Abstract
Beta (β)-blockers are under-prescribed in patients with heart failure (HF) and concurrent chronic obstructive pulmonary disease (COPD) due to concerns about adverse pulmonary effects and a poor understanding of the effects of these drugs. We aimed to evaluate the survival effects of β-blockers in patients with coexistent HF and COPD. Using the Taiwan National Health Insurance Research Database, we conducted a nationwide population-based study. Patients with coexistent HF and COPD diagnosed between 2000 and 2009 were enrolled. Doses of the 3 β-blockers proven to be beneficial to HF (carvedilol, bisoprolol, and metoprolol) during the study period were extracted. The primary endpoint was cumulative survival. Patients were followed until December 31, 2009. The study included 11,558 subjects, with a mean follow-up period of 4.07 years. After adjustment for age, sex, comorbidities, and severity of HF and COPD, bisoprolol use showed a dose–response survival benefit [low dose: adjusted hazard ratio (HR) = 0.76, 95% confidence interval (CI) = 0.59–0.97, P = 0.030; high dose: adjusted HR = 0.40, 95% CI = 0.26–0.63, P < 0.001] compared with nonusers, whereas no survival difference was observed for carvedilol or metoprolol. Compared with patients with HF alone, this special HF + COPD cohort received significantly fewer targeted β-blockers (108.8 vs 137.3 defined daily doses (DDDs)/person-year, P < 0.001) and bisoprolol (57.9 vs 70.8 DDDs/person-year, P < 0.001). In patients with coexisting HF and COPD, this study demonstrated a dose–response survival benefit of bisoprolol use, but not of carvedilol or metoprolol use.
INTRODUCTION
Heart failure (HF) is a major global health concern characterized by high mortality, frequent hospitalization, and poor quality of life, especially in elderly individuals. Improvements in medical treatment have shifted the strategy of HF management from short-term hemodynamic modification to altering the biological properties of the failing heart, greatly improving prognoses.1 Among new treatments, the dramatic rise of beta (β)-blocker therapy, which was initially considered to be contraindicated in HF, is an important milestone.2
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation and associated with an abnormal inflammatory response in the lungs.3 The prevalence of COPD is as high as 39.2% in elderly patients with stable HF, and COPD was identified as an independent risk factor of mortality.4 In turn, patients with COPD were reported to have higher risks for HF, atherosclerosis,4 and death due to cardiovascular diseases.5 Despite the large number of affected patients and greater mortality risk, the coexistence of HF and COPD continues to present a therapeutic dilemma.4,6
The Heart Failure Society of America's comprehensive practice guidelines for HF have recommended the use of β-blockers, even in patients with COPD,1 but concern about the detrimental effect of this therapy on COPD has led to its suboptimal use.6 Because patients with COPD have frequently been excluded from large trials of β-blockers, limited information about the drugs’ effects is available for these patients.4,6–10 More evidence of the survival benefit of this therapy is needed to resolve the treatment dilemma. Ample evidence has demonstrated differences in the effects of β1-selective and nonselective β-blockers, as well as other distinct β-blockers, in patients with COPD,10–15 but whether these differences impact survival remains unknown. Furthermore, the different effects of distinct β-blockers on respiratory systems may result in survival differences. Thus, using a nationwide population-based dataset, this study aimed to analyze the distinct survival effects of the three β-blockers recommended in the guidelines for HF1 (carvedilol, bisoprolol, and metoprolol) in patients with coexistent HF and COPD.
METHODS
Data Source
Taiwan's National Health Insurance (NHI) program, initiated by the government in 1995, provides comprehensive health care to almost all Taiwanese citizens, with a coverage rate of more than 99% of Taiwan's entire population.16 The National Health Research Institute (NHRI) of Taiwan manages and publicly releases, for research purposes, multiple NHI databases that include information about basic patient characteristics; detailed claims data for examinations, disease management, and drug prescriptions; and diagnoses for all admitted patients and outpatients. The NHRI created a research database including a random sample of 1,000,000 subjects from the registry of all NHI enrollees in 2000, with encryption of all data that might allow the identification of any individual. We obtained datasets from this nationally representative cohort from the NHRI for use as our research database. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (VGHIRB No. 201209007BC).
Study Design and Population
We conducted a retrospective cohort study of the period from January 1, 2000 to December 31, 2009. Using the diagnostic codes provided in the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM), we enrolled patients with diagnoses of both HF (ICD-9 codes 425.4, 425.9, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.xx) and COPD (ICD-9 codes 491.xx, 492.xx, 494.xx, and 496.xx). All patients were followed until the date of death, withdrawal from insurance, or December 31, 2009. All daily doses of the 3 β-blockers (carvedilol, bisoprolol, and metoprolol) proven to be beneficial to HF were extracted.1 The average daily doses of the 3 β-blockers in each 3-month period, divided retrospectively from the end of the follow-up date, were applied as time-dependent covariates. A time lag of 3 months was applied to evaluate the effect of β-blocker use in each 3-month block because of the delayed cardiac benefit of this treatment.17–19 Subjects followed for less than a 3-month period of medication use plus a 3-month period of lag time for evaluation (180 days) were excluded. According to the 2010 HF guidelines,1 each 3-month block of the study subjects was assigned to one of the following groups: low-dose carvedilol (≥6.25 and <50 mg/d), high-dose carvedilol (≥50 mg/d), low-dose bisoprolol (≥1.25 and <10 mg/d), high-dose bisoprolol (≥10 mg/d), low-dose metoprolol (≥25 and <200 mg/d), high-dose metoprolol (≥200 mg/d), and nonuser. Patients’ survival outcomes were analyzed after new diagnoses of both HF and COPD, regardless of which occurred first.
Potential Confounders and Classification of Severity
In the analysis of the effects of β-blockers on survival outcomes, we controlled for age, sex, and severity of COPD and HF, and identified the following comorbidities diagnosed before enrollment that might confound the results: diabetes mellitus (ICD-9-CM code 250.xx), dysrhythmia (427.xx), ischemic stroke (433.xx, 434.xx, 436, and 437.1), intracranial hemorrhage (430, 431, 432.x), hypertension (401.xx–405.xx and 437.2), ischemic heart disease (410.xx–414.xx), chronic kidney disease (580.xx–587.xx), and liver cirrhosis (571.2, 571.5, and 571.6).
The severity of COPD was determined by the annual frequency of hospitalization for COPD exacerbation within the follow-up period, as the exacerbation frequency history has been suggested to be an important factor in classifying COPD severity.20 Patients with 2 or more hospitalizations for COPD exacerbation per year were assigned to the severe COPD group and those with no hospitalization for exacerbation were assigned to the mild group; all other patients were assigned to the moderate group. The severity of HF was classified in the same way, using the same cut-off value of admission rate per year for HF exacerbation.
Carvedilol, Bisoprolol, and Metoprolol Usage in Patients With Coexistent HF and COPD
We conducted a retrospective analysis to evaluate the prescription of the 3 β-blockers proven beneficial to HF (carvedilol, bisoprolol, and metoprolol) in patients with coexistent HF and COPD. From January 1, 2000 to December 31, 2009, all patients diagnosed with HF were identified for analysis, and were further divided into 2 cohorts: HF only and HF with concurrent COPD. Prescriptions of β-blockers during HF in the HF cohort were extracted and compared with those during HF with coexistent COPD in the cohort with both diseases. The cumulated doses of carvedilol, bisoprolol, and metoprolol were calculated as defined daily doses (DDDs), according to the 2010 HF guidelines.1 The following DDDs of these β-blockers were used: carvedilol, 6.25 mg; bisoprolol, 1.25 mg; and metoprolol, 25 mg. The mean DDDs per person-year in the 2 cohorts were compared.
Statistical Analyses
Data extraction and computation were performed using the Perl programming language (ver. 5.12.2; Perl Foundation, Walnut, CA). Microsoft SQL Server 2012 (Microsoft Corporation, Redmond, WA) was used for data processing and sampling. All statistical analyses were conducted using the SAS software (ver. 9.2 for windows; SAS Institute, Cary, NC). The analytical endpoints were cumulative survival. The validity of the classification of COPD and HF severity in our study was analyzed by Kaplan–Meier method. Time-dependent Cox proportional-hazards regression analysis was used to determine the effect of β-blocker use on survival outcome during the follow-up period, with β-blocker use calculated as a time-dependent covariate. Other baseline variables that were considered to be potentially confounding factors included age, sex, and comorbidities. Variables with significance levels of P < 0.1 in univariate analysis were included independently in the multivariable analysis using a stepwise selection procedure. P < 0.05 was considered to indicate statistical significance.
RESULTS
Clinical Characteristics of the Study Population
The baseline characteristics of the study groups are shown in Table 1. A total of 13,182 adult patients with diagnoses of both COPD and HF between January 2000 and December 2009 were identified. After excluding patients aged <20 years (n = 4) and those who died within 180 days after diagnosis or were followed for <180 days (n = 1620), the study sample consisted of 11,558 patients. The study subjects were predominantly male (n = 6175, 53.4%) and the median age was 70 [range, 20–101; interquartile range (IQR), 63–76] years. The most common comorbidity was hypertension (87.9%), followed by ischemic heart disease (62.9%), arrhythmia (40.3%), and diabetes mellitus (33.6%). Most (n = 9121, 78.9%) patients had mild COPD, 1180 (10.2%) had moderate COPD, and 1257 (10.9%) patients had severe COPD; 8385 (72.6%) patients had mild HF, 1585 (13.7%) had moderate HF, and 1588 (13.7%) patients had severe HF (Figure 1). The patients were followed for a maximum of 9 (mean, 4.07) years and 2076 (18.0%) patients died during the follow-up period.
TABLE 1.
Baseline Characteristics

FIGURE 1.
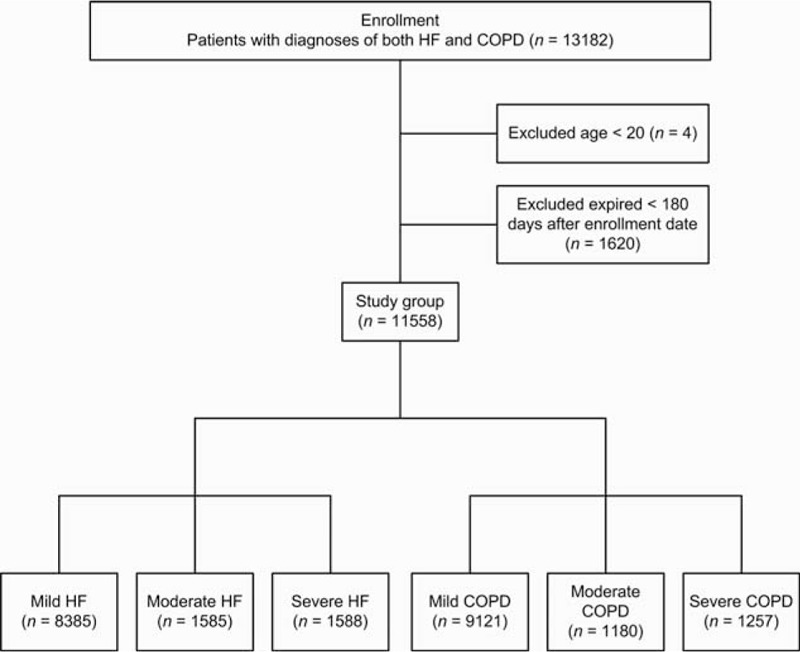
Flow diagram summarizing the process of enrollment.
Survival Effects of Carvedilol, Bisoprolol, and Metoprolol
The survival effects of β-blockers were adjusted in a time-dependent Cox proportional hazards model after correcting for potentially confounding factors, including age, sex, and comorbidities. In the univariate analysis, elderly age, male sex, all identified major comorbidities, and COPD or HF severity significantly affected survival (Table 2). Of note, survival decreased significantly with increasing severity of COPD or HF in the Kaplan–Meier analysis (Figure 2 A and B); this result was sustained after adjustment for other factors (Table 2). Compared with patients with mild COPD, the adjusted hazard ratios (HRs) for mortality in patients with moderate and severe COPD were 1.13 [95% confidence interval (CI), 1.02–1.27; P = 0.026] and 2.56 (95% CI, 2.22–2.95; P < 0.001), respectively. Compared with patients with mild HF, the adjusted HRs for mortality in patients with moderate and severe HF were 1.35 (95% CI, 1.47–1.85; P < 0.001) and 4.11 (95% CI, 3.60–4.70; P < 0.001), respectively. After adjusting for these factors in the time-dependent multivariable Cox regression model, the survival effects of the three β-blockers were analyzed. Compared with nonusers, bisoprolol use was associated with a significantly lower dose-dependent risk of mortality (low-dose bisoprolol: HR = 0.76, 95% CI = 0.59–0.97, P = 0.030; high-dose bisoprolol: HR = 0.40, 95% CI = 0.26–0.63, P < 0.001), whereas no such difference was observed for carvedilol or metoprolol. The subgroup analysis is presented in Figure 3. It is noteworthy that HRs of bisoprolol were statistically significantly lower in most of subgroups, suggesting an independent role of bisoprolol in patient survival.
TABLE 2.
Adjusted Survival Effects of Carvedilol, Bisoprolol, and Metoprolol∗
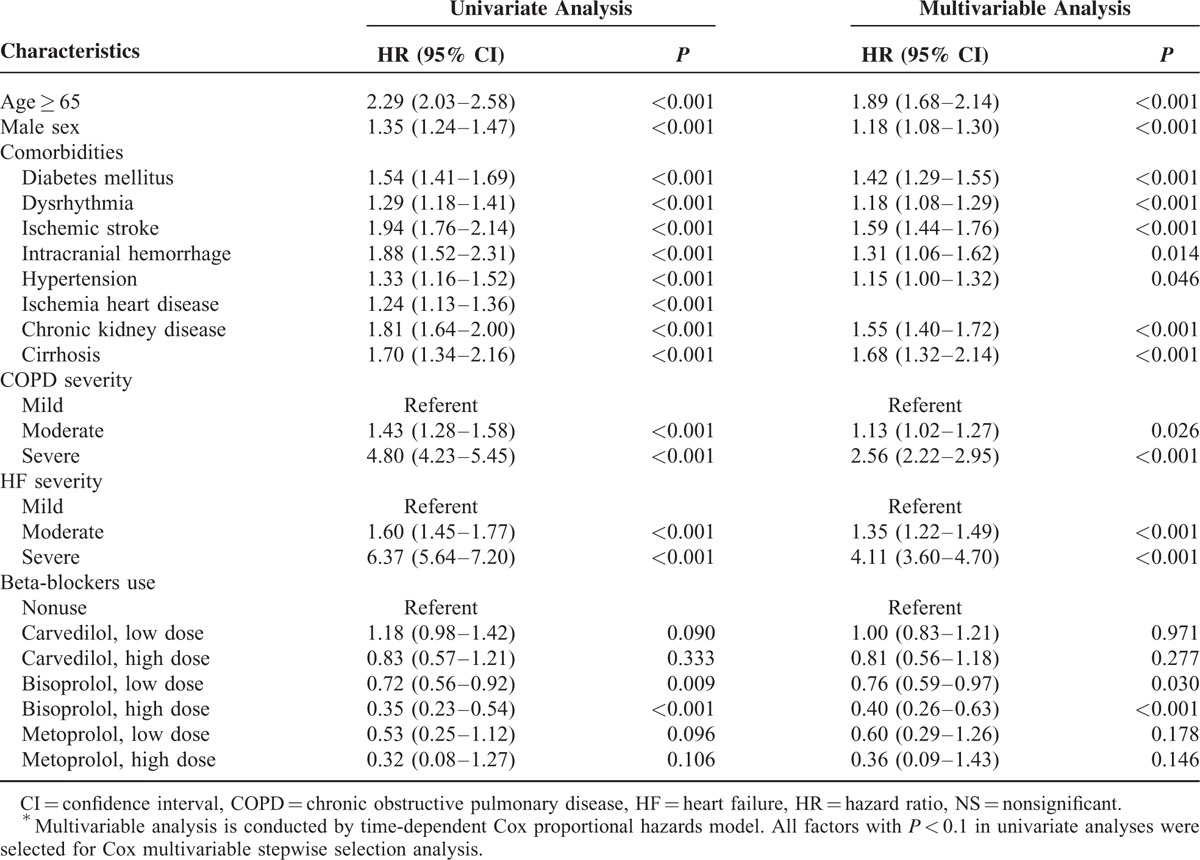
FIGURE 2.
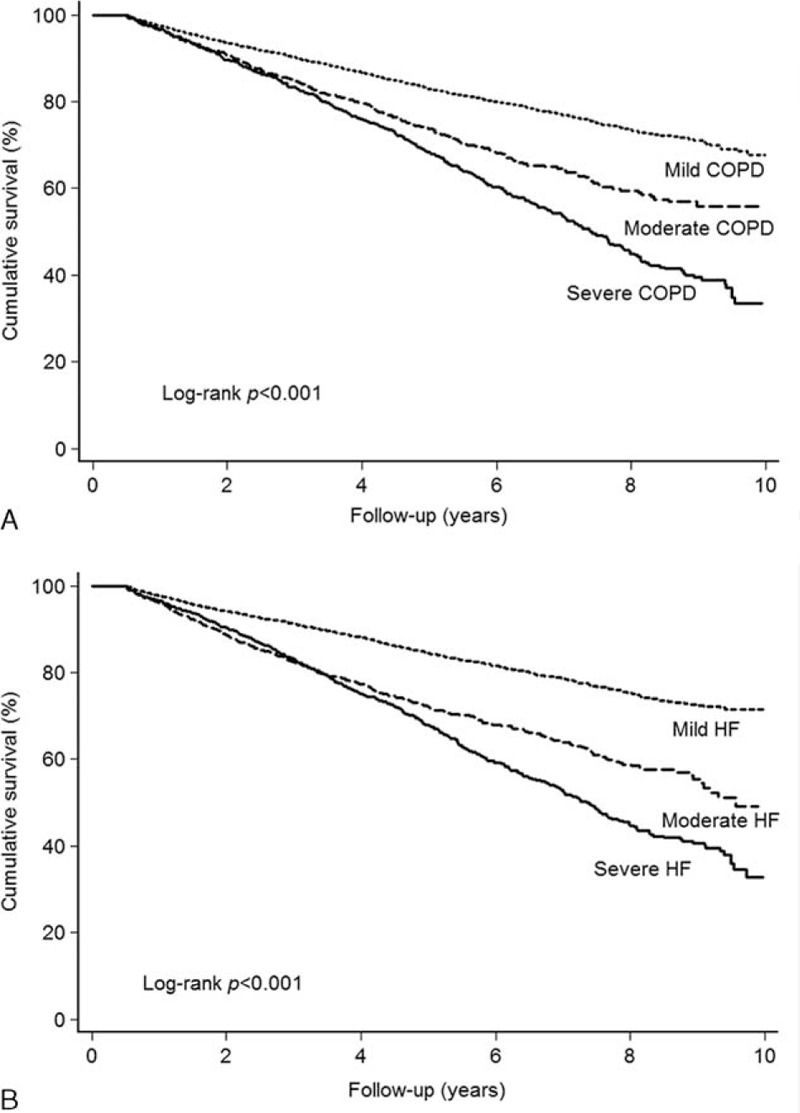
(A) Survival of patients with mild COPD, moderate COPD, or severe COPD. (B) Survival of patients mild HF, moderate HF, or severe HF.
FIGURE 3.
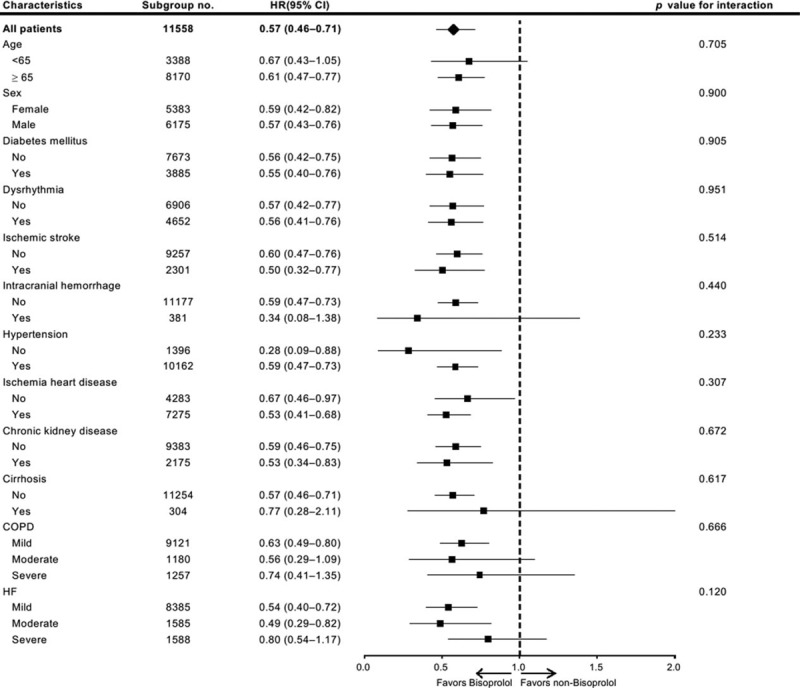
Subgroup analysis.
Carvedilol, Bisoprolol, and Metoprolol Use in Patients With HF Only or Coexistent HF and COPD
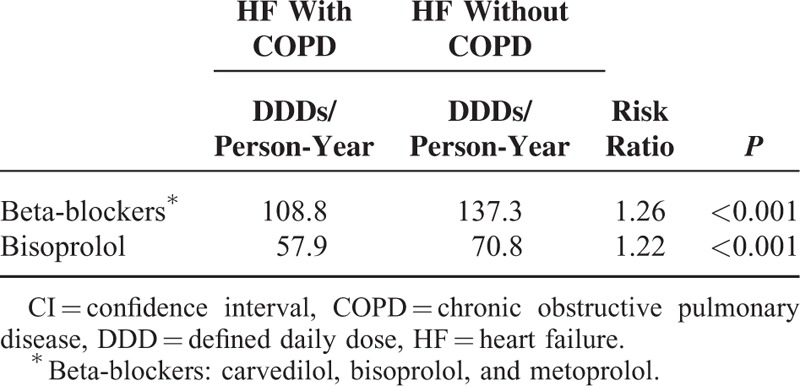
Prescriptions of bisoprolol, metoprolol, and carvedilol for patients with HF alone and coexistent HF and COPD are shown in Table 3. Significantly fewer prescriptions of the 3 β-blockers were given during HF in patients with coexistent HF and COPD than in those with HF alone (108.8 vs 137.3 DDDs per person-year, P < 0.001). We observed a similarly less frequent prescription of bisoprolol, the only β-blocker proven beneficial to patients with COPD and HF (57.9 vs 70.8 DDDs per person-year, P < 0.001).
TABLE 3.
Incidence of Beta-Blockers Prescription in HF Patients With or Without COPD
DISCUSSION
Almost all β-blocker trials in patients with HF have excluded participants with significant pulmonary disease, documented obstructive airway disease, and those using a bronchodilator.4 Observational studies have provided most of the limited evidence for the benefit of β-blockers in this special population.6 This nationwide population-based study revealed the suboptimal prescription of β-blockers in this group of patients and demonstrated a significantly better dose-dependent survival outcome associated with bisoprolol use in 11,558 patients with HF and COPD, whereas metoprolol and carvedilol showed no survival difference compared with nonusers.
To our knowledge, this study included the largest cohort of patients with coexistent HF and COPD. Furthermore, our cohort was adapted from a national database, which minimized selection bias. The validity of this study is also supported by our study design. To eliminate bias, which commonly affects observational studies, we exhaustively corrected for the confounding effects of comorbidities affecting vital organs. Importantly, we corrected for the severity of COPD and HF. In compliance with the Global Initiative for Chronic Obstructive Lung Disease guidelines,20 the severity of COPD was classified according to the average number of admissions for acute exacerbation per year, and the severity of HF was classified in the same manner. The classification of severity was reliable and independently predicted mortality, even after correction for other confounding factors in the time-dependent multivariable Cox regression model. Correcting for the severity of COPD was important because it helped us to eliminate bias caused by the decreased use of β-blockers in patients with severe COPD.
In this study, we demonstrated significantly less prescription of carvedilol, bisoprolol, and metoprolol in patients with coexistent HF and COPD than in those with HF alone. This finding is consistent with those of previous studies conducted worldwide. In Scotland, Hawkins et al21 found that only 18% (n = 1375) of patients with HF and COPD received β-blockers, in contrast to 41% (n = 4451) of those without COPD (P < 0.001). In the Valsartan Heart Failure Trial,9 only 140/628 (22%) participants with coexistent HF and COPD received β-blockers. This suboptimal use is due primarily to fears of respiratory side effects and limited evidence for the benefit of these drugs in this particular cohort.
Previous studies have associated the use of β-blockers with better survival outcomes in patients with COPD and myocardial infarction.22,23 In patients with concurrent HF and COPD, Staszewsky et al9 demonstrated a survival benefit in 140 β-blocker users compared with 488 nonusers. However, the sample size was small and no further comparison among individual β-blockers was performed. We found survival differences among the 3 β-blockers proven to benefit patients with HF in our cohort. Previous studies have noted differences among specific β-blockers in survival outcomes and airway function.12,15 The lack of a survival benefit in association with carvedilol use was not surprising, given the detrimental respiratory effects of this drug.15 Nonselective β-blockers have been found to reduce forced expiratory volume in 1 second (FEV1) and the effect of bronchodilator treatment, and to increase airway hypersensitiveness in patients with COPD.12 In contrast, a meta-analysis found that β1-selective β-blockers produced no adverse respiratory effect in patients with mild to moderate reactive airway disease.11 Furthermore, Salpeter et al13 revealed that β1-selective β-blockers produced no change in FEV1, respiratory symptoms, or treatment response to β2-agonists in patients with COPD. However, metoprolol use also showed no survival benefit in our study. As a β1-selective blocker, metoprolol was shown to increase airway hyperresponsiveness in patients with COPD, whereas celiprolol, another β1-selective β-blocker assessed in the study, demonstrated no negative pulmonary effect.12 These findings imply that pulmonary influences differ among β1-selective β-blockers, some of which continue to have a potential negative pulmonary effect. Our study revealed a superior survival outcome associated with bisoprolol use, which has been proven to not increase the chance of acute COPD exacerbation in patients with concurrent HF and moderate to severe COPD.23 Moreover, Lainscak et al15 not only proved the safety of bisoprolol use, but also found improved FEV1 in patients with concurrent COPD and HF. Interestingly, the differences in negative pulmonary effects demonstrated in previous studies are consistent with the survival differences observed in our cohort in comparisons between β1-selective and nonselective β-blockers and among 3 specific β-blockers. However, this study included 11,558 patients with coexisting HF and COPD. The sample size may be not large enough. So, the negative results of metoprolol might be due to suboptimal power (low-dose metoprolol: HR = 0.60, 95% CI = 0.29–1.26; high-dose metoprolol: HR = 0.36, 95% CI = 0.09–1.43).
The cardioprotective effects of β-blockers result from the blockage of chronic, harmful, compensatory catecholamine stimulation to a failing heart. Most β-blockers accomplish this through β1-receptor signaling, with adverse consequences including impaired systolic function, increased heart rate and myocardial stress, and progression in remodeling.2,24 The benefit of β-blocker use in patients with concurrent HF and COPD is not fully understood. Cardiovascular disease is the leading cause of morbidity and mortality in patients with COPD,5 and COPD is an independent predictor of hospitalization and death due to cardiovascular events in patients with HF.4 Other than sharing a major risk factor, smoking, which might partly explain the high coexistence rate of cardiovascular disease and COPD, increasing evidence suggests that COPD-related systemic inflammation, airway obstruction, and inhaled β-agonist treatment have potential roles in cardiac injury.4 Furthermore, some studies have demonstrated potential benefits of β-blockers in COPD in situ, including reduced mortality and risk of COPD exacerbation.8,10
The results of our study have several implications. Growing evidence has suggested that the use of β-blockers is safe in patients with COPD, including those with moderate to severe COPD and/or advanced age, or even after acute exacerbation.14,25,26 Our study findings strengthen the evidence for the benefits of β-blocker use in patients with coexisting COPD and HF, which might improve the current under-prescription of these drugs in clinical practice. Moreover, we found differences in survival effects among individual β-blockers in this patient group, indicating that bisoprolol is a better choice due to its association with fewer adverse pulmonary effects.
Nevertheless, this study has some limitations. First, the claim-based dataset omitted some personal information, such as smoking, obesity, alcohol use, and family history. Although these factors appear to have no influence on β-blocker selection and we made every effort to correct for confounding factors, especially comorbidities, some factors for which we lacked information might have confounded our results. Furthermore, the effect of smoking on mortality might be reflected by COPD severity in this study. Second, the results of pulmonary and heart function tests were not available. In compliance with the last guidelines of COPD and HF, the annual frequency of admissions for COPD or HF could be used to classify severity. Additionally, the primary endpoint was cumulative survival. Cardiovascular and respiratory complications were not reported in this study. Also, we could not directly evaluate the severity of each comorbidity. Third, the retrospective design restricted and potentially biased our study. However, performance of a randomized control trial to evaluate the survival effects of β-blockers in patients with coexistent HF and COPD would be difficult. Furthermore, because all data that could allow the identification of any individual were encrypted, we could not assess drug adherence and drug–drug interactions. However, this bias is toward the null hypothesis and would lead to underestimation of the actual survival effects of β-blockers. Finally, the external validity of our findings may be of concern because almost all enrollees were Chinese. The generalizability of our results to non-Asian populations must be further verified. Additionally, most patients had mild COPD (78.9%) and HF (72.5%) in this study. The survival benefit of bisoprolol in patients with nonmild COPD and HF should be clarified. More comprehensive studies are needed in order to confirm our findings and identify the potential underlying mechanisms. However, our findings suggest new avenues for future research.
CONCLUSION
In conclusion, this study demonstrated the suboptimal use of β-blockers and revealed a dose-dependent benefit of bisoprolol in patients with coexisting HF and COPD. There is no survival benefit of the other 2 β-blockers recommended by the HF guidelines (carvedilol and metoprolol). Thus, the current HF guidelines do not seem to be suitable for patients with coexisting HF and COPD. Further well-designed studies for these high-risk patients are warranted and should focus not only on the beneficial effects of β-blocker usage, but also on distinguishing among the effects of different β-blockers.
Acknowledgments
The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. This study is supported in part by a grant from Taipei Veterans General Hospital (V101D-001-2).
Footnotes
Abbreviations: β-blockers = beta-blockers, CI = confidence interval, COPD = chronic obstructive pulmonary disease, DDDs = defined daily doses, HF = heart failure, HR = hazard ratio, ICD-9-CM = International Classification of Diseases 9th revision Clinical Modification, IQR = interquartile range, NHI = National Health Insurance, NHRI = National Health Research Institute, NS = nonsignificant, SD = standard deviation.
VY-FS and Y-SC contributed equally to this work.
This study is supported in part by a grant from Taipei Veterans General Hospital (V101D-001-2).
Authors’ contributions: VY-FS, Y-SC, and C-JL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; study concept and design: VY-FS, Y-SC, Y-WH, M-HH, S-MO, K-TC, C-JL; acquisition of data: VY-FSu, Y-SC, and C-JL; analysis and interpretation of data: VY-FS, Y-SC, and C-JL; drafting of the manuscript: VY-FS, Y-SC, and C-JL; statistical analysis: Y-SC, Y-WH, and C-JL; study supervision: F-YL, K-TC, D-WP, and T-JC.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Lindenfeld J, Albert NM, et al. Heart Failure Society of America. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 2010; 16:e1–e194.20610207 [Google Scholar]
- 2.Bristow MR. β-adrenergic receptor blockade in chronic heart failure. Circulation 2000; 101:558–569. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:532–555. [DOI] [PubMed] [Google Scholar]
- 4.Mascarenhas J, Azevedo A, Bettencourt P. Coexisting chronic obstructive pulmonary disease and heart failure: implications for treatment, course and mortality. Curr Opin Pulm Med 2010; 16:106–111. [DOI] [PubMed] [Google Scholar]
- 5.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006; 16:63–70. [DOI] [PubMed] [Google Scholar]
- 6.Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2007; 49:171–180. [DOI] [PubMed] [Google Scholar]
- 7.Sin DD, McAlister FA. The effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failure. Am J Med 2002; 113:650–656. [DOI] [PubMed] [Google Scholar]
- 8.Short PM, Lipworth SI, Elder DH, et al. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ 2011; 342:d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staszewsky L, Wong M, Masson S, et al. Clinical, neurohormonal, and inflammatory markers and overall prognostic role of chronic obstructive pulmonary disease in patients with heart failure: data from the Val-HeFT Heart Failure Trial. J Card Fail 2007; 13:797–804. [DOI] [PubMed] [Google Scholar]
- 10.Rutten FH, Zuithoff NP, Hak E, et al. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med 2010; 170:880–887. [DOI] [PubMed] [Google Scholar]
- 11.Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective β-blockers in patients with reactive airway disease. Ann Intern Med 2002; 137:715–725. [DOI] [PubMed] [Google Scholar]
- 12.van der Woude HJ, Zaagsma J, Postma DS, et al. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; CD003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins NM, MacDonald MR, Petrie MC, et al. Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. Eur J Heart Fail 2009; 11:684–690. [DOI] [PubMed] [Google Scholar]
- 15.Lainscak M, Podbregar M, Kovacic D, et al. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med 2011; 105 Suppl. 1:S44–S49. [DOI] [PubMed] [Google Scholar]
- 16.Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 2001; 103:1428–1433. [DOI] [PubMed] [Google Scholar]
- 18.Kalman J, Buchholz C, Steinmetz M, et al. Safety and efficacy of beta blockade in patients with chronic congestive heart failure awaiting transplantation. J Heart Lung Transplant 1995; 14:1212–1217. [PubMed] [Google Scholar]
- 19.Hall SA, Cigarroa CG, Marcoux L, et al. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol 1995; 25:1154–1161. [DOI] [PubMed] [Google Scholar]
- 20.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins NM, Jhund PS, Simpson CR, et al. Primary care burden and treatment of patients with heart failure and chronic obstructive pulmonary disease in Scotland. Eur J Heart Fail 2010; 12:17–24. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins NM, Huang Z, Pieper KS, et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail 2009; 11:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Radford MJ, Wang Y, et al. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956. [DOI] [PubMed] [Google Scholar]
- 24.Kubon C, Mistry NB, Grundvold I, et al. The role of beta-blockers in the treatment of chronic heart failure. Trends Pharmacol Sci 2011; 32:206–212. [DOI] [PubMed] [Google Scholar]
- 25.Dransfield MT, Rowe SM, Johnson JE, et al. Use of β blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008; 63:301–305. [DOI] [PubMed] [Google Scholar]
- 26.Padeletti M, Jelic S, LeJemtel TH. Coexistent chronic obstructive pulmonary disease and heart failure in the elderly. Int J Cardiol 2008; 125:209–215. [DOI] [PubMed] [Google Scholar]


