Supplemental Digital Content is available in the text
Abstract
Arterial spin labeling (ASL) magnetic resonance imaging analyses allow for the quantification of altered cerebral blood flow, and provide a novel means of examining the impact of dopaminergic treatments. The authors examined the cerebral perfusion differences among 17 Parkinson disease (PD) patients, 17 PD with dementia (PDD) patients, and 17 healthy controls and used ASL-MRI to assess the effects of dopaminergic therapies on perfusion in the patients. The authors demonstrated progressive widespread cortical hypoperfusion in PD and PDD and robust effects for the dopaminergic therapies. Specifically, dopaminergic medications further decreased frontal lobe and cerebellum perfusion in the PD and PDD groups, respectively. These patterns of hypoperfusion could be related to cognitive dysfunctions and disease severity. Furthermore, desensitization to dopaminergic therapies in terms of cortical perfusion was found as the disease progressed, supporting the concept that long-term therapies are associated with the therapeutic window narrowing. The highly sensitive pharmaceutical response of ASL allows clinicians and researchers to easily and effectively quantify the absolute perfusion status, which might prove helpful for therapeutic planning.
INTRODUCTION
Parkinson disease (PD) is a neurodegenerative disease associated with the progressive loss of dopamine neurons, and incidence studies suggest that an annual rate of 10% of PD patients develop dementia (which is sometimes termed PD with dementia (PDD) in such cases).1 Accurate and comprehensive knowledge of the rate of disease deterioration is essential for the design and evaluation of new therapies for this disorder. Current neuroimaging studies, including MRI and radiotracer studies (positron emission tomography, PET; single photon emission computed tomography, SPECT) have identified abnormally decreased levels of cortical metabolism and perfusion in PD.2 Such alterations have been associated with the PD motor-related pattern and the PD cognitive-related pattern,3,4 suggesting that they could potentially be used as disease biomarkers to assess disease diagnoses, progression, and treatment responses in cases of PD.
Although enormous progress has been made in the treatment of PD, including the development of a variety of promising therapies, long-term dopamine replacement therapies as well as the progression of the disease itself might be associated with the gradual development of ineffective motor functional control, the occurrence of adverse drug effects,5 and various cognitive impairments.6 The association between the direct effects of dopaminergic therapies on brain global/regional perfusion and disease status remains unclear. A previous study, however, has reported that as dementia develops in PD patients, those patients exhibit more widespread cortical hypoperfusion.7 By altering dopaminergic neurotransmission, dopaminergic therapy can modulate the cognitive and motor striatal functional networks in healthy individuals and in PD patients.8,9 The spatiotemporal progression of dopamine depletion in PD provides a special model for assessing dopaminergic effects on perfusion in neural systems with different baseline dopamine levels.
Arterial spin labeling (ASL) is a noninvasive MRI perfusion tool that quantitatively measures cerebral blood flow per unit of tissue mass by taking advantage of arterial water as a freely diffusible tracer.10 Arterial spin labelling allows for the acquisition of perfusion data without the fear of nephrogenic systemic fibrosis in patients with significant renal insufficiency. The ability to obtain quantification on an absolute scale further allows for easy recognition of diffuse hypoxic/anoxic states and also for assessments of cortical perfusion before and after a given intervention.11 Unlike processes involving the use of potentially harmful radioactive materials that require long preparation times, ASL can also be easily repeated in routine MRI studies to safely and effectively investigate disease status and progress in PD. Recent studies have demonstrated its clinical applications in cerebral blood flow quantification in PD.3,7
We had 3 priori hypotheses for our study: ASL-MRI would indicate cortical hypoperfusion in the subjects with PD and PDD as compared with the normal controls (NC); dopaminergic therapy might alter acute cerebral blood flow (CBF) changes in particular brain regions in PD and PDD; and differences in regional perfusion resulting from pharmaceutical effects would predict the baseline or interval changes indicated during clinical evaluations. Our results might help prove the utility of ASL in defining a time window for early interventions against PD.
MATERIALS AND METHODS
Participants
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Chang Gung Memorial Hospital human research committee. All participants or their guardians provided written informed consent before participation in the study. Thirty-four right-handed PD and PDD patients (13 men and 21 women, mean age: 62.18 ± 9.1 years) were prospectively enrolled in the Neurology Department of Kaohsiung Chang Gung Memorial Hospital. Patients were included if they had a definitive diagnosis of idiopathic PD12 and had been followed up on at the Neurology Out-Patient Clinic for more than 6 months after titration of their daily dopaminergic medications to a steady dose in accordance with their clinical symptoms. The PDD was diagnosed in accordance with recently published guidelines.1 Patients were excluded if they had newly diagnosed PD or had undergone follow-ups for less than 6 months since their daily dose of dopaminergic medications was still under adjustment; other pyramidal signs, severe sensorial impairment, or ataxia; or impaired consciousness or higher brain dysfunction that precluded following instructions.
All the patients completed 3 tests corresponding to the ON and OFF medication states. All evaluations for the PD and PDD patients, including evaluations of clinical disease status, MRI studies, and neuropsychological tests, were initially assessed in the OFF state achieved by withdrawal of dopaminergic medications 12 to 18 hour before testing. For the ON testing, the study procedures began 1 hour after the patient had taken their daily dopaminergic medications. The daily dose of dopaminergic medications was converted into the equivalent dose of levodopa.13
The disease severity and functional status of each patient were evaluated, as in our previous study,14 with the Unified Parkinson Disease Rating Scale (UPDRS), the modified Hoehn and Yahr stages, and the Schwab and England Activities of Daily Living Scale. The Mini-Mental State Examination (MMSE) was used to assess the general cognitive functions of the subjects.15
The patients’ mean disease duration, defined as the time since the given patient subjectively noticed his or her first symptoms, was 3.1 ± 1.7 years. For comparison, 17 sex- and age-matched NC (8 men and 9 women, mean age: 59.7 ± 7.5 years) with no medical history of neurologic diseases or psychiatric illnesses, alcohol/substance abuse, or head injury, and with similar levels of education, were recruited. These control participants underwent a single testing session without any medication procedure.
Magnetic Resonance Image Acquisition
High-resolution T1-weighted (T1W) imaging was performed on a 3.0T scanner (GE Signa MRI, Milwaukee, WI) with an 8-channel head coil. T1-weighted images were acquired using the three-dimensional fluid-attenuated inversion-recovery fast spoiled gradient recalled echo pulse sequence with the following imaging parameters: TR = 9.5 milliseconds, TE = 3.9 milliseconds, TI = 450 milliseconds, flip angle = 20, field of view = 256 × 256 mm, matrix size = 512 × 512, number of slices = 110, and slice thickness = 1.3 mm.
Perfusion MRI data was performed on a 1.5T scanner (Discovery MR450, GE Healthcare, Milwaukee, WI) with an 8-channel head coil. ASL images were acquired using a pseudocontinuous arterial spin labeling technique with a background suppressed 3D fast spin echo sequence.16 The imaging parameters used were TR = 4548 milliseconds, postlabel delay = 1525 milliseconds, TE = 10.5 milliseconds, matrix size = 128 × 128, number of excitations = 3, number of slices = 38, slice thickness = 4.0 mm (with whole brain coverage), and total acquisition time = 4 minutes. Arterial spin labelling perfusion imaging acquires 2 images: 1 image taken shortly after the inflowing arterial spins are inverted (labeled image) and 1 taken without inverting the arterial spins (unlabeled image). Subtracting the labeled image from the unlabeled image produces an ASL perfusion weighted image, which can be converted to a quantitative image that reflects CBF. For each subject, a CBF map was calculated by scanner console with FuncTool 3DASL (Discovery MR450, GE Healthcare, Milwaukee, WI) within 1 minute and was reported in mL/100 gm/min units.
Image Data Processing
Imaging data were preprocessed using FSL v5.0 (Functional Magnetic Resonance Imaging of the Brain Software Library; http://www.fmrib.ox.au.uk/fsl) and SPM8 (statistical parametric mapping, Wellcome Department of Imaging Neuroscience, London, UK; available online at http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7.3 (MathWorks, Natick, MA). All T1W and ASL images for each participant were carefully checked by an experienced neuroradiologist to ensure that they included no scanner artifacts, motion problems, or gross anatomic abnormalities.
To further ensure the accuracy of cross-modality image registration, the unlabeled ASL images and corresponding T1W images of each participant were skull-stripped using the Brain Extraction (BET, part of FSL, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET) Tool v2.1 (BET and BET2; part of FSL) to remove nonbrain tissues and background noise from the images.17 For each participant, the brain mask that was generated from the unlabeled ASL images was also applied to the CBF map. We used a boundary-based registration algorithm implemented in FMRIB's Linear Image Registration Tool (FLIRT, part of FSL http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT) to obtain accurate and robust ASL and T1W image alignment.18 The high resolution T1W image was used as the reference image of the extracted surfaces to separate tissue types, and then the unlabeled ASL image was aligned to the reference by maximizing the intensity gradient across tissue boundaries. Subsequently, the CBF maps were mapped into the T1W space by using the transformation.
The DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) toolbox (part of SPM8, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/)19 implemented in SPM8 was used to generate group-specific templates for all the subjects based on their segmented gray matter (GM) and white matter (WM) probability maps, and each group-specific template was registered into the Montreal Neurological Institute (MNI) standard space using affine transformation. Each individual subject's T1W image was mapped into the MNI space. With these 2 transformations, CBF maps in the T1W space were normalized to the group-specific template and mapped into the MNI space, and resliced to an isotropic voxel size of 1.5 mm. Because of the partial volume effect (PVE), CBF maps were corrected for volume atrophy according to the proportion of GM and WM in each voxel obtained from the segmented T1W image.20 To eliminate outliers in the perfusion image because of large blood vessels or image processing computations, the threshold was set at a low-value threshold of zero and a high-value threshold of 2 standard deviations above the mean perfusion for each subject.21 Finally, the CBF maps after PVE correction were spatially smoothed using a 6-mm full-width at half-maximum Gaussian kernel for voxel-wise comparisons.
Statistical Analysis
Analysis of Demographic Data
The demographic data, including age, sex, education level, and disease duration, were compared among the study groups using the 1-way analysis of variance test and Pearson χ2 test, were appropriate, and were reported as the mean ± the standard deviation. Differences in disease severity and levodopa equivalent doses were assessed using the Mann–Whitney U test. The MMSE and total intracranial volume (TIV) were analyzed using the analysis of covariance (ANCOVA) model with the participant's age, sex, and education level as covariates. The global tissue volumes, including the GM, WM, and CSF volumes, and global brain mean CBF were analyzed using the ANCOVA model with the participant's age, sex, education level, and TIV as covariates. The associations between the clinical evaluations and the CBF values derived from the differences found via group comparisons were calculated by partial correlation analysis. The threshold for all statistical significance was set at P < .05.
Analysis of Regional Cerebral Blood Flow Differences Between Groups
Comparisons between Normal Controls and Patients in ON and OFF Status
To identify CBF differences between NC and patients in the ON and OFF status, smoothed, PVE corrected CBF maps were analyzed with SPM8 using the framework of general linear model . A voxel-wise ANCOVA design was used with age and sex as covariates to remove the possible effect of these parameters. The differences in CBF maps were compared between the following groups: NC versus PDOFF, NC versus PDDOFF, NC versus PDON, and NC versus PDDON. To prevent possible PVEs between boundaries of different tissue types, a GM mask generated from the group-specific template, including voxels with a GM probability value of less than 0.2 (range from 0 to 1), was excluded. The command-line tool, AlphaSim, which is available in the AFNI toolbox (Analysis of Functional NeuroImages, http://afni.nimh.nih.gov/afni/), was used to correct the problem of image-based multiple comparisons. The statistical threshold for each voxel was set at a corrected Palpha of 0.05 (whereas Puncorrected was 0.005 with a cluster size of at least 275 voxels), based on the results of the Monte Carlo simulation. The coordinates of the voxels of each significant cluster were transformed from MNI coordinates into Talairach coordinates using the GingleALE toolbox (The BrainMap Development Team; http://brainmap.org/ale/index.html). The Talairach and Tournoux atlas (http://www.talairach.org/index.html) was used to identify the anatomic structures of the Talairach coordinates representing significant clusters.
Comparisons Between Parkinson Disease and Parkinson Disease With Dementia
To compare CBF differences between the PD patients and PDD patients, an ANCOVA design with age, sex, and levodopa equivalent dose as covariates was used for the voxel-wise comparisons between PDOFF versus PDDOFF and PDON versus PDDON (Palpha < 0.05).
Comparison Between ON and OFF Status in Patients
One sample t test with levodopa equivalent dose as the covariate was used for the voxel-wise comparisons between PDOFF versus PDON and PDDOFF versus PDDON (Palpha < 0.05).
Relationship Between Cerebral Blood Flow and Clinical Assessments
The mean CBF of the clusters with significant differences between ON and OFF status in the PD and PDD groups was extracted for further correlation analyses. Partial correlation analyses with age, sex, and years of education as nuisance covariates were performed to correlate the effect of levodopa equivalent dose on CBF value changes. A further analysis controlling for the levodopa equivalent dose was carried out to evaluate the relationship between interval changes of CBF values and the clinical characteristics (eg, MMSE and clinical disease severity). The threshold for statistical significance was set at P < 0.05 with Bonferroni correction for multiple comparisons. All statistical analyses were performed by SPSS software (SPSSV.17, Chicago, IL).
RESULTS
Demographic Data
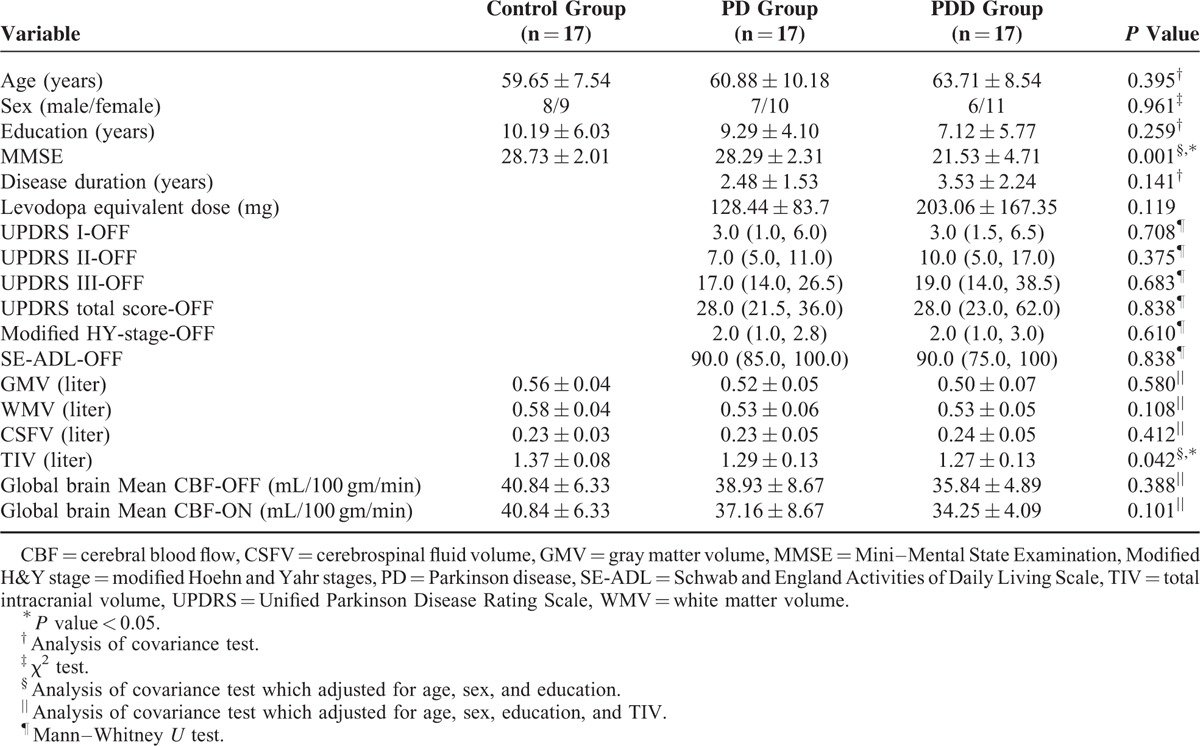
The demographic data are summarized in Table 1. Using ANCOVA analysis, it was determined that there were significant group differences in the MMSE scores (P < 0.001) and TIV (P = 0.042) among the NC, PDOFF and PDDOFF groups. We compared the patients’ performances on the UPDRS between the ON and OFF states using paired t-tests. The UPDRS III and UPDRS total scores were significantly worse in the PDOFF state than in the PDON state (P = 0.001 and P < 0.001, respectively) (data not shown). These results indicate that dopaminergic medications significantly improved the disease severity of the patients, with the improvement being most apparent in terms of motor functions.
TABLE 1.
Comparisons of Demographic Variables, Clinical Variables, Global Anatomic Measurements, and Cognitive Assessment Results for the Healthy Controls, Parkinson Disease Patients, and Parkinson Disease With Dementia Patients
Perfusion Deficits in Parkinson Disease Patients and Parkinson Disease With Dementia Patients
Cerebral Blood Flow Quantification
The calculated whole brain mean CBF in the control group was 40.84 ± 6.33 mL·100 g−1 min−1 (mean ± standard deviation) (Table 1). In the PDOFF and PDDOFF patient groups, the mean CBF values were 38.93 ± 8.67 and 35.84 ± 4.89, values which were 1% and 9% lower, respectively, than that for the control group. These values are in the range of those for perfusion decreases measured in a recent ASL study of PD.22 In the PDON and PDDON patient groups, the mean CBF values were 37.16 ± 8.67 and 34.25 ± 4.09, values which were 9% and 16% lower, respectively, than that for the control group. Using ANCOVA analysis, it was determined that there was no significant group difference of the whole brain mean CBF among the NC, PDOFF, and PDDOFF groups (P = 0.388), nor was there any significant group difference among the NC, PDON, and PDDON groups (P = 0.301). For both the PD group (P = 0.148) and the PDD group (P = 0.112), there were also no significant dopaminergic medication effects indicated via paired t-tests in terms of the whole brain mean CBF values between the ON and OFF states.
Cerebral Blood Flow Comparisons Between Normal Controls and Patients in ON and OFF Status
Normal Controls Versus Parkinson DiseaseOFF
The voxel-wise analysis of the absolute CBF maps revealed no significant perfusion deficits in the PDOFF patient group (Table 2 and Figure 1A).
TABLE 2.
Comparisons of Cerebral Blood Flow Levels in Different Brain Regions for Normal Controls and Patients in ON or OFF Status
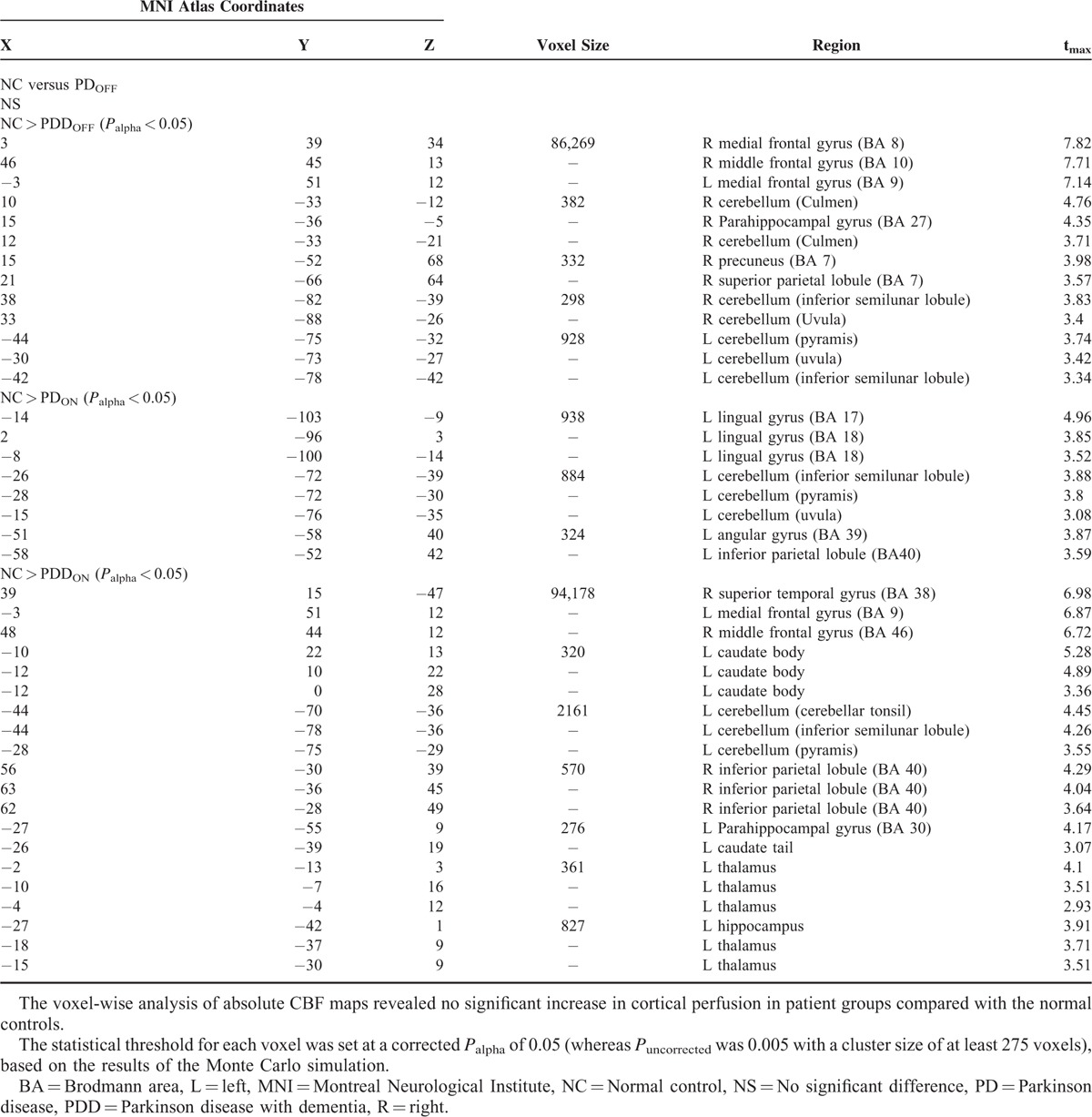
FIGURE 1.
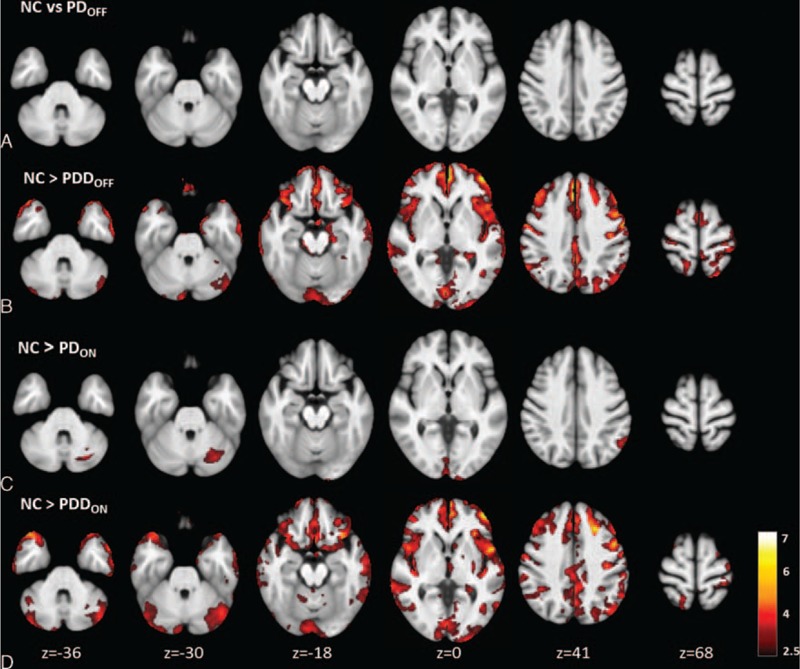
Regions with significantly decreased absolute cerebral blood flow in Parkinson disease and Parkinson disease with dementia patients in the ON and OFF status compared with healthy controls (cluster level statistics, P value < 0.05, family-wise error corrected). The color bar indicates the T scores scale.
Normal Controls Versus Parkinson Disease With DimentiaOFF
Bilateral perfusion decreases were found in the frontal, parietal, and cerebellar regions in the PDDOFF group, whereas only small decreases were found in the temporal and occipital areas in that group (Table 2 and Figure 1B). In the frontal lobe, lower perfusion was found in the medial and middle frontal gyri (Brodmann areas 8, 9, and 10), extending from prefrontal cortex and premotor cortex to the mesial surface of hemisphere and the presupplementary motor area. The area of the presupplementary motor area, the caudal portion of the mesial area BA 6, preserved normal cortical perfusion. In the parietal lobe, lower perfusion was found in the superior parietal lobes and precuneus (BA 7). In the occipital lobe, decreases were found in the cuneus, corresponding to the visual cortex. In the temporal lobe, the parahippocampus gyrus was affected (Table 2 and Figure 1B).
Normal Controls Versus Parkinson DiseaseON
For the PDON patient group, the absolute CBF maps revealed significant perfusion deficits in the occipital lobes, parietal lobes, and cerebellum that were not found before the administration of dopaminergic medications (Table 2 and Figure 1C).
Normal Controls Versus Parkinson Disease With DimentiaON
The CBF patterns in the PDDON patients also demonstrated similar but more widespread neocortical hypoperfusion patterns than those found in the PDOFF patients (Table 2 and Figure 1D). Decreased perfusion was also found in subcortical regions, such as the caudate nucleus and thalamus.
Cerebral Blood Flow Comparisons between Parkinson Disease and Parkinson Disease With Dimentia
Parkinson DiseaseOFF Versus Parkinson Disease With DementiaOFF
Compared with the PD patients, the PDD patients had significantly lower CBF values in the frontal lobe, including the medial (BA 9), inferior (BA 47), paracentral (BA 6), and superior frontal lobe (BA 6) regions. The PDD group also exhibited lower CBF in the parahippocampus (BA 30), anterior cingulate (BA 32), and precuneus (BA 7) (Figure 2A and Supplementary Table 1).
FIGURE 2.
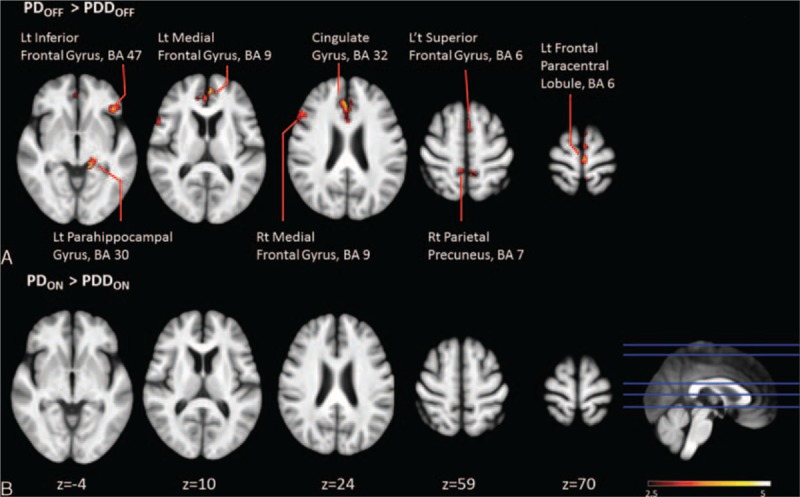
Clusters with significant group differences in absolute cerebral blood flow between the Parkinson disease patients and the Parkinson disease with dementia patients in the ON and OFF status (cluster level statistics, P value < 0.05, family-wise error corrected).
Parkinson DiseaseON Versus Parkinson Disease With DementiaON
A comparison of the PDON and PDDON groups found no significant differences in CBF for the various brain regions (Figure 2B and Supplementary Table 1).
Cerebral Blood Flow Comparison Between OFF and ON Status in Patients
Parkinson DiseaseOFF Versus Parkinson DiseaseON
After the administration of dopaminergic medications, the PD group showed significantly decreased CBF in the left inferior frontal gyrus (BA 47, 13) (Figure 3A and supplementary Table 2).
FIGURE 3.
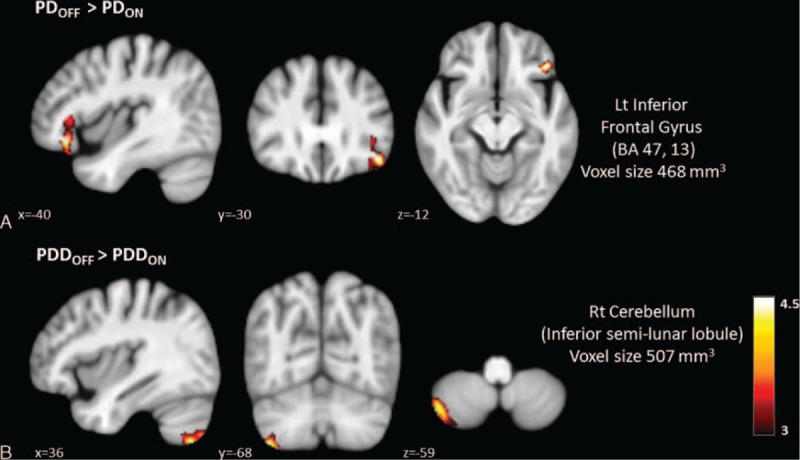
Regions with significantly decreased absolute cerebral blood flow values in ON status compared to OFF status in Parkinson disease and Parkinson disease with dementia patients.
Parkinson Disease With DementiaOFF Versus Parkinson Disease With DementiaON
In patients with PDD, the administration of dopaminergic medications significantly decreased right cerebellar perfusion (inferior semilunar lobule) (Figure 3B and Supplementary Table 2).
Cerebral Blood Flow Comparison Between OFF and ON Status in Patients.
Correlations With Levodopa Equivalent Dose
In the PD patients, the decrease in CBF values from the OFF status to the ON status in the left inferior frontal gyrus was negatively correlated with higher daily levodopa equivalent doses (r = -0.743, P = 0.001). In the PDD patients, the decrease in CBF values from the OFF status to the ON status in the right cerebellum was also negatively correlated with higher daily levodopa equivalent doses (r = −0.616, P = 0.008) (Figure 4).
FIGURE 4.
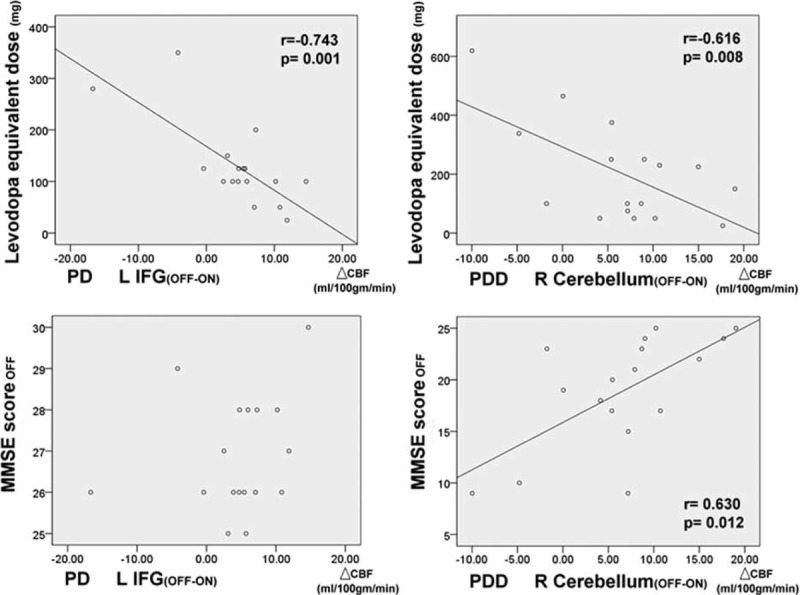
Effect of dosages of dopaminergic medications on regional cerebral blood flow changes (with age and sex as covariates), and correlations between mini-mental state examination scores in OFF status and regional cerebral blood flow changes after dopaminergic medications (with age, sex, education, and levodopa equivalent dose as covariates) (L, left; R, right; IFG, inferior frontal gyrus).
Correlations With Mini-Mental State Examination in the OFF Status
Controlling for the levodopa equivalent dose, the decrease in CBF values from the OFF status to the ON status in the right cerebellum was also positively correlated with lower MMSE scores (r = 0.515, P = 0.041) in the PDD group (Figure 4). For the PD group, in contrast, there was no statistically significant correlation between the MMSE scores and the change in CBF values from the OFF status to the ON status.
Correlations With Clinical Disease Severity in the OFF Status
Controlling for the levodopa equivalent dose, there was no statistically significant correlation between the clinical severity assessment results and the changes in CBF values from the OFF status to the ON status for the various brain regions in the PD group. In the PDD group, however, the decrease in CBF values from the OFF status to the ON status in the right cerebellum was associated with higher disease severity (UPDRS I, r = −0.506, P = 0.045; UPDRS III, r = −0.501, P = 0.049; H&Y stage, r = −0.522, P = 0.038; Schwab and England Activities of Daily Living Scale, r = −0.583, P = 0.018) (Figure 5).
FIGURE 5.
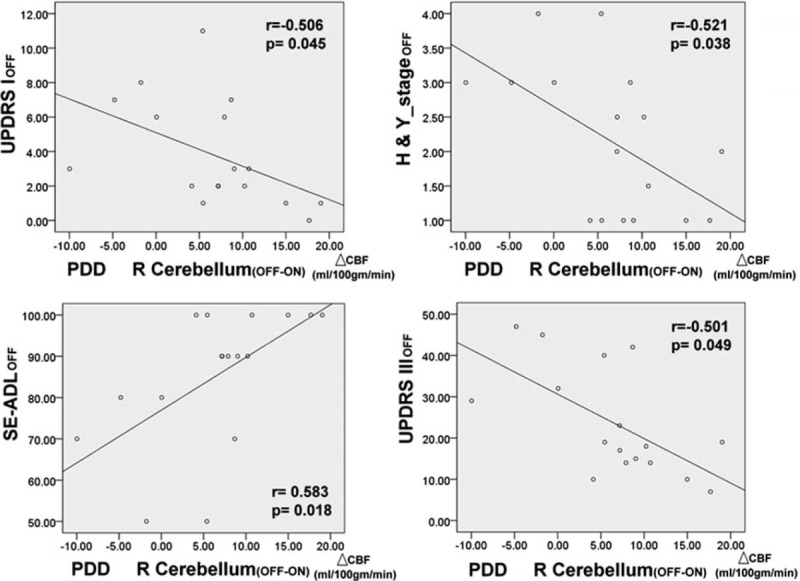
Correlations between disease severity in OFF status and regional cerebral blood flow changes after dopaminergic medications (with age, sex, education, and levodopa equivalent dose as covariates).
Correlations With Clinical Disease Severity in the ON Status
For both the PD and PDD groups, there was no statistically significant correlation between the clinical severity assessment results and the changes in CBF values from the OFF status to the ON status for the various brain regions.
Correlations With Interval Changes of Clinical Disease Severity (OFF–ON)
For both the PD and PDD groups, there was no statistically significant correlation between the degree of clinical severity improvement and the changes in CBF values from the OFF status to the ON status for the various brain regions.
DISCUSSION
Summary
Consistent with our hypothesis and in line with the extant literature, the PD patients and PDD patients experienced progressive cortical hypoperfusion in comparison with the NC. In particular, we also found evidence of dosage-dependent pharmaceutical effects of the dopaminergic therapies on cerebral perfusion in different vulnerable brain regions in the PD and PDD patients. Furthermore, by linking the immediate changes in CBF before and after dopaminergic treatment with measurements of disease severity and cognitive functions, we demonstrated that the effects of dopaminergic therapies on cortical perfusion might be reduced as the disease progresses. Our results highlight the potential value of using ASL as a global marker of overall impairment and for therapeutic monitoring in PD.
Pathophysiology of Hypoperfusion in Parkinson Disease and Parkinson Disease With Dimentia
Our results showed extensive cortical perfusion deficits in the PDD patients, regardless of whether those patients were in the ON or OFF status, as well as mild decreases in perfusion in the occipital and cerebellar regions in the PD group patients when they were in the ON status. The neuroimaging findings of past studies regarding cortical perfusion alterations have varied. Although multiple pathophysiologies support the conclusion that perfusion changes occur in PD, differences in the patient populations studied (ie, in terms of sample size, disease severity, and medication status), different data-analysis procedures, and differences in the statistical criteria selected have led to differences among the observations of different studies. Using a relatively optimal and strict analysis threshold combined with various meta-analyses, Borghammer et al23 recently concluded that PD most likely results in widespread cortical hypometabolism, even at early disease stages.
The underlying mechanism of the extensive reduction of cortical CBF in PD patients remains unknown. The well-known pathologic process in PD is the degeneration of the nigrostriatal pathway, with the consequent reduction of dopamine in the striatum. In addition, Braake et al have proposed that the early degeneration of various subcortical structures corresponding to different neuronal systems, including dopaminergic, cholinergic, noradrenergic, and serotonergic networks, occurs in PD.24 Since those neuronal systems are widespread and connected to different subregions of the striatum and neocortices, it is reasonable that parallel neurodegenerative depletion of these neuron populations and an extensive decrease in cortical perfusion would be observed. Another piece of evidence for an association between the number of existing neurons and cortical perfusion was provided by several studies, in which chemical and electrical stimulations of those neural systems were used to elicit significant increases in regional and global CBF and vasodilatation (Borghammer et al23). Our PDD results showing extensive cortical hypoperfusion may well represent the profound loss of neurons involved in these neuronal systems.
Another finding indicating that no region exhibited hypoperfusion in PD patients in the OFF status seems contradicted by previous reports. There, however, has been some speculation that the cortical hypoperfusion reported in PET and SPECT studies is actually because of a difference in the calculations caused by gray matter atrophy.23 In a previous ASL study, regions with cortical perfusion deficits were found to overlap to a high degree with areas of gray matter volume loss determined by voxel-based morphometry.22 The major concern regarding perfusion measurements comes from the low degree of spatial resolution and the lack of morphologic information imaging modalities. Since nearly all of the previous PET and SPECT studies of PD did not employ partial volume correction, the results of the current study indicating no obvious cortical hypoperfusion in PD are probably strengthened by the use of PVC, which would lessen the confounding influence of cortical atrophy in neurodegenerative diseases. Our results are comparable to those of the existing literature showing that the magnitude of the cortical perfusion decreases in PD are somewhat diminished following PVC.25 Nevertheless, another argument is that the cortical hypoperfusion can probably only be partially explained by atrophy.26 Lots of volumetric studies have demonstrated no loss of gray matter or less extensive clusters of atrophy in early-stage PD subjects.27 In addition, PD exhibits altered functional connectivities, which have previously been shown to be associated with changes in regional/global neurovascular coupling and hemodynamic responses and can precede structural changes.14,28 At present, it is difficult to use a single etiology to completely explain cortical hypoperfusion.
Pharmaceutical Effects of Dopaminergic Therapies on Cortical Perfusion
Comparisons of cerebral blood flow between PDOFF versus PDON and PDDOFF versus PDDON revealed decreased perfusion after the administration of dopaminergic medications in brain regions that are known to be functionally and anatomically connected to different subregions of the striatum.14 In the current study, dopaminergic medications significantly decreased inferior frontal lobe perfusion in PD patients as well as cerebellar perfusion in PDD patients. These diminished perfusion levels were partially in line with a previous functional MRI study indicating that the functional connectivities between the ventral striatum, the ventromedial prefrontal cortex, and the cerebellum were down-regulated by levodopa. That study also, however, found hyperconnectivity in patients with levodopa OFF status compared with controls,9 whereas the cortical perfusion was decreased in the current study. Compared with the normal subjects, the PDON patients showed hypoperfusion in the precuneus and occipital lobes, findings which were somewhat similar to previous results indicating that levodopa disrupts ventral striatal and dorsal caudate functional connectivity with the default mode network in healthy adults.8 It is still difficult to draw firm conclusions regarding the relationship between changes in regional cortical perfusion and the cortico-striatal functional network during dopaminergic medication. According to previous reports, the pathologic state of dopamine depletion is associated with increased synchronous oscillatory activity in the cortico-striatal networks.29 In contrast, dopaminergic medications and deep brain stimulation were demonstrated to eliminate these increases in coherent neural activity across networks with clinical improvement.30 The decrease in cortical perfusion might thus reflect a decrease in the energetic need for cortico-cortical synchronous oscillatory activity after dopamine resupply.
Specifically, we found that dopaminergic therapy reduced cortical perfusion in different anatomies in the PD and PDD groups. According to the Braak model, alpha synuclein is deposited in specific brain regions and neuronal types, giving rise to Lewy pathology in a stereotypic, temporal pattern that ascends caudo-rostrally from the lower brainstem through susceptible regions of the midbrain and forebrain and into the cerebral cortex.24 In addition, a recent study reported that brain atrophy occurs in early PD in inferior frontal regions related to primary olfactory and/or secondary integrative olfactory processing, such as the olfactory bulb, piriform cortex, and orbitofrontal cortex.31 Furthermore, frontal lobe hypometabolism might exceed gray matter atrophy in the early stages of PD with mild cognitive impairment.32 Therefore, treatment effects in early PD might substantially contribute to the down regulation of fronto-striatal hyperconnectivity with subsequent lessening of the need for cortical perfusion in the frontal lobe.
Our results were consistent with those of a previous study showing a decline of frontal lobe, anterior cingulate, and parahippocampal perfusion in PDDOFF patients compared with the PDOFF patients.33 This can be explained by the fact that PD is associated with better dopaminergic function reserves in the fronto-striatal and mesolimbic system than PDD is. Increasing evidence suggests that the major role of the cerebellum in PD is likely to include both pathologic and compensatory effects. In contrast to the functioning of the frontal lobes, which present in the preclinical or early stages of PD with lesions and corresponding frontal lobe symptoms, the pseudo-normal cerebellar functioning of PD patients is actually because of a balance between the effects of lesions, compensation by the cerebellum, and dopaminergic effects.34 Strengthened connections between cortico-cerebellar motor regions have previously been shown to counterbalance weakened striatal-cortical and striatal-cerebellar connections to compensate for basal ganglia dysfunction.34 Extensive neocortical degeneration with a decrease number of dopamine neurons in PDD weakens the capacity to respond to the dopaminergic treatment with neocortical perfusion. Therefore, a compensatory increase in the cortico-cerebellar connections or a hyperactive cerebello-thalamo-cortical loop in PDD may help to maintain better motor and nonmotor functions. Levodopa had been shown to decrease functional connectivity between the ventral striatum and cerebellum,9 which might be associated with cerebellar hypoperfusion in PDON patients. Nonetheless, further research is needed to support this concept.
Desensitization to the Effects of Dopaminergic Therapies on Cortical Perfusion as the Disease Progresses
The disabling “on-off” fluctuations are among the most frequent and troublesome complications of chronic levodopa therapy in PD. In addition to investigating the responses of the main anatomies to dopaminergic medications, we also explored whether alterations in their cortical perfusion were inversely related to dosages. Furthermore, higher disease severities, including poorer motor and cognitive functions, were strongly associated with lower cortical perfusion responses to dopaminergic medications even after controlling for differing dosages of the medications. Our results were compatible with the general concept that supplying dopamine pharmaceutically over a long period of time is associated with a narrowing of the therapeutic window.35 The G protein-coupled receptors phosphorylation and down regulation can be associated with a loss of response to dopaminergic treatments when a given drug is administered repeatedly.36 Since PD and PDD both revealed an inversed dosage related cortical perfusion response, the desensitization of dopaminergic receptors might partially explain the current findings.
In addition, our perfusion results might indicate deteriorating presynaptic functions of dopamine neurons because of a decrease in dopamine neuronal survival. Dopaminergic drugs, such as levodopa, are potentially toxic to dopaminergic neurons.37 Two studies of patients with PD have evaluated the effect of levodopa compared with a dopamine agonist on surrogate neuroimaging biomarkers of dopamine neuronal function.38,39 Levodopa was demonstrated to be associated with an accelerated rate of decline in biomarker activity compared with the dopamine agonist.38,39 Although dosage-related perfusion changes were observed in both the PD and PPD groups, a significant correlation between perfusion changes and disease severity was only found among the PDD subjects. It is possible that these findings might have resulted from a differential pharmacological effect of the drug on target receptors, since early PD patients are more likely to take dopamine agonists whereas PDD patients are more likely to take levodopa.40 Nevertheless, our result might simply reflect the natural degeneration process of PD. Such an effect, however, was difficult to clarify in the current study.
The interpretation of the findings here must be tempered by some of the limitations of the current study. First, the number of subjects was relatively small. It is possible that our results could not be regenerated in all disease spectrums. There were also mild imbalances in the mean age and mean disease duration for the PD and the PDD patients who might have confounded the statistical analysis. Our results need to be replicated. Much larger sample sizes may permit the identification of perfusion effects specific to degeneration versus dementia subpopulations. Furthermore, the direct effect of different dopaminergic medications on cortical perfusion and their characteristic patterns in this study are unknown because all medicated patients were assessed and imaged with no disruption to their normal drug regimens. Since all the patients underwent MRI scanning under a stable medication control condition (ie, when continuing the same dopaminergic medications for at least 3 months), our group contrasts reveal different disease severities rather than different medication effects. This topic awaits further investigation.
CONCLUSIONS
In conclusion, we noninvasively explored PD- and PDD-related cortical blood flows via ASL. The perfusion patterns were characterized by extensively decreased neocortical and preserved subcortical perfusion. The development of dementia in PD further worsens the perfusion status. In addition, the highly sensitive temporal responses to the direct effect of dopaminergic medications shown by ASL allow clinicians and researchers to easily, safely, and effectively quantify the absolute perfusion values. The availability and repeatability of ASL might prove useful for therapeutic planning and offer a useful platform for translation research, particularly in clinical trials of new antiparkinsonian therapies.
Supplementary Material
Footnotes
Abbreviations: AFNI = Analysis of Functional NeuroImages, ANCOVA = analysis of covariance, ASL = arterial spin labeling, BET = Brain Extraction Tool, CBF = cerebral blood flow, DARTEL = Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra, FLIRT = FMRIB's Linear Image Registration Tool, GLM = general linear model, MMSE = Mini-Mental State Examination, MNI = Montreal Neurological Institute, NC = normal controls, PD = Parkinson disease, PDD = Parkinson disease with dementia, PET = positron emission tomography, PVE = partial volume effect, SPECT = single photon emission computed tomography, SPM = statistical parametric mapping, UPDRS = Unified Parkinson Disease Rating Scale.
This work was supported by funds from Chang Gang Memorial Hospital (Chang Gang Medical Research Project CMRPG891511 and CMRPG8B0831 to W-C Lin) and from the National Science Council (NSC101-2314-B-182A-078 to W-C Lin).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Goetz CG, Emre M, Dubois B. Parkinson's disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 2008; 64:S81–S92. [DOI] [PubMed] [Google Scholar]
- 2.Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nature reviews. Neurology 2014; 10:708–722. [DOI] [PubMed] [Google Scholar]
- 3.Melzer TR, Watts R, MacAskill MR, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain 2011; 134:845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson's disease. Brain 2007; 130:1834–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herz DM, Haagensen BN, Christensen MS, et al. The acute brain response to levodopa heralds dyskinesias in Parkinson disease. Ann Neurol 2014; 75:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson's disease: a review. Ther Adv Psychopharmacol 2013; 3:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamagata K, Motoi Y, Hori M, et al. Posterior hypoperfusion in Parkinson's disease with and without dementia measured with arterial spin labeling MRI. J Magn Reson Imaging 2011; 33:803–807. [DOI] [PubMed] [Google Scholar]
- 8.Kelly C, de Zubicaray G, Di Martino A, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci 2009; 29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak Y, Peltier S, Bohnen NI, et al. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci 2010; 4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deibler AR, Pollock JM, Kraft RA, et al. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol 2008; 29:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirasol RV, Bokkers RP, Hernandez DA, et al. Assessing reperfusion with whole-brain arterial spin labeling: a noninvasive alternative to gadolinium. Stroke 2014; 45:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobson DE, Lang AE, Martin WR, et al. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. J Am Med Assoc 2002; 287:455–463. [DOI] [PubMed] [Google Scholar]
- 14.Chou KH, Lin WC, Lee PL, et al. Structural covariance networks of striatum subdivision in patients with Parkinson's disease. Hum Brain Mapp 2015; 36:1567–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008; 60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009; 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113. [DOI] [PubMed] [Google Scholar]
- 20.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 2008; 26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Seara MA, Mengual E, Vidorreta M, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI. Neuroimage 2012; 59:2743–2750. [DOI] [PubMed] [Google Scholar]
- 23.Borghammer P, Chakravarty M, Jonsdottir KY, et al. Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: probably even at early disease stages. Brain Struct Funct 2010; 214:303–317. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24:197–211. [DOI] [PubMed] [Google Scholar]
- 25.Firbank MJ, Colloby SJ, Burn DJ, et al. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage 2003; 20:1309–1319. [DOI] [PubMed] [Google Scholar]
- 26.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 2005; 64:224–229. [DOI] [PubMed] [Google Scholar]
- 27.Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. European journal of neurology: the official journal of the European Federation of Neurological Societies 2012; 19:199–206. [DOI] [PubMed] [Google Scholar]
- 28.Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 2009; 30:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 2007; 30:357–364. [DOI] [PubMed] [Google Scholar]
- 30.Silberstein P, Pogosyan A, Kuhn AA, et al. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain 2005; 128:1277–1291. [DOI] [PubMed] [Google Scholar]
- 31.Lee EY, Eslinger PJ, Du G, et al. Olfactory-related cortical atrophy is associated with olfactory dysfunction in Parkinson's disease. Mov Disord 2014; 29:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Redondo R, Garcia-Garcia D, Clavero P, et al. Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: a two-step process. Brain 2014; 137:2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, Nagano-Saito A, Kato T, et al. Striatal and extrastriatal dysfunction in Parkinson's disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain 2002; 125:1358–1365. [DOI] [PubMed] [Google Scholar]
- 34.Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain 2013; 136:696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block G, Liss C, Reines S, et al. Comparison of immediate-release and controlled release carbidopa/levodopa in Parkinson's disease. A multicenter 5-year study. The CR First Study Group. Eur Neurol 1997; 37:23–27. [DOI] [PubMed] [Google Scholar]
- 36.Maggio R, Barbier P, Corsini GU. Apomorphine continuous stimulation in Parkinson's disease: receptor desensitization as a possible mechanism of reduced motor response. J Neural Transm Suppl 1995; 45:133–136. [PubMed] [Google Scholar]
- 37.Olanow CW, Obeso JA. Levodopa toxicity and Parkinson disease: still a need for equipoise. Neurology 2011; 77:1416–1417. [DOI] [PubMed] [Google Scholar]
- 38.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003; 54:93–101. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson Study G. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. J Am Med Assoc 2002; 287:1653–1661. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia K, Brooks DJ, Burn DJ, et al. Guidelines for the management of Parkinson's disease. The Parkinson's Disease Consensus Working Group. Hosp Med 1998; 59:469–480. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


