Abstract
The aim of the present study was to compare differential impacts of bariatric surgery and exercise-induced weight loss on excessive abdominal and cardiac fat deposition.
Excessive fat accumulation around the heart may play an important role in the pathogenesis of cardiovascular disease. Recent evidences have suggested that bariatric surgery results in relatively less decrease in epicardial fat compared with abdominal visceral fat and paracardial fat.
Sixty-four consecutive overweight or obese subjects were enrolled in the study. Clinical characteristics and metabolic profiles were recorded. The volumes of abdominal visceral adipose tissue (AVAT), abdominal subcutaneous adipose tissue (ASAT), epicardial (EAT), and paracardial adipose tissue (PAT) were measured by computed tomography in the bariatric surgery group (N = 25) and the exercise group (N = 39) at baseline and 3 months after intervention. Subjects in both the surgery and exercise groups showed significant reduction in body mass index (15.97%, 7.47%), AVAT (40.52%, 15.24%), ASAT (31.40, 17.34%), PAT (34.40%, 12.05%), and PAT + EAT (22.31%, 17.72%) (all P < 0.001) after intervention compared with baseline. In both the groups, the decrease in EAT was small compared with the other compartments (P < 0.01 in both groups). Compared with the exercise group, the surgery group had greater loss in abdominal and cardiac visceral adipose tissue (AVAT, ASAT, PAT, EAT+PAT) (P < 0.001), but lesser loss in EAT (P = 0.037).
Compared with the exercise group, bariatric surgery results in significantly greater percentage loss of excessive fat deposits except for EAT. EAT, but not PAT, was relatively preserved despite weight reduction in both the groups. The physiological impact of persistent EAT deserves further investigation.
INTRODUCTION
The prevalence of obesity increased in adults and children during the last decades and is now a leading cause of avoidable illnesses and deaths in developed countries.
In addition, obesity has been linked to metabolic syndrome and related cardiometabolic disorders through subsequent excessive fat accumulation in the liver, skeletal muscle, and abdominal cavity that leads to insulin resistance, glucose intolerance, and dyslipidemia.1–4 About 80% of total body fat comprises subcutaneous adipose tissue depots. Another 10% is contained within body cavities as visceral adipose tissue (VAT), mainly abdominal VAT. Epicardial adipose tissue (EAT) is defined as the adipose tissue between the surface of myocardium and the visceral layer of the pericardium (visceral epicardium). Paracardial adipose tissue (PAT) is defined as the adipose tissue situated on the outer surface of the parietal pericardium within the mediastinum.5 Recently, some studies have focused on the potential role of EAT as an important modulator of cardiovascular function and disorders, owing to its immediate anatomic proximity to the coronary arteries and its shared embryologic origin with intra-abdominal VAT.6,7
Emerging evidence suggests that excessive EAT accumulation is linked to increased risk of heart disease through paracrine secretion of proinflammatory adipocytokines and other bioactive molecules.5,8–14 Therefore, epicardial fat may serve as an indicator of cardiovascular risk. A recent study has shown that weight loss of around 10% of the initial body weight in an abdominally obese person can reduce visceral fat by almost 30%.15 It is suggested that excessive visceral fat deposits are very responsive to weight loss. The observation of the interval change of visceral fat through volumetric CT-measurement of adipose tissue can potentially reflect consequences of weight loss and reduction of cardio-metabolic risks.
A recent meta-analysis has demonstrated that bariatric surgery leads to greater body weight loss and higher remission rates of type 2 diabetes and cardiometabolic syndrome, compared with nonsurgical treatment of obesity.16 Although 2 previous studies have demonstrated lesser decrease in epicardial fat compared with visceral abdominal fat after bariatric surgery,17,18 changes in excessive visceral fat after weight reduction are still controversial because some studies did not actually address or observe this phenomenon by volumetric or thickness measurement.19–24
Previous studies demonstrated that CT-based measurements of volumetric EAT are more reproducible than CT-based measurements of EAT thickness.25,26 In addition, some studies on EAT thickness were mainly based on echocardiography that has been reported to have less reproducibility and accuracy than CT EAT measurements.27
Moreover, a comprehensive comparison of the changes of CT-measured excessive visceral fat deposits, especially that of EAT, after bariatric surgery and exercise has not been well reported. We hypothesize that bariatric surgery has differential effects on excessive visceral fat deposition compared with exercise program due to different physiological changes. In addition, the change of EAT may not parallel the changes of other compartments. In this prospective study, we measured the excessive visceral fat deposition before and after intervention of bariatric surgery or exercise program in 2 groups of patients. We aimed to comprehensively compare the decrease and relative distribution of CT-measured excessive visceral fat deposit in these 2 groups, which may shed light on differential mechanisms and potential metabolic impacts of weight loss after bariatric surgery or exercise program.
METHODS
Study Population
Between October 2009 and October 2011, 66 consecutive subjects who met the inclusion criteria were enrolled: the bariatric surgery group was composed of the 27 subjects who met standard eligibility criteria for bariatric surgery (BMI > 40 kg/m2 or >35 kg/m2 with comorbidities); the exercise group was composed of the 39 overweight/obese (BMI ≥ 30 kg/m2) subjects. A flowchart of our proposed scheme is shown in Figure 1, and detailed descriptions of the 2 groups are given in the following subsections. Subjects in the exercise group were on an 800 kcal Optifast diet and participated in an exercise program using aerobic steps that consisted of 3 to 5 sessions per week for 12 weeks with each session lasting at least 40 minutes in duration.
FIGURE 1.
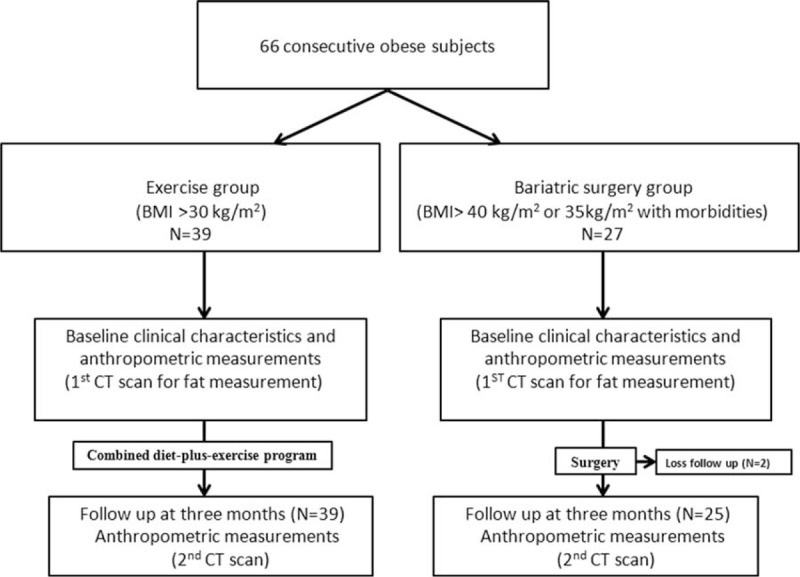
The flowchart scheme of the study.
The combined program of aerobic exercise and low calorie diet of the study followed the current nutrition and physical activity recommendations by the National Institutes of Health Obesity Education Initiative Expert Panel.28
All bariatric surgery procedures were performed using laparoscopic Roux-en-Y gastric bypass surgical techniques in the E-Da Hospital. Two subjects in the bariatric surgery group were excluded because of lack of follow-up.
At baseline and 3 months of postintervention (after the bariatric surgery or after initiation of exercise program), a medical record of anthropometric and biochemical parameters was obtained by the treating physician including age, sex, weight, height, BMI, waist circumference (WC), total cholesterol, glucose, high density lipoprotein, homeostatic model assessment (HOMA) index, and triglycerides. All subjects who agreed to participate signed an informed consent form and the research protocol was approved by the Ethics Committee of our hospital.
Measurement of Abdominal and Cardiac Fat Volume
All of the study subjects underwent a computed tomography (CT) scan (64-detector raw CT scanner based in Kaohsiung Veterans General Hospital, Aquilion 64; Toshiba Medical Systems, Tokyo, Japan; 64-detector raw CT scanner based in E-Da Hospital, LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA) at baseline and 3 months after intervention to assess abdominal and cardiac adipose tissue. Abdominal and cardiac visceral fat measurements were all performed on noncontrast enhanced CT scans using a workstation (Volume Analysis, Advantage Workstation 4.3, GE Healthcare). Adipose tissue was defined as attenuation ranging from −50 and −200 HU, as reported in previous studies.29,30
The program automatically interpolated the interval space. EAT was defined as adipose tissue located within the pericardial sac. PAT was defined as adipose tissue located outside of the pericardial sac. Total pericardial fat volume (PAT + EAT) was measured in axial images starting from the level of the left main pulmonary artery to the level of the left ventricular apex by manual tracing shown in Figure 2 (red boundary). EAT was measured in axial images starting from the level of the left main pulmonary artery to the level of the left ventricular apex by manual tracing (blue boundary). PAT was defined as total pericardial fat volume (PAT + EAT) minus EAT. Abdominal visceral adipose tissue (AVAT) was defined as adipose tissue located in the peritoneal or retroperitoneal region. Abdominal subcutaneous adipose tissue (ASAT) was defined as adipose tissue outside the visceral region. Total abdominal fat volume (ASAT + AVAT) was measured on axial images starting from L2 to L5 levels in each subject with 0.5 mm slice thickness at 8 mm intervals by manual tracing shown in Figure 2 (red boundary). AVAT was measured on axial images starting from L2 to L5 levels in each subject with 0.5 mm slice thickness at 8 mm intervals by manual tracing (blue boundary). ASAT was defined as total abdominal fat volume (ASAT + AVAT) minus AVAT. Regional EAT thickness was also measured at right ventricular (RV) free wall as described in our previous study.30
FIGURE 2.
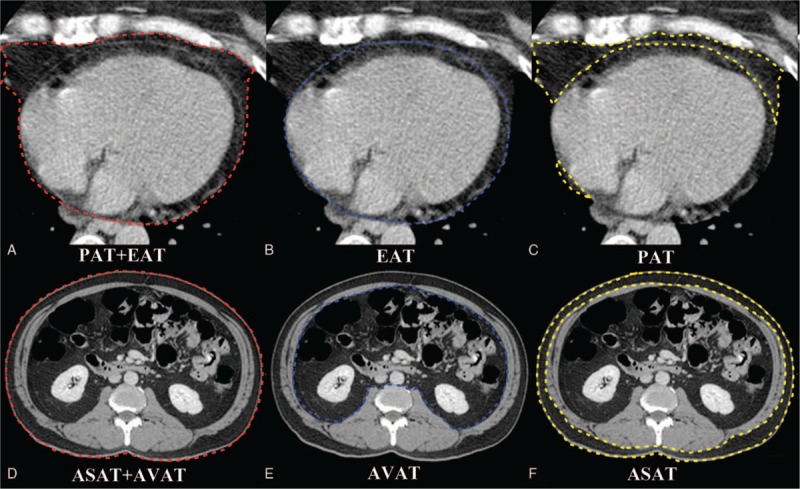
Quantification of different fat deposits by CT. Total pericardial volume = total EAT volume + total PAT volume. (A–C) Measurement of total EAT volume was performed on axial images by manual tracing (blue boundary) of the parietal pericardium from the left main pulmonary artery level to the left ventricular apex. Measurement of total pericardial fat volume by manual tracing (red boundary) of the area of the pericardial fat from the left main pulmonary artery level to the left ventricular apex. Total PAT volume (yellow boundary) = total pericardial fat volume (PAT + EAT) − total EAT volume. (D–F) Measurement of total AVAT volume was performed on axial images by manual tracing (blue boundary) of the parietal peritoneum from L2 to L5 levels. Measurement of total abdominal fat volume by manual tracing (red boundary) of the area of the abdominal fat from L2 to L5 levels. Total ASAT volume (yellow boundary) = total abdominal fat volume (AVAT + ASAT) − total AVAT volume. ASAT = abdominal subcutaneous adipose tissue, AVAT = abdominal visceral adipose tissue, EAT = epicardial adipose tissue, PAT = paracardial adipose tissue.
Statistical Analysis
Summary data are expressed as means standard deviation for continuous variables. Because all the continuous variables are normally distributed, student t test was used to test the differences between groups. Comparison of baseline and postintervention parameters was performed using paired t test or Wilcoxon signed-rank test when appropriate. A P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc, Chicago, IL). For the analysis of the variables, analysis of variance-repeated measures (with a set of the covariates of age, sex, and baseline WC) followed by Bonferroni correction were used to compare the percentage changes of anthropometric measurements in both the groups. In addition, the percentage changes of anthropometric measurements between the bariatric surgery group and the exercise group were compared using the t test for independent variables. Pearson correlation coefficients were calculated between the percentage change in BMI and percentage change of excessive fat loss in different regions.
RESULTS
Clinical Characteristics and Changes in Anthropometric Variables
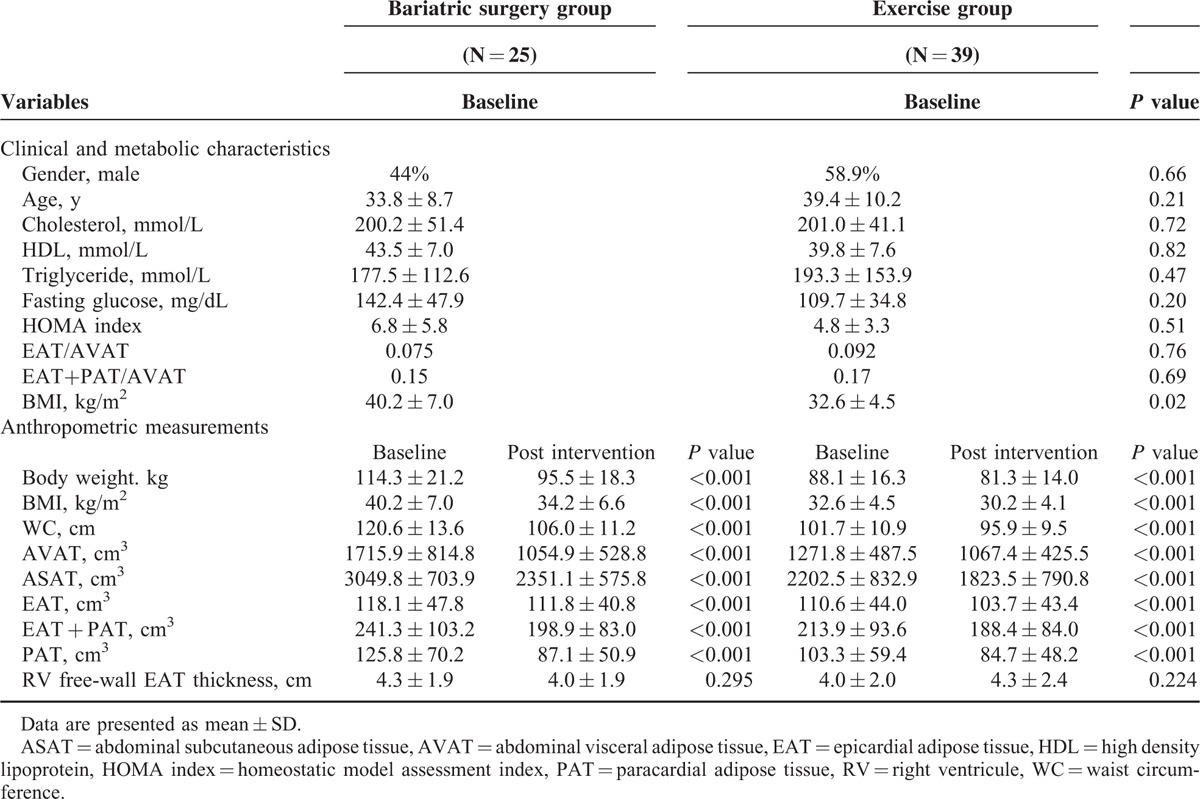
Clinical profile and anthropometric measurements in both the bariatric surgery group and the exercise groups at baseline and after intervention are listed in Table 1. At baseline, the 2 groups were comparable with respect to clinical and metabolic parameters including sex, age, fasting glucose, HOMA index, EAT/AVAT ratio, (EAT + PAT)/AVAT ratio and lipid profiles. BMI was higher in the bariatric surgery group. The bariatric surgery group had significant reduction of BMI, AVAT, ASAT, PAT, and EAT compared with baseline (15.97%, 40.52%, 31.40%, 34.40%, and 12.40% of baseline values respectively, all P < 0.001). The exercise group also had reduced BMI, AVAT, ASAT, PAT, and EAT (7.47%, 15.24%, 17.34%, 12.05%, and 6.82% of baseline values respectively, all P < 0.001). There was no significant reduction in the RV free-wall EAT thickness in either groups.
TABLE 1.
Anthropometric measurements, clinical, and metabolic characteristics of subjects
Changes in Different Regions of Excessive Fat Deposits After Surgery and Exercise
Comparison of relative percentage changes in clinical profile and anthropometric measurements in different regions of excessive fat deposits after intervention for both the bariatric surgery group and the exercise group are listed in Table 2 and Figure 3 A and B. Group comparison showed that percentages of decrease were significantly smaller in the exercise group except for ΔEAT (all P < 0.001, and P = 0.037, respectively). ΔEAT was −6.8% in the exercise group and −12.4% in the bariatric surgery group (P = 0.037). Compared with all of the other regions of excessive fat deposit, EAT had a relatively small percentage decrease in volume after intervention in both the groups (Figures 3A and 2B).
TABLE 2.
Comparison of relative change (%) at different excessive fat distribution and anthropometric measurements after intervention versus baseline in both the bariatric surgery group and the exercise group
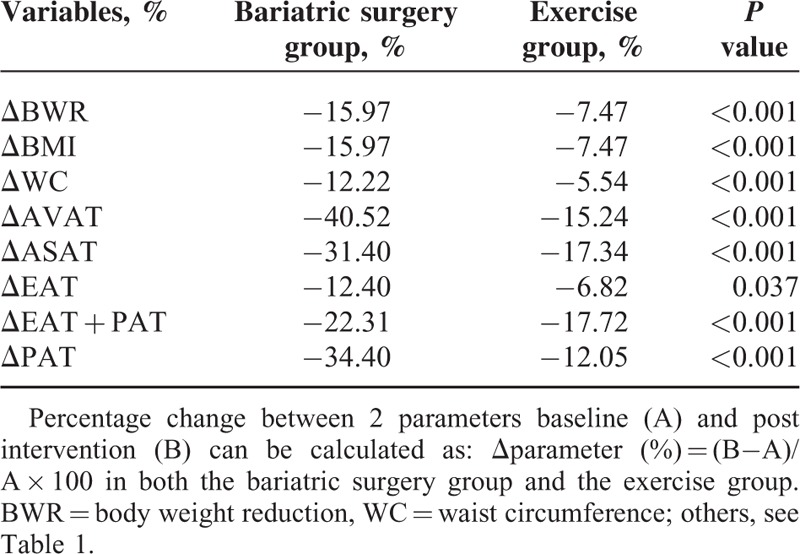
FIGURE 3.
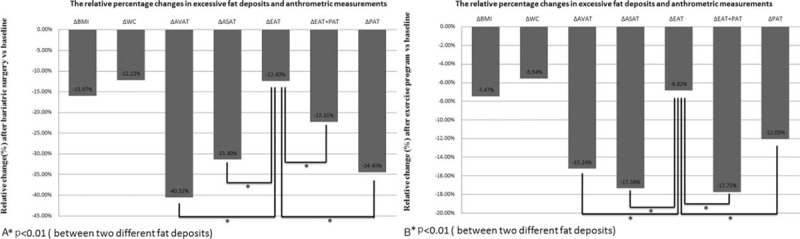
(A) Comparison of relative change (%) at different excessive fat deposits in the bariatric surgery group. (B) Comparison of relative change (%) at different excessive fat deposits in the exercise group. ASAT = abdominal subcutaneous adipose tissue, AVAT = abdominal visceral adipose tissue, BMI = Body Mass Index, EAT = epicardial adipose tissue, PAT = paracardial adipose tissue, WC = waist circumference.
Correlation Between Changes in BMI and Fat Deposits
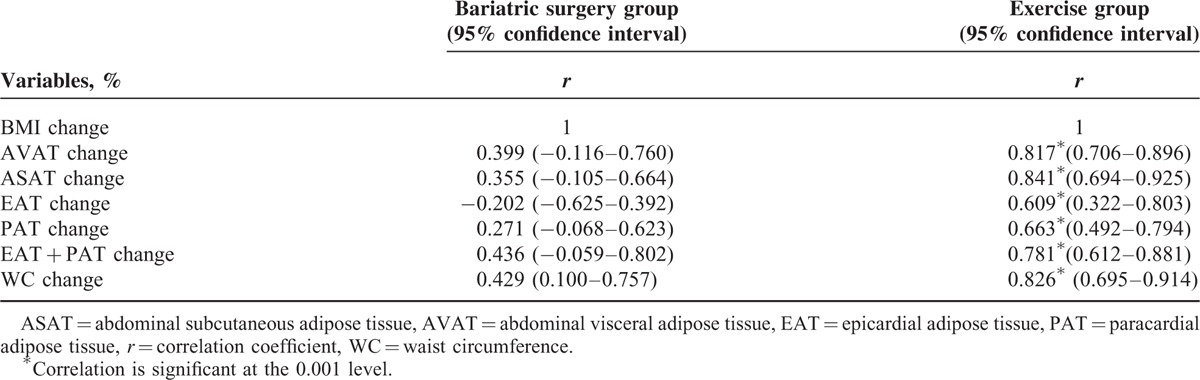
Correlations between the percentage change of different regions of excessive fat deposits and change in BMI in 2 groups are listed in Table 3. Unexpectedly, percentage changes of different excessive fat deposits did not correlate with the percentages of BMI decrease in the bariatric surgery group (all P > 0.05). But the percentage changes of different regions of fat deposits strongly correlated with the percentages of BMI loss in the exercise group.
TABLE 3.
Correlations of BMI change (%) with the percentage change of anthropometric measurements and different excessive fat deposits
DISCUSSION
There are 4 main findings in the current study. First, all anthropometric variables such as body weight, BMI, WC, and all abdominal and cardiac excessive adipose tissue measurements, except for RV free-wall EAT thickness, significantly decreased from baseline 3 months after intervention in both the groups. Second, the percentage loss of EAT was relatively limited, compared to other regions of excessive fat in both the groups. Third, as compared with the exercise group, bariatric surgery group showed greater percentage changes in all anthropometric adipose measurements, except for EAT. Fourth, percentage changes in the anthropometric parameters and excessive visceral adipose measurements correlated with percentage of BMI change in the exercise group, but not in the bariatric surgery group. Our findings are in agreement with previous results of “lesser decrease in epicardial adiposity.”17,18 Furthermore, we were able to demonstrate efficacy of both bariatric surgery and exercise program in terms of decrease in weight and excessive visceral adiposity in a single comparative, prospective clinical interventional study.
Obesity, associated with increased risks for cardiometabolic disorders and mortality, is one of the greatest public health challenges in developed countries. Treatment options for overweight or obese individuals include nonsurgical treatments (such as low-caloric diet, exercise, and pharmacotherapy alone or combined) and bariatric surgery. Previous studies have shown that bariatric surgery leads to greater body weight loss and higher remission rates of type 2 diabetes and metabolic syndrome in comparison to nonsurgical treatments of obesity, possibly due to underlying alterations in the gastrointestinal anatomy, gastrointestinal hormones, and regulatory factors of energy homeostasis following bariatric surgery.1,4,16
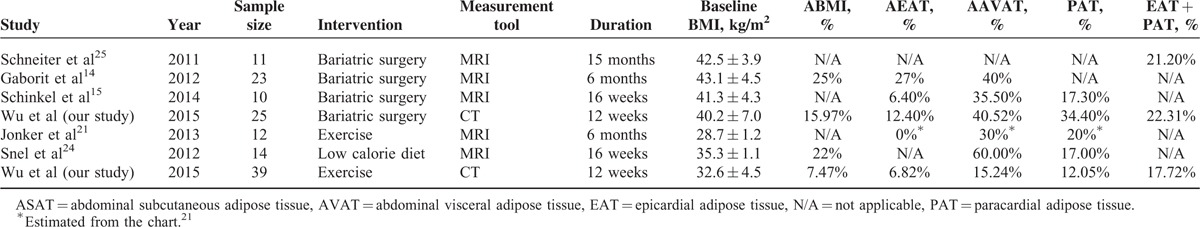
Previous studies have examined the effect of nutritional, exercise, or surgical interventions on visceral adiposity, including volumetric measurements of abdominal and epicardial visceral adipose tissues. The study by Gaborit et al17 on the effects of bariatric surgery on pericardial fat, visceral fat, and myocardial triglyceride content assessed by advanced magnetic resonance imaging (MRI) technology, showed a preferential loss of visceral abdominal fat (decrease in AVAT of 40% ± 19%) relative to epicardial fat (27% ± 11%) after 6 months. The study by Schinkel et al31 also demonstrated that surgical-induced weight loss leads to larger decrease in visceral fat volume (decrease in AVAT of 35.5% ± 9.6%) compared to epicardial fat volume (6.4% ± 6.0%) after 16 weeks. Jonker et al24 showed that no significant change in epicardial fat volume after 6 months of exercise in subjects with type 2 diabetes mellitus (4.7 ± 0.4 to 4.7 ± 0.4 mL, P = 0.9). The study by Snel et al31 demonstrated that 16 weeks of very low calorie diet reduced body weight, PAT, AVAT, ASAT to 78, 83, 40, and 53% of baseline values, respectively. Schneiter et al32 showed that BMI, EAT, and PAT + EAT were significantly reduced after gastric banding intervention. It is unknown whether exercise program and bariatric surgery have similar effects on the different visceral excessive fat deposits and their relative distribution. Our work, to our knowledge, is the first to compare the effects of exercise versus bariatric surgery on the reduction and relative distribution of excessive visceral fat deposition in a single comparative, prospective clinical study. There are 6 major studies that described the different visceral fat deposits by volumetric measurements after weight reduction interventions shown in Table 4. In summary, these studies have shown that percentage loss of EAT is limited compared with the other regions of visceral fat after weight reduction interventions. The study by Jonker et al24 demonstrated that no significant change in epicardial fat volume after 6 months of exercise in subjects with type 2 diabetes mellitus. It is probably because the baseline and relative change of BMI were lower than in other studies. Second, previous studies and our findings suggest differential effects on PAT and EAT after weight reduction interventions by bariatric surgery or exercise. We have shown that the percentage loss of EAT was relatively limited compared with PAT after weight reduction interventions. On the one hand, epicardial fat and paracardial fat have different embryonic origins. Epicardial fat shares a common embryonic origin with intra-abdominal visceral fat and gets its blood supply from the coronary arteries microcirculation.33 On the other hand, the paracardial fat originates from the embryonic primitive thoracic mesenchyme and gets its blood supply from noncoronary arteries.33,34 Thus, it is important to distinguish epicardial and paracardial fat deposits in evaluating effects of weight-loss intervention.
TABLE 4.
Summary of studies included the different viscera fat deposits by volumetric measurement after weight reduction intervention
Our study demonstrates that both bariatric surgery and exercise program lead to larger percentage decrease in visceral fat compared with epicardial fat volume (40.52% ± 11.15% compared with 12.40% ± 7.82% in the bariatric surgery group; and 15.24% ± 17.43% compared with 6.82% ± 9.38% in the exercise program group). Our study, similar to other studies, showed that reduction in total body weight (>5%) is associated with stabilization or reduction in epicardial fat burden.20 It is important to emphasize that the percentage decrease of EAT was smaller than other excessive fat regions. These results in the current study are consistent with those of Gaborit and Schinkel.17,18 Some authors have suggested that EAT adiposity plays a long-term sustained and protective effect in response to weight loss.13,17,18,20 Epicardial fat may display cardioprotective properties and function as a buffer to protect the heart against exposure to excessively high levels of free fatty acids through local secretion of anti-inflammatory and anti-atherogenic adipokines.7 An alternative explanation proposed by Gaborit et al17,35,36 is that EAT is very sensitive to intermittent hypoxia, and sequent fibrosis may decreases its capacity to be modulated by weight loss through bariatric surgery or exercise. Nevertheless, further studies are needed to investigate the causes and long-term effects of persistent EAT, and changes in endocrine/paracrine mediators secretion by EAT through EAT biopsy sampling37 after weight loss through bariatric surgery or exercise.
Unexpectedly, the percentage of EAT loss did not correlate with the percentage of BMI loss in the bariatric surgery group. However, there was significant correlation of percentages of EAT loss with the percentage of BMI loss in the exercise group. The results of the bariatric surgery group in our study is consistent with those findings of Gaborit et al,17 and the discrepant results between the 2 intervention groups suggest different mechanisms in weight loss. We postulated that this is due to the alternation of the gastrointestinal anatomy, gastrointestinal hormone, and regulatory factors of energy homeostasis following bariatric surgery,38 which leads to greater body weight loss and higher remission rates of diabetes in the bariatric group, compared with nonsurgical treatments of obesity. However, the underlying mechanism of bariatric surgery on gut hormones is far from clear, and the various types of surgery differ very much in terms of physiological effects. Future work in this area is warranted. On the other hand, that also means that weight loss by exercise may be more physiological and natural. All together, these observations suggest that the unclear multifactorial mechanisms of variation and relative distribution of excessive fat deposits between the 2 groups warrant further investigation.
LIMITATIONS OF THE STUDY
There were several limitations in the study. First, this study does not evaluate the blood or tissue levels of proinflammatory adipokines or address the underlying mechanisms of change in EAT or other visceral adipose tissue in weight loss through bariatric surgery or exercise. In addition, the resulting lack of investigation of association between nonalcoholic fatty liver disease and the visceral adiposity after bariatric surgery or exercise plan in our study should be interpreted with caution because there are several factors, which are required to be considered strictly.39,40 Second, there is disparity between the 2 groups with regard to baseline characteristics such as BMI and HOMA index, despite of the similar ratio of EAT/AVAT. It implies that a cause-effect relationship cannot be strongly concluded from the current study design. Third, the study is also limited by the small sample size and the short follow-up period. The current evidences suggest that sustained maintenance of physical activity after bariatric surgery or nonsurgical weight loss programs as an essential part of many health benefits such as lowering all-cause and cardiovascular mortality risk, even in the absence of weight loss.41–45 It also plays an important role in the amount of weight loss and maintenance following successful weight loss.44 Further study should enroll more subjects, control the baseline characteristics such as BMI and HOMA index, apply quasi-experimental design, and try to investigate the underlying mechanism and long-term effects of different abdominal and cardiac fat deposition between the 2 groups.
CONCLUSIONS
Compared with nonsurgical exercise program treatment of obesity, bariatric surgery leads to significantly greater reduction of anthropometric variables (BMI, WC, and body weight) and abdominal and cardiac visceral adipose tissue volume, but relative smaller decrease in EAT. In addition, the percentage decrease in EAT is less than in other areas of adipose tissue deposition in both the groups. These findings contribute to the existing evidences suggesting differential impacts of weight loss on adipose tissue of various locations after bariatric surgery or exercise program. However, further studies are needed to investigate the long-term effects of weight loss with bariatric surgery and exercise intervention on different visceral adiposity.
Acknowledgments
The authors thank RT, Chen-Shying Chen, Chiung-Chen Chuo, for CT scanning; and research assistants, Yu-Jeng Ju, Min-Tao Hsu, Yu-Ying Lin for data management.
Footnotes
Abbreviations: ASAT = abdominal subcutaneous adipose tissue, AVAT = abdominal visceral adipose tissue, CT = computed tomography, EAT = epicardial adipose tissue, HDL = high density lipoprotein, HOMA = homeostatic model assessment, MRI = magnetic resonance imaging, NAFLD = nonalcoholic fatty liver disease, PAT = paracardial adipose tissue, VAT = visceral adipose tissue, WC = waist circumference.
Authors’ contributions: FZW and CCW performed the data collection, article drafting, data and statistical analysis, and interpretation of the results. CSC and YLH contributed to data analyses and interpretation of the results. FZW, YY, and GYM contributed to article drafting, statistical analyses, and interpretation of the results. FZW and MTW conducted the development of the conception and design of the experiments. All authors read and approved the final article.
This study is supported by Grants from National Science Council NSC97–2314-B-010–045-MY3 and Kaohsiung Veterans General Hospital, VGHKS101–020, VGHKS100–074, VGHKS104–048, Taiwan, R.O.C.
The authors have no conflicts of interest to disclose. The authors report no potential conflicts of interest with any companies or organizations whose products or services are mentioned in this article.
REFERENCES
- 1.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13:214. [DOI] [PubMed] [Google Scholar]
- 2.Despres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444:881–887. [DOI] [PubMed] [Google Scholar]
- 3.Després J-P, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28:1039–1049. [DOI] [PubMed] [Google Scholar]
- 4.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 2008; 32:619–628. [DOI] [PubMed] [Google Scholar]
- 5.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007; 153:907–917. [DOI] [PubMed] [Google Scholar]
- 6.Ngo DT, Gokce N. Epicardial adipose tissue: a benign consequence of obesity? Circ Cardiovasc Imaging 2015; 8:e003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol 2011; 43:1651–1654. [DOI] [PubMed] [Google Scholar]
- 8.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117:605–613. [DOI] [PubMed] [Google Scholar]
- 9.Djaberi R, Schuijf JD, van Werkhoven JM, et al. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol 2008; 102:1602–1607. [DOI] [PubMed] [Google Scholar]
- 10.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006; 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 12.Kankaanpää M, Lehto H-R, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metabol 2006; 91:4689–4695. [DOI] [PubMed] [Google Scholar]
- 13.de Roos A. Reversibility of intrathoracic lipotoxicity in obesity after bariatric surgeryuse of magnetic resonance imaging. J Am Coll Cardiol 2012; 60:1390–1392. [DOI] [PubMed] [Google Scholar]
- 14.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011; 124:e837–e841. [DOI] [PubMed] [Google Scholar]
- 15.Borel A-L, Nazare J-A, Smith J, et al. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity 2012; 20:1223–1233. [DOI] [PubMed] [Google Scholar]
- 16.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013; 347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol 2012; 60:1381–1389. [DOI] [PubMed] [Google Scholar]
- 18.van Schinkel LD, Sleddering MA, Lips MA, et al. Effects of bariatric surgery on pericardial ectopic fat depositions and cardiovascular function. Clin Endocrinol 2014; 81:689–695. [DOI] [PubMed] [Google Scholar]
- 19.Foppa M, Pond KK, Jones DB, et al. Subcutaneous fat thickness, but not epicardial fat thickness, parallels weight reduction three months after bariatric surgery: a cardiac magnetic resonance study. Int J Cardiol 2013; 168:4532–4533. [DOI] [PubMed] [Google Scholar]
- 20.Nakazato R, Rajani R, Cheng VY, et al. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis 2012; 220:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willens HJ, Byers P, Chirinos JA, et al. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol 2007; 99:1242–1245. [DOI] [PubMed] [Google Scholar]
- 22.Kokkinos A, Alexiadou K, Liaskos C, et al. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg 2013; 23:31–38. [DOI] [PubMed] [Google Scholar]
- 23.Fu C-P, Sheu WHH, Lee IT, et al. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clinica Chimica Acta 2013; 421:98–103. [DOI] [PubMed] [Google Scholar]
- 24.Jonker JT, de Mol P, de Vries ST, et al. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 2013; 269:434–442. [DOI] [PubMed] [Google Scholar]
- 25.Chaowalit N, Somers VK, Pellikka PA, et al. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis 2006; 186:354–359. [DOI] [PubMed] [Google Scholar]
- 26.Gorter PM, van Lindert ASR, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008; 197:896–903. [DOI] [PubMed] [Google Scholar]
- 27.Saura D, Oliva MJ, Rodríguez D, et al. Reproducibility of echocardiographic measurements of epicardial fat thickness. Int J Cardiol 2010; 141:311–313. [DOI] [PubMed] [Google Scholar]
- 28.NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The Evidence Report. National Institutes of Health. Obesity Res 1998; 6 suppl 2:51S–209S. [PubMed] [Google Scholar]
- 29.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999; 211:283–286. [DOI] [PubMed] [Google Scholar]
- 30.Wu F-Z, Huang Y-L, Wang Y-C, et al. Impact of location of epicardial adipose tissue, measured by coronary artery calcium-scoring computed tomography on obstructive coronary artery disease. Am J Cardiol 2013; 112:943–949. [DOI] [PubMed] [Google Scholar]
- 31.Snel M, Jonker JT, Hammer S, et al. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity 2012; 20:1572–1576. [DOI] [PubMed] [Google Scholar]
- 32.Schneiter SM. Effects of weight loss on pericardial fat and left ventricular mass assessed with cardiac magnetic resonance imaging in morbid obesity. Int J Clin Med 2011; 02:360–366. [Google Scholar]
- 33.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 2009; 22:1311–1319. [DOI] [PubMed] [Google Scholar]
- 34.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab 2011; 22:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-Inducible Factor 1 (Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol Cell Biol 2009; 29:4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010; 59:2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes 2007; 32:268–274. [DOI] [PubMed] [Google Scholar]
- 38.Ionut V, Burch M, Youdim A, et al. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity 2013; 21:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finelli C, Tarantino G. Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease? World J Gastroenterol 2012; 18:6790–6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 2015; 63:174–182. [DOI] [PubMed] [Google Scholar]
- 41.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One 2015; 10:e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014; 56:369–381. [DOI] [PubMed] [Google Scholar]
- 43.Pack QR, Rodriguez-Escudero JP, Thomas RJ, et al. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc 2014; 89:1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014; 56:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ades PA, Savage PD. Potential benefits of weight loss in coronary heart disease. Prog Cardiovasc Dis 2014; 56:448–456. [DOI] [PubMed] [Google Scholar]


