A and B); the summary ROC was 0.939 for the single-gene assay and 0.980 for multigene assays, respectively (Figure 5A and B). According to AJCC classification, the pooled DOR was 20.03 for the single-gene assay and 37.45 for multigene assays (Figure 4C and D); the summary ROC was 0.890 for the single-gene assay and 0.933 for multigene assays, respectively (Figure 5C and D).
FIGURE 4.
Pooled DOR according to Duke criteria for (A) single-gene assay, (B) multiple-gene assays; pooled DOR according to AJCC classification for (C) single-gene assay, (D) multiple-gene assays.
FIGURE 5.
Summary ROC according to Duke criteria for (A) single-gene assay, (B) multiple-gene assays; summary ROC according to AJCC classification for (C) single-gene assay, (D) multiple-gene assays.
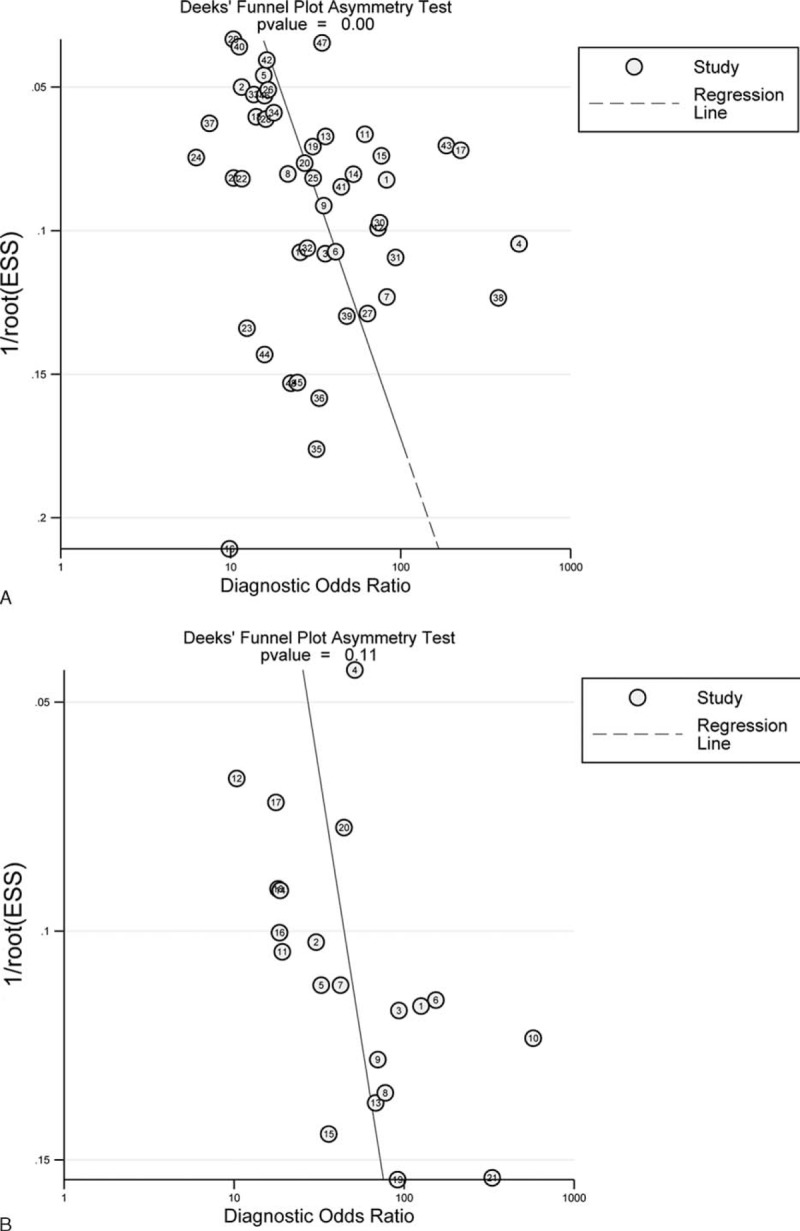
Publication Bias
The results via Deeks funnel plot showed that there was publication bias for the single-gene assay in regards to DOR value (P < 0.001, Figure 6A). However, there no publication bias for multigene assays was found (P = 0.11, Figure 6B).
FIGURE 6.
Publication bias for (A) single-gene assay, (B) multiple-gene assays.
Quality Assessment
Quality assessment of the different studies found the greatest potential risk of bias came from patient selection as most of the studies did not collect a consecutive or random sample (Figure 7). Furthermore, some of the included studies did not prespecify the threshold of the index test.
FIGURE 7.
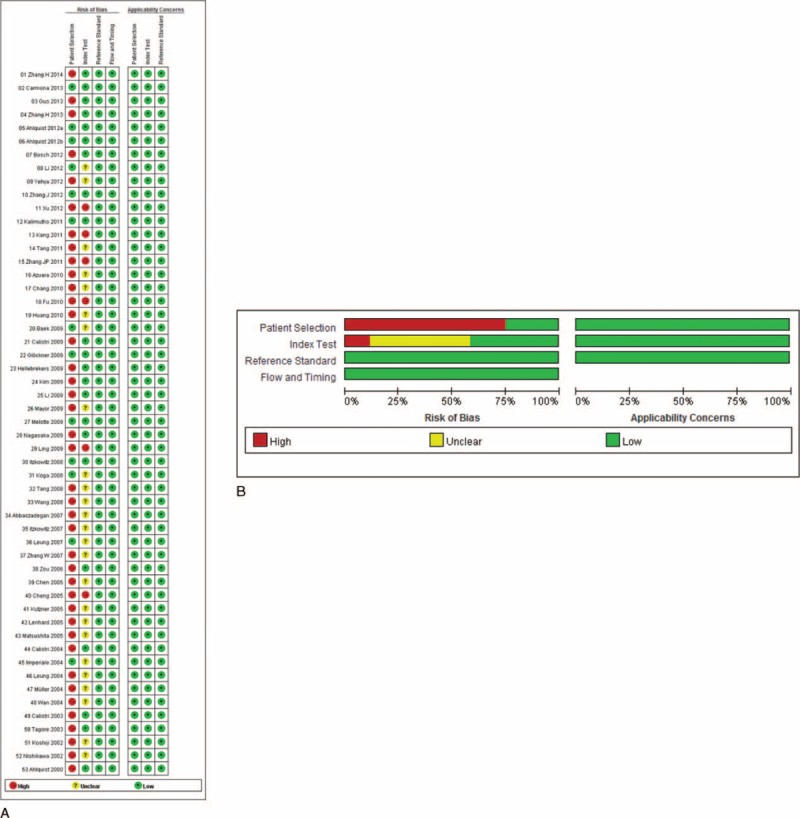
Results of quality assessment. A, Potential risk of bias of individual study. B, Summarized risk of bias of all included studies.
DISCUSSION
This meta-analysis evaluated the diagnostic performance of stool DNA testing for screening for CRC and compared the diagnostic performance or single-gene and multiple-genes assays. The pooled sensitivities were found to be 48.0% for single-gene and 77.8% for multiple-gene assays, while the pooled specificities for the single-gene and multiple-gene assays were 97.0% and 92.7%, respectively. There was no significant difference between single- and multigene tests regarding the pooled sensitivity and specificity by ROC curve analysis. However, multiple-gene assays were noted to have higher sensitivity than the single-gene assays, implying that the former testing may have advantages for screening CRC. Although previous studies reported that assays based on a combination of biomarkers had a high detection rates of both CRC and advanced adenomas,67 no large-scale comparison between the single-gene and multigene stool DNA testing has previously been reported. Our study updated and expanded the prior information by including recent studies, and to the best of our knowledge, is the first study to compare the diagnostic accuracy of single-gene and multiple-gene testing for CRC using stool DNA.
A possible reason why the multiple-gene assay has higher sensitivity but similar specificity as the single-gene assay is that the multiple-gene assay has the ability to detect methylation or genetic changes across multiple CRC-related genes, so the chance of detecting CRC-related changes is higher than detecting changes of only 1 gene. The similar but high specificity indicates that both assays have few false positives suggesting the individual assays used across the studies for detecting the stool DNA have high accuracy for only evaluating the particular genes of interest.
Several prior meta-analyses have assessed the diagnostic value of stool DNA testing.68–70 Yang et al68 performed a meta-analysis that evaluated the diagnostic abilities of testing stool for multiple DNA markers of CRC. They included 20 studies that comprised 5876 patients. They found that multiple marker tests had a sensitivity for CRC of 0.676 (95% confidence interval [CI]: 0.642–0.708) and a specificity of 0.928 (95% CI: 0.917–0.939). Subgroup analysis indicated that the detection sensitivity and specificity for advanced adenoma was 0.329 (95% CI: 0.294–0.365) and 0.939 (95% CI: 0.927–0.949). In addition, subgroup analysis indicated methylation DNA testing had a significantly higher sensitivity (0.753 [95% CI: 0.685–0.812]) for CRC and a relatively similar specificity (0.913 [95% CI: 0.860–0.95] than evaluating genetics. The authors concluded that the methylated markers may have better diagnostic value than genetic markers. The sensitivity, we found in our multiple-gene biomarker analysis for CRC, was higher than that reported by Yang et al. The difference between the 2 meta-analyses may reflect differences in included studies, as our study included a larger number of studies than that of Yang et al.
Zhang et al69 evaluated the accuracy of gene methylation analysis of DNA in stool samples for diagnosing CRC. They included 37 articles that comprised 4484 patients. The sensitivity and specificity for detection of CRC were 73% (95% CI: 71%–75%) and 92% (90%–93%), respectively. They also found that for adenoma the sensitivity was 51% (95% CI: 47%–54%) and the specificity was 92% (95% CI: 90%–93%). Pooled diagnostic performance for methylation of the SFRP2 gene indicated the sensitivity of an SFRP2-based methylation assay was 70% (95% CI: 75%–82%) and the specificity was 93% (95% CI: 90%–96%).
Luo et al70 also evaluated the use of measuring the methylation state of biomarkers for detecting CRC. Their analysis included 19 studies comprising 2356 patients. They found that the sensitivity and specificity for detecting CRC were 0.62 (95% CI: 0.51–0.71) and 0.89 (95% CI: 0.86–0.92), respectively. The sensitivity and specificity for adenoma were 0.54 (95% CI: 0.39–0.68) and 0.88 (95% CI: 0.83–0.92). Luo et al found a lower sensitivity of methylation-based assays for detecting CRC than did Yang et al and Zhang et al. Luo et al concluded that the use of hypermethylated gene panels was not yet currently sufficiently accurate to be used alone for CRC screening, and future studies and evaluation of additional biomarkers were mandatory to improve sensitivity and specificity.
This analysis has several limitations that should be considered when interpreting the results. We did not perform subgroup analysis to compare the diagnostic abilities of methylation-based and mutation-based assays or the sensitivity or specificity of the single-gene or multigene assay for detecting advanced adenoma or adenoma highly potential for neoplastic change. All these issues are important and deserve additional studies. Most of the included studies were not prospective randomized controlled trials and there was a great heterogeneity across the studies, including CRC staging criteria and methods targeted genes, and analysis methods, which may confound study findings. Finally, the cost effectiveness of the clinical use of fecal DNA testing for CRC screening was not evaluated. A prior meta-analysis that included 7 studies found that fecal DNA testing was not cost-effective if compared with other CRC-screening tools; it would only become cost-effective when compared with no screening.71
In conclusion, no statistically significant difference was observed from the ROC curves between both tests for detecting CRC. Compared with the single-gene testing, multiple-gene stool DNA testing was shown to confer no better diagnostic performance in the screening of CRC. The high specificity of the assaying stool DNA for CRC-related genes suggested these assays may not only be of benefit to diagnosing CRC but also for evaluation recurrence of the disease.
Footnotes
Abbreviations: CRC = colorectal cancer, DOR = diagnostic odds ratio, FOBT = fecal occult blood testing, QUADAS = Revised Quality Assessment of Diagnostic Accuracy Studies = ROC, receiver operator curves.
Both multiple- and single-gene testing assays were shown to have similar sensitivity and specificity in detecting CRC-related DNA changes from stool samples. The 2 assays had similar diagnostic performance for CRC.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Pfister DG, Benson AB, 3rd, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med 2004; 350:2375–2382. [DOI] [PubMed] [Google Scholar]
- 3.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol 2005; 23:378–391. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger BM, Ahlquist DA. Stool DNA screening for colorectal neoplasia: biological and technical basis for high detection rates. Pathology 2012; 44:80–88. [DOI] [PubMed] [Google Scholar]
- 6.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008; 135:1079–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med 2009; 361:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller HM, Oberwalder M, Fiegl H, et al. Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet 2004; 363:1283–1285. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–94. [DOI] [PubMed] [Google Scholar]
- 10.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536. [DOI] [PubMed] [Google Scholar]
- 11.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993; 12:1293–1316. [DOI] [PubMed] [Google Scholar]
- 12.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148:839–843. [DOI] [PubMed] [Google Scholar]
- 14.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58:882–893. [DOI] [PubMed] [Google Scholar]
- 15.Abbaszadegan MR, Tavasoli A, Velayati A, et al. Stool-based DNA testing, a new noninvasive method for colorectal cancer screening, the first report from Iran. World J Gastroenterol 2007; 13:1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology 2000; 119:1219–1227. [DOI] [PubMed] [Google Scholar]
- 17.Ahlquist DA, Taylor WR, Mahoney DW, et al. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol 2012; 10:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlquist DA, Zou H, Domanico M, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology 2012; 142:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azuara D, Rodriguez-Moranta F, de Oca J, et al. Novel methylation panel for the early detection of colorectal tumors in stool DNA. Clin Colorectal Cancer 2010; 9:168–176. [DOI] [PubMed] [Google Scholar]
- 20.Baek YH, Chang E, Kim YJ, et al. Stool methylation-specific polymerase chain reaction assay for the detection of colorectal neoplasia in Korean patients. Dis Colon Rectum 2009; 52:1452–1459. [DOI] [PubMed] [Google Scholar]
- 21.Bosch LJ, Oort FA, Neerincx M, et al. DNA methylation of phosphatase and actin regulator 3 detects colorectal cancer in stool and complements FIT. Cancer Prev Res (Phila) 2012; 5:464–472. [DOI] [PubMed] [Google Scholar]
- 22.Calistri D, Rengucci C, Bocchini R, et al. Fecal multiple molecular tests to detect colorectal cancer in stool. Clin Gastroenterol Hepatol 2003; 1:377–383. [DOI] [PubMed] [Google Scholar]
- 23.Calistri D, Rengucci C, Lattuneddu A, et al. Detection of colorectal cancer by a quantitative fluorescence determination of DNA amplification in stool. Neoplasia 2004; 6:536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calistri D, Rengucci C, Molinari C, et al. Quantitative fluorescence determination of long-fragment DNA in stool as a marker for the early detection of colorectal cancer. Cell Oncol 2009; 31:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmona FJ, Azuara D, Berenguer-Llergo A, et al. DNA methylation biomarkers for noninvasive diagnosis of colorectal cancer. Cancer Prev Res (Phila) 2013; 6:656–665. [DOI] [PubMed] [Google Scholar]
- 26.Chang E, Park DI, Kim YJ, et al. Detection of colorectal neoplasm using promoter methylation of ITGA4, SFRP2, and p16 in stool samples: a preliminary report in Korean patients. Hepatogastroenterology 2010; 57:720–727. [PubMed] [Google Scholar]
- 27.Chen WD, Han ZJ, Skoletsky J, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst 2005; 97:1124–1132. [DOI] [PubMed] [Google Scholar]
- 28.Cheng ZH. Stool SFRP2 methylation in the diagnosis of colorectal cancer. Shandong Yiyao Zazhi 2007; 47:10–12. [Google Scholar]
- 29.Fu L, Sheng JQ, Meng XM, et al. Stool Vimentin methylation in the diagnosis of colorectal cancer. Weichangbing Ji Ganzabingxue Zazhi 2010; 19:601–603. [Google Scholar]
- 30.Glockner SC, Dhir M, Yi JM, et al. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res 2009; 69:4691–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Q, Song Y, Zhang H, et al. Detection of hypermethylated fibrillin-1 in the stool samples of colorectal cancer patients. Med Oncol 2013; 30:695. [DOI] [PubMed] [Google Scholar]
- 32.Hellebrekers DM, Lentjes MH, van den Bosch SM, et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res 2009; 15:3990–3997. [DOI] [PubMed] [Google Scholar]
- 33.Huang ZH, Li LH, Yang F, et al. Detection of aberrant methylation in fecal DNA as a molecular screening tool for colorectal cancer and precancerous lesions. World J Gastroenterol 2007; 13:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004; 351:2704–2714. [DOI] [PubMed] [Google Scholar]
- 35.Itzkowitz S, Brand R, Jandorf L, et al. A simplified, noninvasive stool DNA test for colorectal cancer detection. Am J Gastroenterol 2008; 103:2862–2870. [DOI] [PubMed] [Google Scholar]
- 36.Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol 2007; 5:111–117. [DOI] [PubMed] [Google Scholar]
- 37.Kalimutho M, Del Vecchio Blanco G, Cretella M, et al. A simplified, non-invasive fecal-based DNA integrity assay and iFOBT for colorectal cancer detection. Int J Colorectal Dis 2011; 26:583–592. [DOI] [PubMed] [Google Scholar]
- 38.Kang YP, Cao FA, Chang WJ, et al. Gene methylation in stool for the screening of colorectal cancer and pre-malignant lesions. Zhonghua Wei Chang Wai Ke Za Zhi 2011; 14:52–56. [PubMed] [Google Scholar]
- 39.Kim MS, Louwagie J, Carvalho B, et al. Promoter DNA methylation of oncostatin m receptor-beta as a novel diagnostic and therapeutic marker in colon cancer. PLoS One 2009; 4:e6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koga Y, Yasunaga M, Moriya Y, et al. Detection of colorectal cancer cells from feces using quantitative real-time RT-PCR for colorectal cancer diagnosis. Cancer Sci 2008; 99:1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koshiji M, Yonekura Y, Saito T, et al. Microsatellite analysis of fecal DNA for colorectal cancer detection. J Surg Oncol 2002; 80:34–40. [DOI] [PubMed] [Google Scholar]
- 42.Kutzner N, Hoffmann I, Linke C, et al. Non-invasive detection of colorectal tumours by the combined application of molecular diagnosis and the faecal occult blood test. Cancer Lett 2005; 229:33–41. [DOI] [PubMed] [Google Scholar]
- 43.Lenhard K, Bommer GT, Asutay S, et al. Analysis of promoter methylation in stool: a novel method for the detection of colorectal cancer. Clin Gastroenterol Hepatol 2005; 3:142–149. [DOI] [PubMed] [Google Scholar]
- 44.Leung WK, To KF, Man EP, et al. Detection of epigenetic changes in fecal DNA as a molecular screening test for colorectal cancer: a feasibility study. Clin Chem 2004; 50:2179–2182. [DOI] [PubMed] [Google Scholar]
- 45.Leung WK, To KF, Man EP, et al. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol 2007; 102:1070–1076. [DOI] [PubMed] [Google Scholar]
- 46.Li BS, Wang XY, Xu AG, et al. High-resolution melting assay (HRMA) is a simple and sensitive stool-based DNA test for the detection of mutations in colorectal neoplasms. Clin Colorectal Cancer 2012; 11:280–290. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Chen WD, Papadopoulos N, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol 2009; 27:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling ZA, Chen LS, He CG. Stool P16 methylation in the diagnosis of colorectal cancer. Jiezhichang Gangmen Waike 2009; 15:144–148. [Google Scholar]
- 49.Matsushita H, Matsumura Y, Moriya Y, et al. A new method for isolating colonocytes from naturally evacuated feces and its clinical application to colorectal cancer diagnosis. Gastroenterology 2005; 129:1918–1927. [DOI] [PubMed] [Google Scholar]
- 50.Mayor R, Casadome L, Azuara D, et al. Long-range epigenetic silencing at 2q14.2 affects most human colorectal cancers and may have application as a non-invasive biomarker of disease. Br J Cancer 2009; 100:1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melotte V, Lentjes MH, van den Bosch SM, et al. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst 2009; 101:916–927. [DOI] [PubMed] [Google Scholar]
- 52.Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst 2009; 101:1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa T, Maemura K, Hirata I, et al. A simple method of detecting K-ras point mutations in stool samples for colorectal cancer screening using one-step polymerase chain reaction/restriction fragment length polymorphism analysis. Clin Chim Acta 2002; 318:107–112. [DOI] [PubMed] [Google Scholar]
- 54.Tagore KS, Lawson MJ, Yucaitis JA, et al. Sensitivity and specificity of a stool DNA multitarget assay panel for the detection of advanced colorectal neoplasia. Clin Colorectal Cancer 2003; 3:47–53. [DOI] [PubMed] [Google Scholar]
- 55.Tang D, Wang D, Li H. Combination analysis of hypermethylated SFRP1 and SFRP2 gene in fecal as a novel epigenetic biomarker panel for colorectal cancer screening. J Nanjing Medical University 2008; 22:96–101. [Google Scholar]
- 56.Tang D, Liu J, Wang DR, et al. Diagnostic and prognostic value of the methylation status of secreted frizzled-related protein 2 in colorectal cancer. Clin Invest Med 2011; 34:E88–95. [DOI] [PubMed] [Google Scholar]
- 57.Wan J, Zhang ZQ, You WD, et al. Detection of K-ras gene mutation in fecal samples from elderly large intestinal cancer patients and its diagnostic significance. World J Gastroenterol 2004; 10:743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang DR, Tang D. Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol 2008; 14:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu MH, Cai KY, Tu Y. Stool DNA methylation analysis in the early diagnosis of colorectal cancer. Linchang Xiaohuabing Zazhi 2012; 24:7–19. [Google Scholar]
- 60.Yehya AH, Yusoff NM, Khalid IA, et al. Pilot study of the sensitivity and specificity of the DNA integrity assay for stool-based detection of colorectal cancer in Malaysian patients. Asian Pac J Cancer Prev 2012; 13:1869–1872. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Zhu YQ, Wu YQ, et al. Detection of promoter hypermethylation of Wnt antagonist genes in fecal samples for diagnosis of early colorectal cancer. World J Gastroenterol 2014; 20:6329–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Song YC, Dang CX. Detection of hypermethylated spastic paraplegia-20 in stool samples of patients with colorectal cancer. Int J Med Sci 2013; 10:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Yang S, Xie Y, et al. Detection of methylated tissue factor pathway inhibitor 2 and human long DNA in fecal samples of patients with colorectal cancer in China. Cancer Epidemiol 2012; 36:73–77. [DOI] [PubMed] [Google Scholar]
- 64.Zhang JP, Wang J, Gui YL, et al. Stool DNA methylation in the diagnosis of colorectal cancer. Zhonghua Yixue Zazhi 2011; 95:2482–2484.22321844 [Google Scholar]
- 65.Zhang W, Bauer M, Croner RS, et al. DNA stool test for colorectal cancer: hypermethylation of the secreted frizzled-related protein-1 gene. Dis Colon Rectum 2007; 50:1618–1626. [DOI] [PubMed] [Google Scholar]
- 66.Zou H, Harrington JJ, Klatt KK, et al. A sensitive method to quantify human long DNA in stool: relevance to colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2006; 15:1115–1119. [DOI] [PubMed] [Google Scholar]
- 67.Ahlquist DA, Sargent DJ, Loprinzi CL, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med 2008; 149:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H, Xia BQ, Jiang B, et al. Diagnostic value of stool DNA testing for multiple markers of colorectal cancer and advanced adenoma: a meta-analysis. Can J Gastroenterol 2013; 27:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Qi J, Wu YQ, et al. Accuracy of early detection of colorectal tumours by stool methylation markers: a meta-analysis. World J Gastroenterol 2014; 20:14040–14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo YX, Chen DK, Song SX, et al. Aberrant methylation of genes in stool samples as diagnostic biomarkers for colorectal cancer or adenomas: a meta-analysis. Int J Clin Pract 2011; 65:1313–1320. [DOI] [PubMed] [Google Scholar]
- 71.Skally M, Hanly P, Sharp L. Cost effectiveness of fecal DNA screening for colorectal cancer: a systematic review and quality appraisal of the literature. Appl Health Econ Health Policy 2013; 11:181–192. [DOI] [PubMed] [Google Scholar]


