Supplemental Digital Content is available in the text
Abstract
The aim of our study was to develop a Spanish-structured HIV risk of exposure and indicator conditions (RE&IC) questionnaire. People attending to an emergency room or to a primary clinical care center were offered to participate in a prospective, 1 arm, open label study, in which all enrolled patients filled out our developed questionnaire and were HIV tested. Questionnaire accuracy, feasibility, and reliability were evaluated.
Valid paired 5329 HIV RE&IC questionnaire and rapid HIV tests were performed, 69.3% in the primary clinical care center, 49.6% women, median age 37 years old, 74.9% Spaniards, 20.1% Latin-Americans. Confirmed hidden HIV infection was detected in 4.1%, while HIV RE&IC questionnaire was positive in 51.2%. HIV RE&IC questionnaire sensitivity was 100% to predict HIV infection, with a 100% negative predictive value. When considered separately, RE or IC items sensitivity decreases to 86.4% or 91%, and similarly their negative predictive value to 99.9% for both of them. The majority of people studied, 90.8% self-completed HIV RE&IC questionnaire. Median time to complete was 3 minutes. Overall HIV RE&IC questionnaire test-retest Kappa agreement was 0.82 (almost perfect), likewise for IC items 0.89, while for RE items was lower 0.78 (substantial).
A feasible and reliable Spanish HIV RE&IC self questionnaire accurately discriminated all non–HIV-infected people without missing any HIV diagnoses, in a low prevalence HIV infection area. The best accuracy and reliability were obtained when combining HIV RE&IC items.
INTRODUCTION
Late HIV diagnosis has been identified in most world regions,1–4 women sex, older age, people acquiring HIV infection through heterosexual contact, or being immigrant were, among others, at higher risk.4,5 Consequences of late HIV presentation are serious for HIV-infected individuals, because it leads to a higher morbidity and mortality.6 Also from a Public health perspective, late diagnosis favors HIV transmission7 and increases the use of resources and costs.8,9
Testing for HIV has become a critical component of HIV/AIDS prevention strategies because of the fact that an alarming number of people afflicted with HIV remain unaware of their infection.1 The reduction of new HIV infections is one of the main goals UNAIDS (United Nations AIDS) has set for the coming years,10 an earlier HIV diagnosis plus adequate engagement in care of most of the world's HIV-infected individuals are essential to achieving this goal.
HIV testing guidelines vary from a more universal HIV screening for people aged 13 to 64 in all health care settings after the patient is notified that testing will be performed, unless the patient declines (opt-out screening)11 to a more targeted strategy,12,13 in which all individuals presenting to any health care setting with one of the indicator conditions (ICs) identified should be highly recommended an HIV test. The main area of discrepancy between HIV screening guidelines is whether to routinely screen populations not known to be at increased risk.11–13 In any of the HIV testing strategies, we focus on; investigation of HIV Risk of Exposure or IC; is recommended to indicate first HIV testing12 or a more frequent retesting, as indicated in US Preventive Services Task Force Screening for HIV statement,13 where persons at higher risk of HIV infection should be screened for HIV at least annually.14
Many barriers have been identified with HIV screening, some of which are common to a variety of health care settings such as insufficient time, burdensome consent process, lack of training, lack of patient acceptance and pretest counseling requirements, competing priorities, and inadequate reimbursement.15
The question of “who, how, and where” HIV RE&IC should be assessed has been poorly investigated. In fact, inquiring systematically about HIV RE&IC is poorly observed in clinical practice.
In health care settings with heavy workload and limited resources, a systematic investigation of exposure to HIV in all individuals, attending a health care setting, usually for other reasons not related with HIV, can be time consuming and difficult to meet.
Sexually HIV RE behavior questionnaires have been studied in the first years of HIV epidemics, mostly for public health purposes,16 and also as a tool for targeted HIV testing.17
Recently, HIV infection risk assessment has been more extensively studied with the purpose of helping health care providers to recognize people with increasing HIV risk. An easy score has been developed, based on epidemiological characteristics and behaviors associated with the risk of HIV acquisition.18 This initiative has proved to be strongly associated with new HIV diagnoses compared with nontargeted screening,19 and to be more efficient as well. On the other hand, a strategy is being developed in collaboration with European Centre for Disease Prevention and Control and WHO Europe to guide the implementation of identifying and HIV screening individuals with HIV IC associated with higher risk of HIV infection.12
Different tools to assess HIV risk, scores, and IC studies can be extrapolated to be used in different world regions, although a validating process is necessary. For a questionnaire, language construction and wording validation is essential. UNAIDS estimations reported that around, 1.4 to 2.4 million people are living with HIV in Latin America, most of them Spanish speakers.20 No tool has been designed and validated in Spanish language.
The aim of our study was to evaluate the accuracy, feasibility, and reliability of a Spanish-structured HIV RE&IC questionnaire among people attending an emergency room or a primary clinical care center.
METHODS
DRIVE study (Diagnóstico Rápido de la Infección por VIH en España, in English Rapid HIV testing in Spain) was designed to investigate the different aspects of HIV testing.
From July 2012 to June 2013, people attending the hospital emergency room (HER) at Ramon y Cajal Hospital or the Hermanos García Noblejas Primary Care Center (PCC), both settings in the same Health Care Area, (in Madrid, Spain) were given the opportunity to participate in the DRIVE Study. Prevalence of HIV infection per 1000 inhabitants, in Madrid, was 0.35. Inclusion criteria were as follows: having attended HER or PCC, aged between 18 and 60 years, having understood, accepted, and signed the written informed consent, and in case of inability, it had to be signed by the legal representative. Exclusion criteria were as follows: a prior HIV diagnosis, having already been included in the DRIVE study, or inability to understand the Spanish language.
Study Procedures
A commercial rapid HIV test (Insti, Byolitical lab Inc based in Richmond, BC, Canada) with high sensitivity (Sn) and specificity (Sp)21 and an HIV RE&IC questionnaire were performed to all people admitted to the study. The Questionnaire, drawn up by 2 investigators, was designed specifically for this study. In order to easily identify any possible risks of HIV exposure or IC known to be related to HIV acquisition, a committee of HIV specialists, researchers, practitioners, primary care providers, and nurses from the study team voted the items that should be maintained, then revised and corrected the first version. Corrections were made to improve readability and comprehension, in attempt to cover maximum of RE&IC. Then it was tested in a set of the first 10 patients to check readability and comprehension. In its final version, the first 6 items of HIV RE&IC questionnaire investigated HIV RE (based on identified HIV routes of transmission)22 and the last 14 items HIV IC (based on a selection of HIV Indicator Diseases across Europe Study).12 All items permitted only 2 answers, yes or no: the first column for “yes” and the second one for “no.” If any answered item was yes, it was considered positive, indicating some risk for HIV infection, (Supplemental digital content SDC Table 1 and Table-2, original and translated HIV RE&IC questionnaire versions). Interpretation was easily obtained as only 1 X marked in the right column (yes) indicated a positive questionnaire. A priori, all patients should fill out the questionnaire by themselves, but testing staff could assist participants to fill out the whole HIV RE&IC questionnaire if necessary, or to help only with some questions. Because the aim was to assess the questionnaire itself, to verify if there were any problems with understanding the IC, testing staff was trained to answer, if the respondent was not familiar with the IC, that he/she would probably, have never been diagnosed with this IC.
Furthermore, in all questionnaires the number of study inclusion, sex, year, and country of birth were recorded. Other questions about health care assistance frequency and prior HIV screening were also included but not analyzed in the present substudy.
To evaluate feasibility of HIV RE&IC questionnaire, testing staff completed a short questionnaire of feasibility (SQF) while proceeding with 107 participants from HER and 100 from PCC (207 total) English translated version of SQF (Supplemental digital content Table 2). SQF consisted of 9 items, 5 of which referred specifically to HIV RE&IC questionnaire: time in minutes it was filled out, format in which it was filled out (paper or electronic), who filled it out (subject or testing staff), and if any problems were detected and which ones. To evaluate reliability, a subset of 100 individuals from PCC answered an identical questionnaire, and a second one, with the order of items changed. It was performed around 20 to 30 minutes after the original was completed. All HIV RE&IC questionnaires were filled out before rapid HIV test and their results were known.
DRIVE Program Description
Both research ethical committees, Ramon y Cajal Hospital and Madrid Primary Care, approved the study including the written informed consent, and all the materials used in the study.
In a confidential setting, all participants were provided a written informed consent, answered the RE&IC questionnaire, and HIV tested.
The study activities were carried out by the trained testing staff: 9 nurses in emergency room and 2 nurses in primary care center. The nurses explained all the procedures to participants, made sure they filled the RE&IC or helped them to answer the questions when needed. Subsequently, they numbered, performed, and photographed the rapid HIV test. All of the data were transmitted to a central web database via electronic tablets. People could choose to fill out the RE&IC questionnaire on paper or in electronic format.
There were 2 project coordinators who periodically checked the database, confirmed results with photographs, as well as verifying that the HIV positives were confirmed with a Western blot test and that they had not been previously diagnosed. Furthermore, they queried for possible errors or duplicated uncompleted records.
All staff from settings, HER, and PCC, including medical assistants and nurses, were informed of the study with written summaries, flyers, and slide presentations at meetings explaining the main concepts of HIV screening and study design. People could be included in the study through active recruitment by testing staff or referred by physicians or nurses to the testing staff, who were placed in a specific room, mobiles were also used to locate testing staff. Posters and brochures were also designed to be placed in both the settings to announce the study and to draw in participants.
All people with an HIV positive result were informed and counseled about the result, at that moment testing staff informed by phone the coordinating personnel at the HIV Infectious Diseases Department of Ramon y Cajal Hospital, where they were referred to, for a full HIV evaluation within the following 1 to 48 hours. Firstly, an HIV Enzyme Immunoassay and a Western Blot were obtained. Only confirmed HIV-positive people, without any evidence of a prior HIV diagnosis, were considered in the study.
Statistical Analysis
For present analyses, only participants with valid HIV-rapid test were selected. Only 4 participants were excluded: 2 came out as false positives, while 2 indeterminate. No cases were excluded because of invalid questionnaires. Three other participants were excluded because a prior diagnosis was identified.
Variables considered in this study were: sex, age, categorized in 3 strata (<30, 30–50, and >50 years) for some analyses, country of birth categorized into 2 (Spanish or non-Spanish) for some analyses, setting of inclusion in DRIVE study (HER or PCC), HIV rapid test and RE&IC questionnaire results (positive or negative). Each RE&IC item was also analyzed (yes or no) for different analyses.
Accuracy was assessed in the overall population enrolled in the study by calculating Sn, Sp, positive predictive value (PPV), and negative predictive value (NPV) using the gold standard confirmed HIV infection. Sample size was calculated for a maximum of 5300 rapid HIV tests assuming a hidden HIV infection in our environment of 0.35%,23 an NPV of 100% and a difference of 0.25% for a power of 75%.
Association between each HIV RE&IC item result and HIV-confirmed infection was assessed by Chi-square and Fisher exact test when appropriate. Student t test or Mann–Whitney test was used to compare continuous variables. To determine the association between baseline characteristics and HIV RE&IC questionnaire result, a logistic regression multivariate analysis was performed adjusting for sex, age, and country of birth. The Statistical Package for Social Sciences (SPSS 17.0; SPSS Inc, Chicago, IL) and STATA 13.1 software (Stata-Corp LP, College Station, TX) were used. Everything was calculated for a 95% CI (confidence interval) and P < 0.05 values were considered statistically significant.
SQF 5 items regarding HIV RE&IC questionnaire were described. The reliability of the test-retest procedure was measured by the Cohen Kappa (κ) Index of Reliability24 and the criteria for the κ suggested by Landis and Koch,25 including the overall result of the questionnaire, along with partial results of RE items and IC items.
RESULTS
Baseline Participant Characteristics and HIV RE&IC Questionnaire
Overall 5,329 valid paired HIV RE&IC questionnaires and rapid HIV tests were performed, 3694 patients 69.3% in the PCC. Overall population baseline characteristics were 49.6% women, median age 37 (28–47) interquartile range years old, 74.9% Spaniards, 20.1% Latin-Americans, and 2.5% from Eastern Europe. Similar characteristics were observed in HER and PCC populations, relating to sex, age, and country of birth. For the feasibility and reliability subsets of individuals, a slightly higher proportion of women were included, while age and country of origin was similar (Table 1). Confirmed hidden HIV infection was detected in 4.1%.
TABLE 1.
Distribution of baseline variables and HIV RE&IC questionnaire results in overall population, in HER, in PCC, in feasibility eliminate semicolon and in reliability subsets of individuals
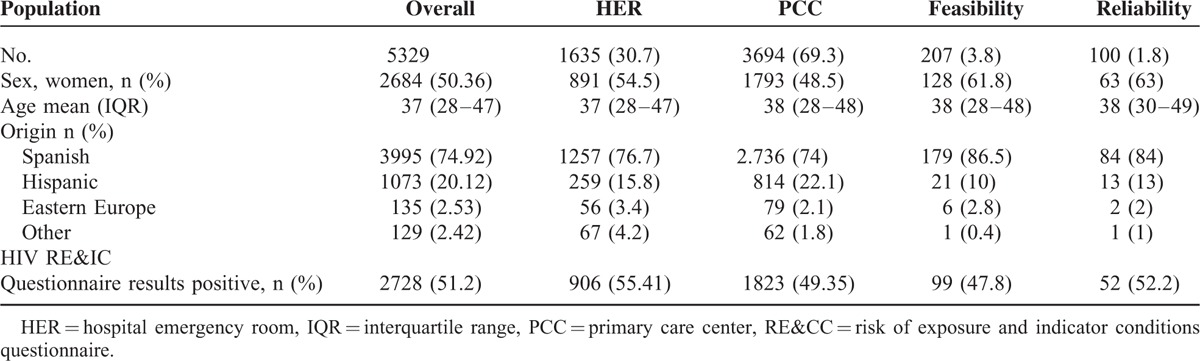
Overall 51.2% of the population answered at least 1 item of the HIV RE&IC questionnaire as “yes,” indicating a possible risk of HIV infection. In the multivariate analysis, women, odds ratio (OR) 0.76, CI 95% (0.68; 0.85), P < 0.001; having been recruited in the PCC, OR 0.77, CI 95% (0.68;0.85), P < 0.001; and aged over 50 years as compared to 30 to 50 years, OR 0.83, CI 95% (0.71;0.6), P = 0.013; had lower odds of having a positive HIV RE&IC questionnaire, whereas younger people, below 30 years had higher odds of having a positive HIV RE&IC than 30- to 50-year olds, OR 1.2, CI 95% (1.07;1.36), P = 0.002. No differences were found with respect to patient's country of birth.
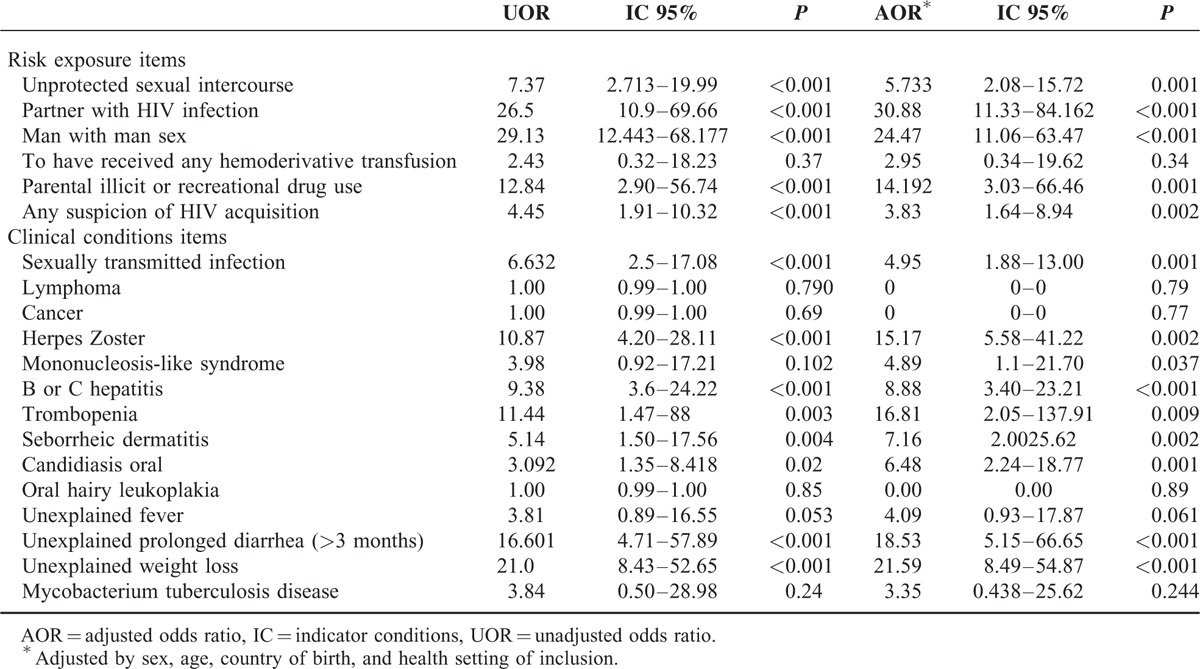
HIV RE&IC questionnaire answers were compared between HIV-diagnosed individuals and non-HIV infected. Unadjusted and adjusted analyses showed that infected HIV participants had significantly more positive answers indicating risk for HIV infection for the majority of the items (Table 2). Considering only individuals with HIV RE&IC positive questionnaire, mean number of affirmative answers was significantly higher for HIV-infected people versus noninfected (4.18 ± 1.74 vs 1.88 ± 1.11, P < 0.001).
TABLE 2.
Unadjusted and adjusted analyses of HIV RE&IC questionnaire items comparing yes answers between HIV diagnosed individuals and non–HIV-infected
Accuracy, Feasibility, and Reliability
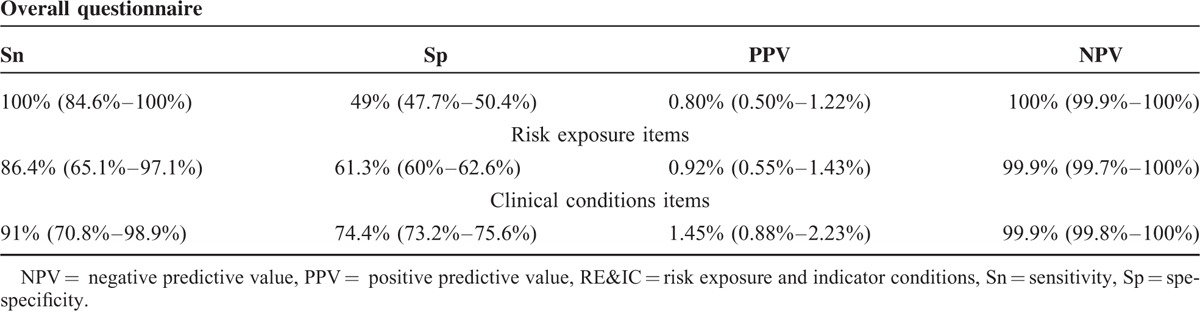
HIV RE&IC questionnaire Sn was 100% to predict HIV infection, with an Sp of 49%. PPV was 0.80%, and NPV reached 100%, positive likelihood-ratio test (LLR) was 1.96, and negative LLR 0. Considering only 6 risk exposure items or only 14 IC items, Sn was 86.4% or 91%, and NPV 99.9% or 99.9%, respectively (Table 3).
TABLE 3.
HIV RE&IC predictability: Sn, Sp, PPV, and NPV for overall questionnaire and separately for risk exposure or clinical conditions items
According to the 207 SQF analyzed; 188 (90.8%) HIV RE&IC questionnaires were self administered. Most of them (203) 98.1% were filled out on paper. Median time to complete the HIV RE&IC questionnaire was 3 (IQR, 2–4) minutes. Some clarifications about HIV RE&IC questionnaire were addressed to the testing staff by 37 (17.9%) participants (of which 17 related to HIV IC, 2 to language difficulties, and 18 to other questions).
Overall, HIV RE&IC questionnaires 1 and 2 Kappa index agreement was 0.8 (almost perfect), similarly for IC items 0.89, while for RE items was lower 0.78 (substantial).
DISCUSSION
Our main finding was that an easy fill-it-out-yourself HIV RE&IC questionnaire accurately predicted which individuals were not HIV infected. This easy tool, which can be filled out in a short time and interpreted instantly, only 1 positive question is considered a positive questionnaire, could be useful for discriminating people with almost no risk of HIV infection, and thus excluding them to be HIV tested. The interpretation used in this analysis was that only 1 positive question is enough to confer risk of HIV exposure, and this is the recommended interpretation of the questionnaire, because it resulted in 100% Sn.
Other authors had developed a score, the Denver HIV Risk Score, that included only demographics (age, sex, race/ethnicity), 2 risk behaviors (men-with-men sex and injection drug use), and the history of HIV testing, which have been shown to accurately stratify individuals into 5 distinct risk groups from several different clinical settings, including emergency departments and sexually transmitted diseases clinics. This score accurately predicted HIV infection with the same aim, helping physicians to target HIV screening.18
No studies to date have designed tools or scores that evaluate risk of HIV infection combining both HIV RE and IC.12,16–18 In our study, HIV RE&IC questionnaire exploring both risk of HIV exposure and IC, it has been observed that this combined approach improved HIV RE&IC questionnaire accuracy, with better results in both Sn and NPV, which is of utmost importance for not missing HIV infections. This characteristic is essential if HIV RE&IC questionnaire is used as an HIV testing prescreening tool, to potentially exclude individuals from HIV testing.
One of the innovative approaches of this HIV RE&IC questionnaire was its high feasibility and self-management feature, with practically no need for any professional support for most individuals or, if need be, only a minimal assistance, as well as being easy to interpret. All this is in sharp contrast with the current time consuming assessment, where all the questions or score items are filled in by a nurse or a physician, which by the way is possibly less reliable. Self-administered questionnaires, it has been suggested, may reduce barriers to HIV risk exposure reporting commonly found in face-to-face interviews.26 An easy fill-it-out-yourself questionnaire is crucial as heavy workload and time factors have been identified as some of the main barriers to HIV testing.12,15 Some questionnaires evaluated in past studies had many more items, more complex questions, and with in-depth inquiry about sexual risk behaviors.16,26 Because those were designed for epidemiological research, they seemed unsuitable for our purpose. Other questionnaires could easily be self-administered, reliable, and valid; however, their accuracy has not been tested.17
The HIV RE&IC questionnaire's main focus is on how to accurately predict non-HIV infection, making it possible to exclude individuals from HIV screening. In keeping with this objective, it was designed to have a high Sn, considering as HIV risk individuals those with only 1 affirmative item. The low predictive positive value obtained reflects this.
In the HIV RE&IC-positive individuals, differences shown in the median number of affirmative answers between HIV-infected and noninfected individuals open up the possibility of using the questionnaire to indicate which people were in a higher risk of infection, or to indicate more frequent HIV testing.
Reliability is one of the key points for a questionnaire to be valid. Our results provided a high agreement between test and retest, when considering overall results and IC items result, while in RE questions reliability is slightly reduced. Other studies, focused on HIV risk factors, have observed a lower reliability of self-reported sexual behavior than other more constant information.16
Some limitations were found in our study. Subsets of feasibility and reliability were selected in an exploratory manner and a higher number of individuals could have been necessary, but due to the similarity with the overall population, in terms of percentage of HIV RE&IC questionnaire positivity, the age and the origin, we were able to assume the validity of our results. Other possible limitation is the short time laps between the test-retest questionnaires, because memory of the previous answers could have biased the response, influencing the good results.27 However, for our purposes it was important that both questionnaires were answered before knowing the HIV rapid test result. Finally, the high level of accuracy observed in our HIV RE&IC questionnaire could have been the result of the voluntary individual participation, while in other settings or scenarios there is an every likelihood that the respondents might not have answered as sincerely as possible. Clinical judgment of health care personnel should be essential to interpret HIV RE&IC questionnaire results, in other settings and populations, where validation has not been assessed. Furthermore, test should be performed to any individual who requests it, irrespective of whether the individual gives any underlying reason or not.
A wider validation of this questionnaire, in other settings and populations, will make it easy to implement targeted HIV testing, by reducing problems previously associated with this strategy.
For this targeted strategy to work, it should cover the maximum number of people attending a health care setting. Therefore, it will be necessary to voluntarily offer and systematically assess HIV RE&IC to all the attending individuals and subsequently try to HIV test all individuals within risk of HIV infection. Some recent studies have provided more evidence in support of using more structured approaches to screen for HIV infection in health care settings.28,29
Targeted HIV testing, using our RE&IC questionnaire, as it focuses on HIV testing only on higher HIV risk individuals, will be more resource saving in low HIV prevalence populations. Taking into account our results, around half the population could be spared from HIV testing. In populations with very low HIV RE&IC prevalence, the number of HIV tests that can be avoided will increase even more, while in settings of very high prevalence of HIV RE&IC, the questionnaire may be unnecessary as it is better to assume that all the population have RE to HIV and therefore to perform the HIV test on all the population.
Many clinics are understaffed and underfunded, making it difficult to provide and administer adequately testing and care for people seeking rapid HIV screenings. In this situation, it is critical to ensure that testing resources are directed toward high-risk populations.30
Finally, we can conclude that a feasible and reliable Spanish HIV RE&IC self-questionnaire accurately discriminated all non–HIV-infected people without missing any HIV diagnoses, in a low prevalence HIV area. The best accuracy and reliability were obtained when combining HIV RE&IC items.
Supplementary Material
Footnotes
Abbreviations: κ = Kappa, CI = confidence interval, DHRS = Denver HIV Risk Score, DRIVE = Diagnóstico Rápido de la Infección por VIH en España in English Rapid HIV testing in Spain, EIA = Enzyme Immunoassay, HER = hospital emergency room, HIDES = HIV Indicator Diseases Across Europe Study, HIV = human immunodeficiency virus, IC = indicator conditions, LLR = likelihood-ratio test, NPV = negative predictive value, PCC = primary care center, PPV = positive predictive value, RE = risk of exposure, RE&IC = risk of exposure and indicator conditions, SDC = supplemental digital content, Sn = sensitivity, Sp = specificity, SPSS = Statistical Package for Social Sciences, SQF = short questionnaire of feasibility, UNAIDS = United Nations AIDS.
DRIVE study composition. Organizing Committee: María Jesús Pérez Elías, María Martinez-Colubi, and Cristina Gomez Ayerbe. Field work, data management: Rafael Barea, Lidia Polo, Gema Robledillo, Statistician Alfonso Muriel; Inclusion of people, management of HIV-positive patients, contributions to the analysis, and critical reading the article: Pilar Perez Elías, Cristina Santos Alvarez, Ana Moreno, Alberto Díaz de Santiago, Almudena Uranga, Agustina Cano Tinoco, Santiago Moreno, Fernando Dronda, Carmen Quereda, Jose Casado, Centers and participating investigators of the DRIVE Study Group.
Working group Primary Care Center García Noblejas: Esmeralda Alonso, Arancha Alonso, Josefa Araujo, Alberto Barbado, Fernando Barcala, Rafael Barea, Rosario Blanco, Raquel Blázquez, María Eugenia Calonge, Agustina Cano, Fuencisla Consuegra, Lino Cota, María Teresa Cuenca, Maria Escribano, Concepcion Falcón, Mercedes Fernández, Mercedes Fraile, Pilar García, Rocio Garrido, María Isabel González, Juan Jose González, María Jesus González, Pilar González, Carmen Gutiérrez, Adelaida Iglesias, Victoria Izquierdo, Juan Jose Jiménez, Eduardo Llamazares, Rafaela Lerín, María Jose López Bonillo, Luisa Lorente, Antonia Martin, Eva Martín Gracia, Jose Luis Martínez, Silvia Medrano, Lucia Naranjo, Juana Pascual, Jose Parra, Rosa Pavo, Pilar Pérez Elías, Lidia Polo, Rosario Ruíz Giardín, Cristina Santos, Alberto Serrano, Luis Miguel Serrano, Pilar Sanz, Rosario Sobrino, Isabel Susaeta, Mariano Torres, Araceli Treceño, Julio Turrientes, Almudena Uranga.
Working group Hospital Ramón y Cajal: Santiago Moreno, Cristina Gómez-Ayerbe, Alberto Diaz, Ana Moreno, Alfonso Muriel Beatriz Hernandez, Carolina Gutierrez, Jose Luis Casado, Angela Trueba, Ana Arizcorreta, Fernando Dronda, Gema Robledillo, Marta Fernandez, Sandra Ibarra Lorenzo, Beatriz Sanz Arias, Rocio Curiel Serradilla, Cristina Fernández San Pedro, M Bueno Pozo, María Teresa sanchez leiva, María Dolores López Pérez, Carmen Quereda, Paloma Martí-Belda, Isabel Hornero, Laura Etxeberria, Juan Carlos Galán, Jose María Gonzalez-Alba, Dolores pastor Pinilla, Pilar Regojo Dans, Ana Belen Sanchez Rubio, Marta Gonzalez Gómez, Serafina Perez Figueroa.
Gestion Avanzada de Tecnologías working Group Fernando Cerezal, Jose David Allona, Fernando Allona. Software design and Support.
Dr M Jesús Pérez-Elías, Dr Santiago Moreno, Dr Fernando Dronda, and Dr Ana Moreno have received honoraria for lectures or for participation in advisory boards from Abbott, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, ViiV, MSD and Janssen Cilag; and have received payment to develop educational presentations for Bristol-Myers Squibb, ViiV, Abbott laboratories and unrestricted grants from Abbott, ViiV, Gilead and Janssen-Cilag.
This study was supported by 2 competitive Grants; of the Instituto de Salud Carlos III (Plan Estatal de I+D+i 2013-2016), Grant PI12-00995 and the Ministerio de Sanidad, Seguridad Social e Igualdad Project code: EC11-144 both cofinanced by the European Development Regional Fund “A way to achieve Europe” (ERDF) and partially funded by the RD12/0017/0017 project as part of the Plan Nacional R + D + I and cofinanced by ISCIII- Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis 2010; 50:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolbers M, Bucher HC, Furrer H, et al. Swiss HIV Cohort Study. Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med 2008; 9:397–405. [DOI] [PubMed] [Google Scholar]
- 3.Likatavicius G, Van de Laar MJW. HIV and AIDS in the European Union, 2009. Eurosurveillance 2010; 15:19737. [DOI] [PubMed] [Google Scholar]
- 4.Sobrino-Vegas P, Rodríguez-Urrego J, Berenguer J, et al. on behalf of CoRIS. Educational gradient in HIV diagnosis delay, mortality, antiretroviral treatment initiation and response in a country with universal health care. Antivir Ther 2012; 17:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Smith RD, Delpech VC, Brown AE, et al. HIV transmission and high rates of late diagnoses among adults aged 50 years and over. AIDS 2010; 24:2109–2115. [DOI] [PubMed] [Google Scholar]
- 6.Lucas SB, Curtis H, Johnson MA. National review of deaths among HIV-infected adults. Clin Med 2008; 8:250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450. [DOI] [PubMed] [Google Scholar]
- 8.Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med 2004; 5:93–98. [DOI] [PubMed] [Google Scholar]
- 9.Martínez Colubi M, Pérez Elías MJ, Muriel A, et al. Delayed diagnosis of HIV Infection: prevalence, risk factors and high costs. Belgrade, Serbia: 13th European AIDS Conference/EACS; 2011; 12–15 [Google Scholar]
- 10.UNAIDS Strategy 2011–2015. http://www.unaids.org/en/aboutunaids/unaidsstrategygoalsby2015 Accessed 2 Feb 2015. [Google Scholar]
- 11.Branson BM, Handsfield HH, Lampe MA, et al. Revised Recommendations for, HIV, Testing of Adults, Adolescents, Pregnant Women in Health-Care Settings. MMWR 2006; 55:1–17.Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 12.Sullivan AK, Raben D, Reekie J, et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV Indicator Diseases across Europe Study). PLoS ONE 2013; 8:e52845.doi:10.1371/jopurnal.pone.0052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S., Preventive Services Task Force. Screening for HIV: recommendation statement. Ann Intern Med 2005; 143:32–37. [DOI] [PubMed] [Google Scholar]
- 14.US Preventive Services Task Force. Screening for HIV: Recommendation Statement. American Family Physician 2014; 89:666AD http://www.uspreventiveservicestaskforce.org/ Accessed 25 March 2015. [Google Scholar]
- 15.Burke RC, Sepkowitz KA, Bernstein KT, et al. Why don’t physicians test for HIV? A review of the US literature. AIDS 2007; 21:1617–1624. [DOI] [PubMed] [Google Scholar]
- 16.Saltzman SP, Stoddard AM, McCusker J, et al. Reliability of selfreported sexual behavior risk factors for HIV infection in homosexual men. Public Health Rep 1987; 102:692–697. [PMC free article] [PubMed] [Google Scholar]
- 17.Gerbert B1, Bronstone A, McPhee S, et al. Development and testing of an HIV-risk screening instrument for use in health care settings. Am J Prev Med 1998; 15:103–113. [DOI] [PubMed] [Google Scholar]
- 18.Haukoos JS, Lyons MS, Lindsell CJ, et al. Derivation and validation of the Denver Human Immunodeficiency Virus (HIV) risk score for targeted HIV screening. Am J Epidemiol 2012; 175:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haukoos JS, Hopkins E, Bender B, et al. Comparison of enhanced targeted rapid HIV screening using the Denver HIV risk score to nontargeted rapid HIV screening in the emergency department. Ann Emerg Med 2013; 61:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global AIDS epidemic facts and figures. http://www.unaids.org/sites/default/files/media_asset/20140716_FactSheet_es_0.pdf Accessed March 2015. [Google Scholar]
- 21.Pavie J, Rachline A, Loze B, et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS ONE 2010; 19 5:e11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe HW, Lifson AR. Acquisition and transmission of HIV. Infect Dis Clin North Am 1988; 2:299–306. [PubMed] [Google Scholar]
- 23.Moreno S, Ordobás M, Sanz JC, et al. Prevalence of undiagnosed HIV infection in general population having blood tests within primary care in Madrid, Spain. Sex Trans Infect 2012; 88:522–524. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. A coefficient of agreement for nominal scales. Education Psychol Measure 1960; 20:37–46. [Google Scholar]
- 25.1977; Landis JR, Koch GG. Biometrics. 33:159–174. [PubMed] [Google Scholar]
- 26.Locke SE, Kowaloff HB, Hoff RG, et al. Computer-based interview for screening blood donors for risk of HIV transmission. JAMA 1992; 268:1301–1305. [PubMed] [Google Scholar]
- 27.d’Almeida KW, Kierzek G, de Truchis P, et al. Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch Intern Med 2012; 172:12–20. [DOI] [PubMed] [Google Scholar]
- 28.Klein P, Messer L, Myers E, et al. Impact of a routine, opt-out HIV testing program on HIV testing and case detection in North Carolina sexually transmitted disease clinics. Sex Transm Dis 2014; 41:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraenkel JR, Wallen NE. How to design and evaluate research in education. 6th edNew York: Mc-Graw-Hill; 2006. [Google Scholar]
- 30.Haukoos JS, Hopkins E, Bucossi MM. Routine opt-out HIV screening: more evidence in support of alternative approaches? Sex Transm Dis 2014; 41:403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


