Abstract
Combination therapy of intravenous immunoglobulin (IVIG) and rituximab showed a good transplant rate in highly sensitized wait-listed patients for deceased donor kidney transplantation (DDKT), but carried the risk of antibody-mediated rejection. The authors investigated the impact of a new combination therapy of bortezomib, IVIG, and rituximab on transplantation rate.
This study was a prospective, open-labeled clinical trial. The desensitization regimen consisted of 2 doses of IVIG (2 g/kg), a single dose of rituximab (375 mg/m2), and 4 doses of bortezomib (1.3 mg/m2). The transplant rate was analyzed. Anti-Human leukocyte antigen (HLA) DRB antibodies were determined by a Luminex solid-phase bead assay at baseline and after 2, 3, and 6 months in the desensitized patients.
There were 19 highly sensitized patients who received desensitization and 17 patients in the control group. Baseline values of class I and II panel reactive antibody (%, peak mean fluorescence intensity) were 83 ± 16.0 (14952 ± 5820) and 63 ± 36.0 (10321 ± 7421), respectively. Deceased donor kidney transplantation was successfully performed in 8 patients (42.1%) in the desensitization group versus 4 (23.5%) in the control group. Multivariate time-varying covariate Cox regression analysis showed that desensitization increased the probability of DDKT (hazard ratio, 46.895; 95% confidence interval, 3.468–634.132; P = 0.004). Desensitization decreased mean fluorescence intensity values of class I panel reactive antibody by 15.5% (20.8%) at 2 months. In addition, a liberal mismatch strategy in post hoc analysis increased the benefit of desensitization in donor-specific antibody reduction. Desensitization was well tolerated, and acute rejection occurred only in the control group.
In conclusion, a desensitization protocol using bortezomib, high-dose IVIG, and rituximab increased the DDKT rate in highly sensitized, wait-listed patients.
INTRODUCTION
Deceased donor kidney transplantation (DDKT) has rapidly grown to approximate 750 cases per year in Korea. The mean waiting time for DDKT in Korea in 2013, however, was 1861 days (5.1 years), which is longer than that in the United States.1 Moreover, highly sensitized patients are prone to have longer waiting times and lower transplant rates because of a positive crossmatch response.2 Highly sensitized patients form a significant percentage (16.8%) of kidney wait-listed patients in Korea, which poses an important clinical problem.3
Previous strategies, including high-dose intravenous immunoglobulin (IVIG) and rituximab, have been reported to yield successful results for desensitization and conversion to kidney transplantation.4–7 High-dose IVIG-based desensitization increases the probability of transplantation conversion with reduction of preformed donor-specific antibody (DSA). In other reports, the efficacy of desensitization based on high-dose IVIG, however, was questioned, especially for patients with calculated panel reactive antibody (cPRA) levels higher than 90%; these agents cannot eliminate the bone marrow-resident, long-lived, plasma cells and the risk of subsequent antibody-mediated rejection was significant.8–13 Bortezomib, a proteasome inhibitor, induces apoptosis of plasma cells.14 Recently, Woodle et al15 reported a successful outcome of desensitization by bortezomib and plasmapheresis without high-dose IVIG in a mixed group of deceased and living kidney transplantations. Plasmapheresis, however, is not a convenient procedure compared with high-dose IVIG. Here, we present the results of desensitization using high-dose IVIG and rituximab with bortezomib, which reduced the waiting time for DDKT and facilitated successful transplantation.
MATERIALS AND METHODS
Study Population
The study was a prospective, open-labeled trial. The inclusion criteria required all of the following: age ≥19 years, waiting duration ≥4 years, history of failure to receive allocated kidney because of positive crossmatch response, and high degree of sensitization defined as peak panel reactive antibody (PRA) class I or II >50%. Subjects were excluded if any of the following exclusion criteria were present: history of live attenuated vaccination within 4 weeks, evidence of viral hepatitis B, C, or Human immunodeficiency virus infection, active infection, pregnancy or lactation, history of malignancy within 5 years, history of treatment for psychiatric problems within 6 months, or hematologic or biochemical abnormalities (hemoglobin <7 g/dL, platelet <50,000/mm3, and aspartate aminotransferase or alanine transaminase >80 IU).
The study flow is presented in Figure 1. At the screening stage, there were 53 highly sensitized patients, among them 16 were excluded (13 for not meeting inclusion criteria, 3 for participating other trials). A total of 20 patients agreed to receive desensitization treatment. Among the 20 patients, 1 was lost after day 30 because of noncompliance and was excluded from further analysis. Seventeen patients did not receive desensitization because of personal rejection. Ultimately, the 19 desensitized patients in the treatment group were compared with the 17 patients without desensitization in the control group.
FIGURE 1.
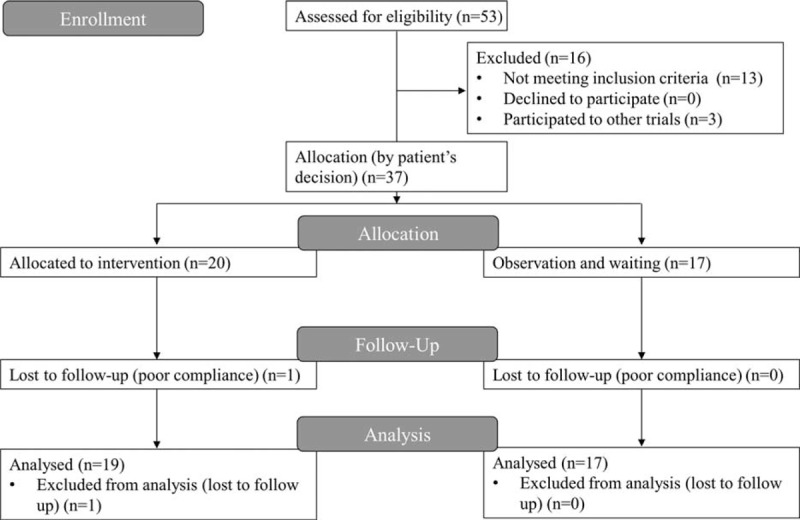
The CONSORT study flow diagram.
An Institutional Review Board of Seoul National University Hospital approved this study (IRB approval numbers H-0910–082–298), and informed consent was obtained from all study participants. The study was performed in accordance with the Declaration of Helsinki 2000 and was registered at ClinicalTrials.gov (NCT01502267). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Study Design and Desensitization Regimen
The desensitization regimen consisted of 2 doses of high-dose IVIG (2 g/kg, on D0 and D30), a single dose of rituximab (375 mg/m2, on D1), and a single cycle of bortezomib (1.3 mg/m2, on D15, 18, 21, and 24). Antibacterial and antiviral prophylaxes were given with sulfamethoxazole–trimethoprim and acyclovir during the 3 to 6 months after desensitization.
Study Outcomes
The primary study outcome was kidney transplantation rate among the wait-listed population. The secondary outcomes were reduction in anti-HLA antibodies, renal allograft survival rate and acute rejection rate among patients who received DDKT, and the profile of adverse events. The profile of adverse events were graded by the Common Terminology Criteria for Adverse Events, v3.0.16 Kidney allocations were performed by the Korean network for organ sharing, which was blinded to the desensitization enrollment. Crossmatch results at the allocation step were collected for the all transplant attempts. For the desensitized group, after completion of desensitization treatment, the changes in PRA values were measured at 2, 3, 6, and 12 months after initiation of desensitization. For the control group, the date of transplant and crossmatch results were collected. Posttransplant renal allograft pathology was assessed according to Banff 07 classification by 2 independent pathologists who were blinded to allocation status.17
Anti-HLA Antibody Assays
Levels of anti-HLA antibody were serially assessed by HLA phenotype (LIFECODES class I and II ID panels, Immucor Gen-Probe, San Diego, CA) and single-antigen bead assays (LIFECODES LSA class I and II panels, Immucor Gen-Probe, Norcross, GA) on a Luminex platform. Anti-HLA antibody status was measured by counting positive probe (%) and determining peak mean fluorescence intensity (MFI) values for phenotype antigen bead assays. For single-antigen bead assays, anti-HLA antibody status was measured by determining MFI values of each donor antigen and calculating cPRA values, which were derived by using UNet allocation calculators.18
T- and B-cell crossmatch tests were performed by complement-dependent cytotoxicity crossmatch (CDC-XM) and flow cytometry crossmatch (FC-XM). The criterion for negative FC-XM was an MFI ratio below 2.0 in both T- and B-cell crossmatch. In our center, the threshold of positive FC-XM was correlated with a moderate degree of DSA levels (3000–9000 MFI), and positive CDC-XM was correlated with stronger DSA levels (MFI > 9000).19
Statistical Analysis
For categorical variables, a χ2 test or Fisher exact test were used. For continuous variables, independent or paired t tests were used. A linear mixed model was used in the longitudinal analysis to measure changes in anti-HLA antibody levels. To measure the effect of desensitization on waiting time, a time-varying covariate Cox regression model was used.20 A P value of <0.05 was regarded as significant. All statistical analyses were done with Stata 12 (StataCorp LP, College Station, TX).
RESULTS
Clinical Characteristics of the Study Population
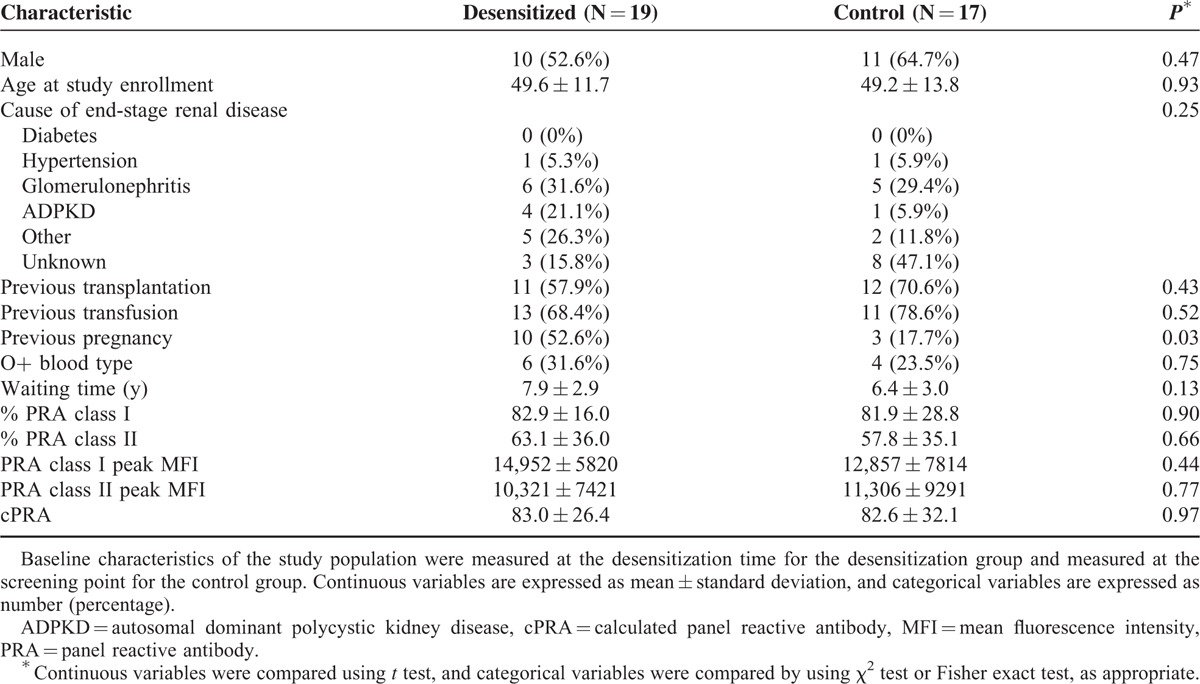
Table 1 summarizes the clinical characteristics of the study populations. The mean percent values of PRA class I and II in the desensitization group were 82.9% and 63.1%, respectively. The mean peak MFI values of class I and II PRA were 14,952 and 10,321, respectively, indicating the severity of sensitization in the study population. The cPRA values were 83.0% and 82.6% in the desensitization group and the control group, respectively. The overall clinical characteristics were comparable between the 2 groups except history of pregnancy.
TABLE 1.
Baseline Clinical Characteristics of the Study Population
Desensitization Facilitated Transplantation Conversion
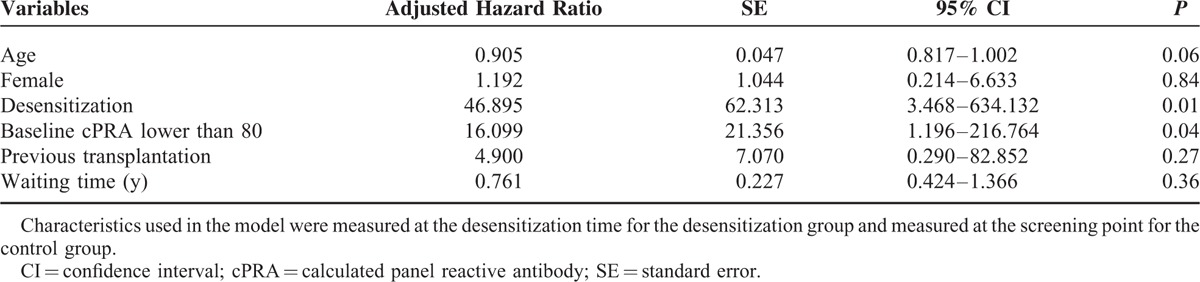
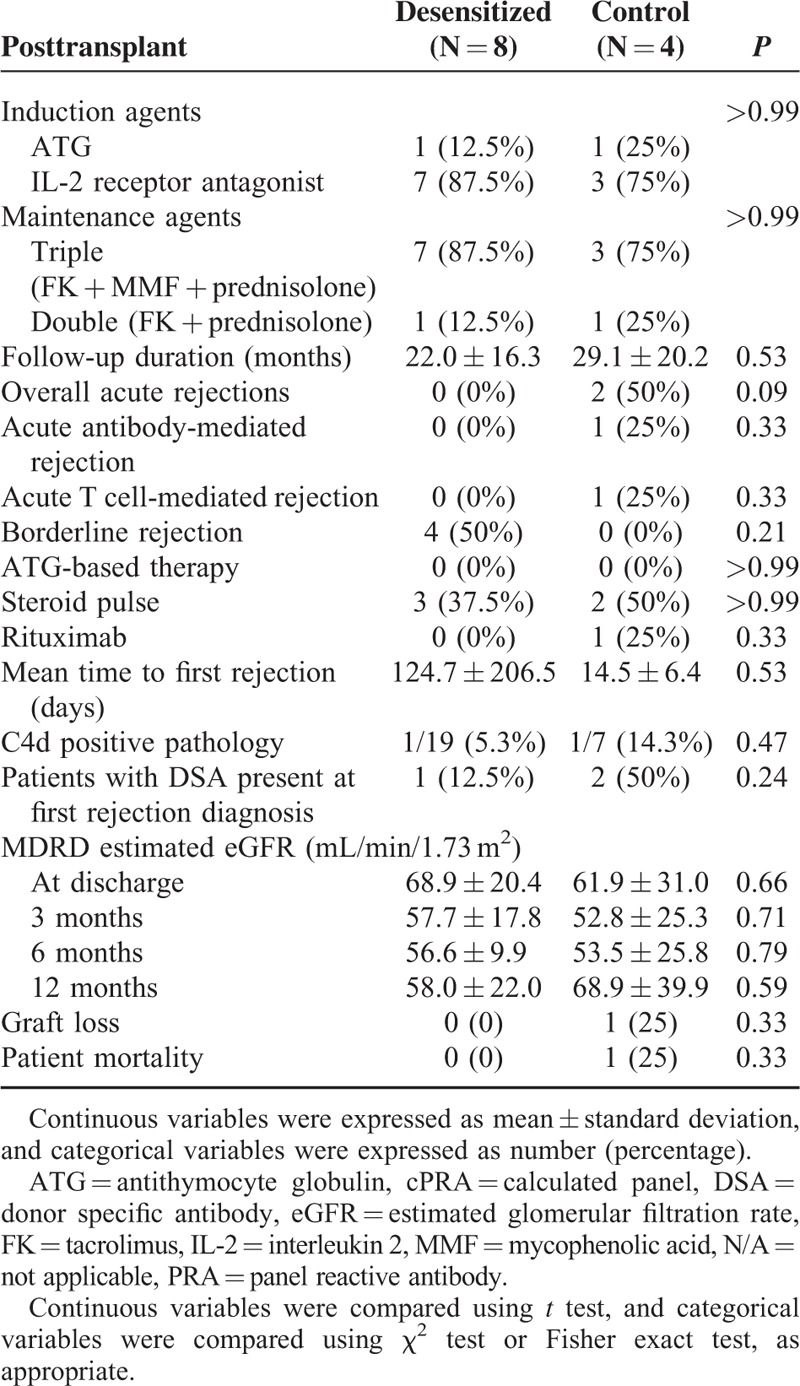
Among the 19 patients in the desensitization group, 8 (42.1%) received DDKT, compared with 4 (23.5%) patients of DDKT among the 17 patients in the control group. There were no HLA full-matched cases, and there were no differences in the number of HLA antigen mismatch (Table 2). When time interval to kidney transplantation was estimated from the study enrollment, the desensitization group received DDKT at an earlier time point than the control group (Table 2 and Figure 2A). Most transplantation conversion occurred within 6 months after desensitization, indicating that the desensitization effects were immediate. Next, time-varying covariate Cox regression analysis was performed to estimate the time interval to kidney transplantation from the wait-list registration. Desensitization significantly increased the probability of receiving DDKT for highly sensitized, wait-listed patients (P < 0.001, Figure 2B). When the hazard ratio (HR) was adjusted for age, sex, baseline cPRA lower than 80%, history of transplantation, and waiting time was estimated by the time-varying covariate Cox regression model, the adjusted HR of desensitization to receive DDKT was 46.90 (95% confidence interval [CI], 3.47–634.13; P = 0.004, Table 3). Among covariates, a baseline cPRA lower than 80% was also a significant predictor for the transplant conversion with an HR of 16.10 (95% CI, 1.20– 216.76; P = 0.04).
TABLE 2.
Pretransplant Clinical Characteristics of Kidney Transplant Recipients
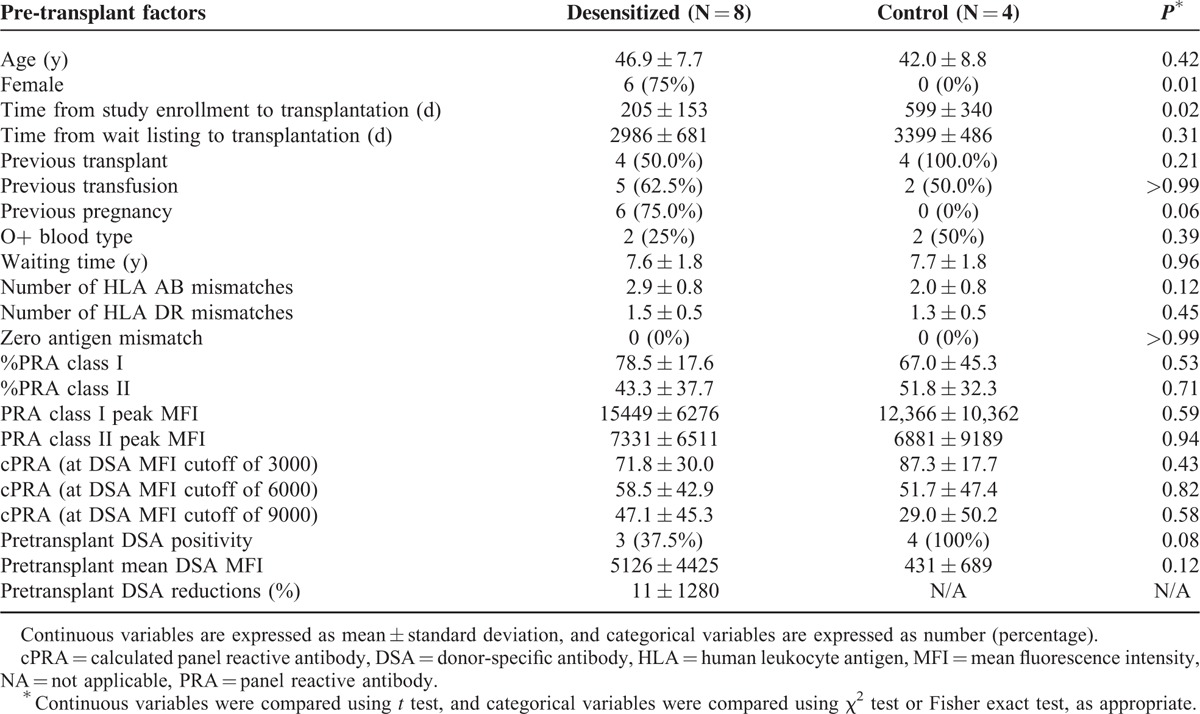
FIGURE 2.
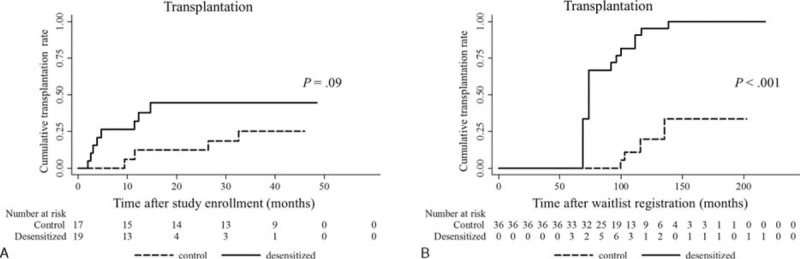
Transplantation rate according to desensitization. A, Kaplan–Meier curves show time interval to kidney transplantation from the study enrollment. Transplantation occurred early after desensitization (P = 0.09, log-rank test). B, Kaplan–Meier curves show time interval to kidney transplantation from the wait-list registration. Desensitization increased the probability of receiving deceased donor kidney transplantation during the waiting period when estimated with time-varying covariate Cox regression analysis (P < 0.001, log-rank test).
TABLE 3.
Adjusted Hazard Ratio of Receiving Kidney Transplantation in Time Varying Covariate Cox Regression Model
Desensitization Reduced Levels of Preformed Anti-HLA Antibodies
Figure 3 shows sequential change in PRA class I and II (%) and their peak MFI values in the desensitized group. Desensitization reduced PRA class I at 2 months after the initiation of desensitization. Using a linear mixed model, the regression coefficient for percent values of PRA class I was −6.34 per month (95% CI, −9.22 to −3.46; P < 0.001), and that for peak MFI values of PRA class I was −1528.02 per month (95% CI, −2509.65 to −546.39; P = 0.002). These data reveal a 15.5% reduction in percent values of PRA class I and a 20.8% reduction in peak MFI values of PRA class I (Table 4). The rebounds of percent and peak MFI values of PRA abrogated the effect of desensitization at 6 months after desensitization. The cPRA values at different cutoff points were not changed after desensitization at any interval (Table 4). The impact of desensitization was effective for class I HLA antibodies with both high (>9999 MFI, P = 0.05) and intermediate (5000–9999, P = 0.02) levels of MFI (Figure 4A). Along with the refractory response of class II HLA antibodies, antibodies to public epitope HLA DRB 3, 4, and 5 did not show any reduction (Figure 4B and C).
FIGURE 3.
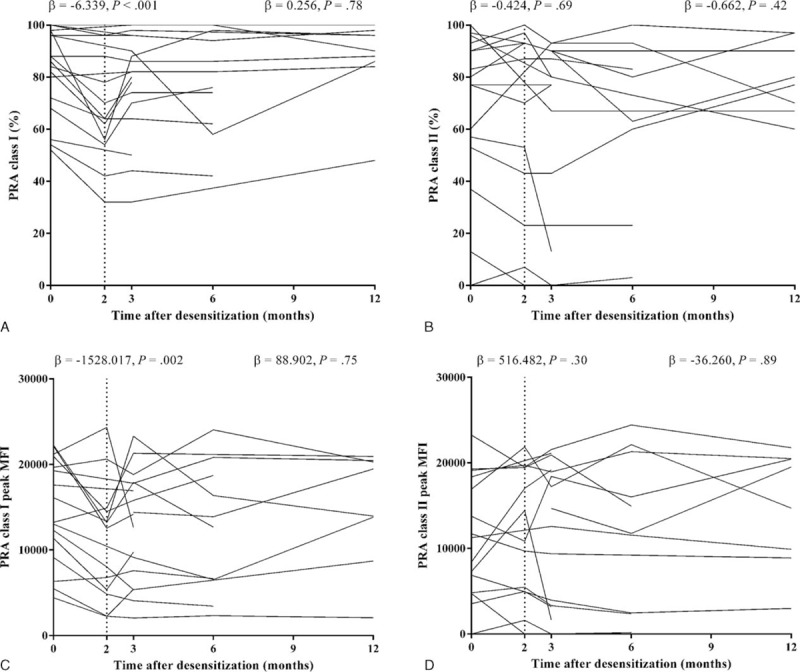
Changes of PRA after desensitization. A, The serial measurements of percent values of PRA class I after desensitization. B, The serial measurements of percent values of PRA class II after desensitization. C, The serial measurements of peak MFI values of PRA class I after desensitization. D, The serial measurements of peak MFI values of PRA class II after desensitization. Vertical dashed line indicates the time point of 2 months after desensitization. Text values located at the left side of the vertical dashed line indicate linear regression coefficient of PRA change and P value for comparison between baseline and 2 months after desensitization. Text values located at the right side of vertical dashed line indicate linear regression coefficient of PRA change and its P value for comparing between 2 and 12 months after desensitization. MFI = mean fluorescence intensity, PRA = panel reactive antibodies.
TABLE 4.
Changes in Panel Reactive Antibodies After Desensitization
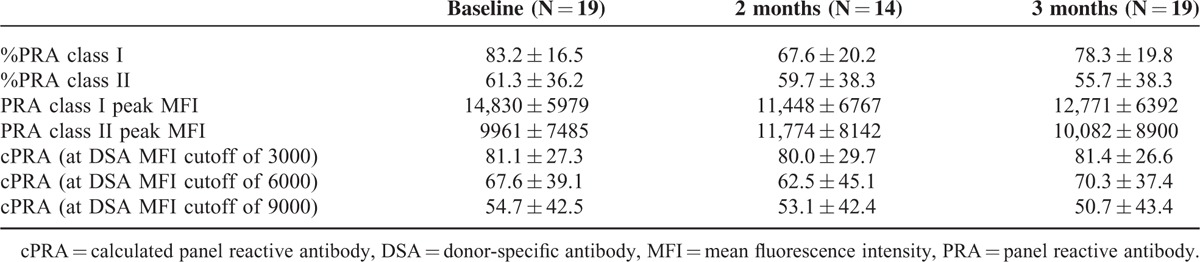
FIGURE 4.
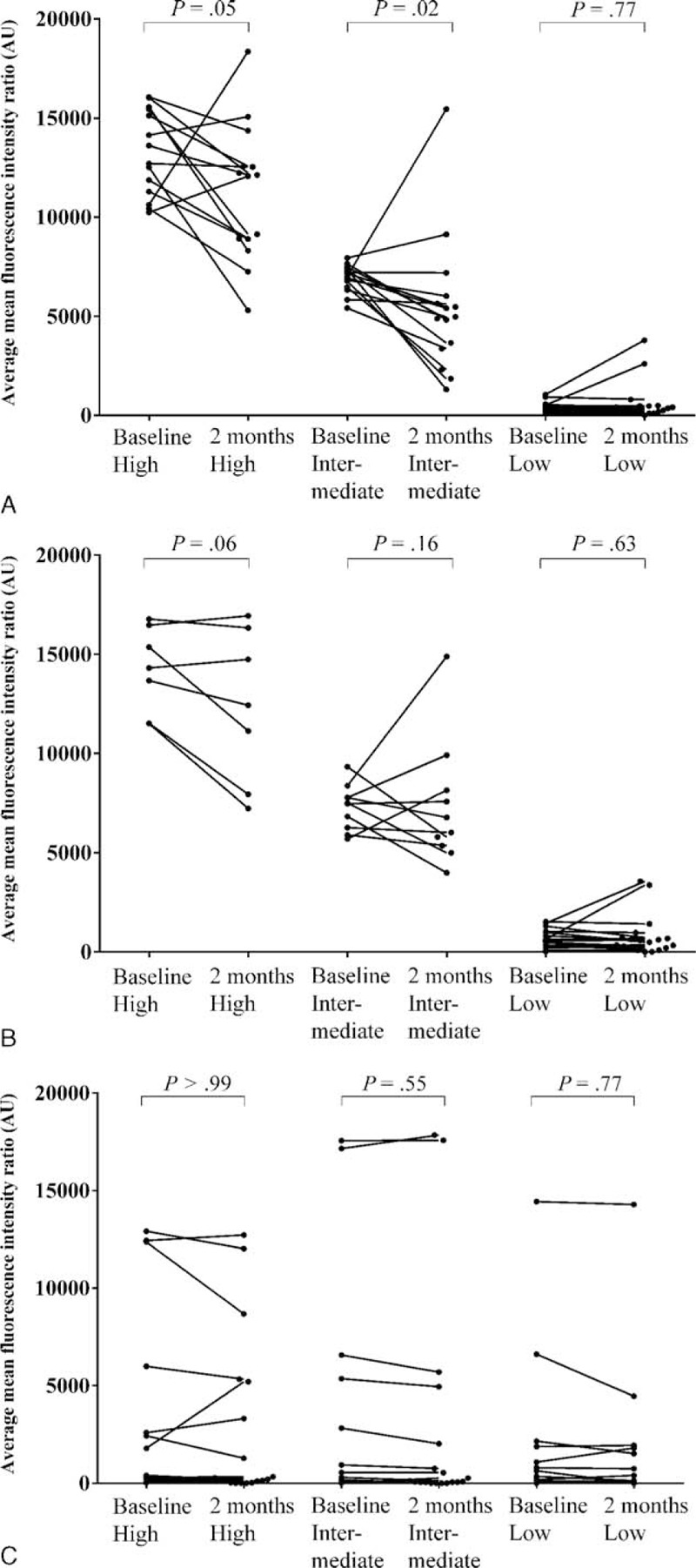
Changes in the MFI values of anti-HLA antibodies according to different baseline antibody intensity. A, Change of MFI levels in class I anti-HLA antibodies. Antibody levels were significantly reduced in groups of both high and intermediate MFI levels. B, Change of MFI levels in class II anti-HLA antibodies. Class II antibodies did not show significant decrement in any intensity criteria. C, Change of MFI among public epitope, HLA DRB 3, 4, and 5. Along with the refractoriness of class II anti-HLA antibodies, public epitopes were not decreased by desensitization. human leukocyte antigenMFI = mean fluorescence intensity, PRA = panel reactive antibodies.
Desensitization Reduced the Donor-specific Antibody Level Only in Patients With Significant Donor-specific Antibody Levels Before Desensitization
We performed DDKT according to the strict criteria of FC-XM negativity (MFI ratio <2.0). When intensity of FC-XM was estimated in transplant patients, desensitization reduced B-cell crossmatch intensity in 2 patients among 8 desensitized patients (Figure 5A). The other patients in the desensitization group had low FC-XM intensity before desensitization, as did all patients in the control group (Figure 5A and B). Only a single case showed a 27.8% reduction of DSA after desensitization (Figure 5C). The other desensitized patients had MFI values of DSA less than 2000 before desensitization. These results indicate that desensitization reduced DSA levels only in patients with significant levels before desensitization.
FIGURE 5.
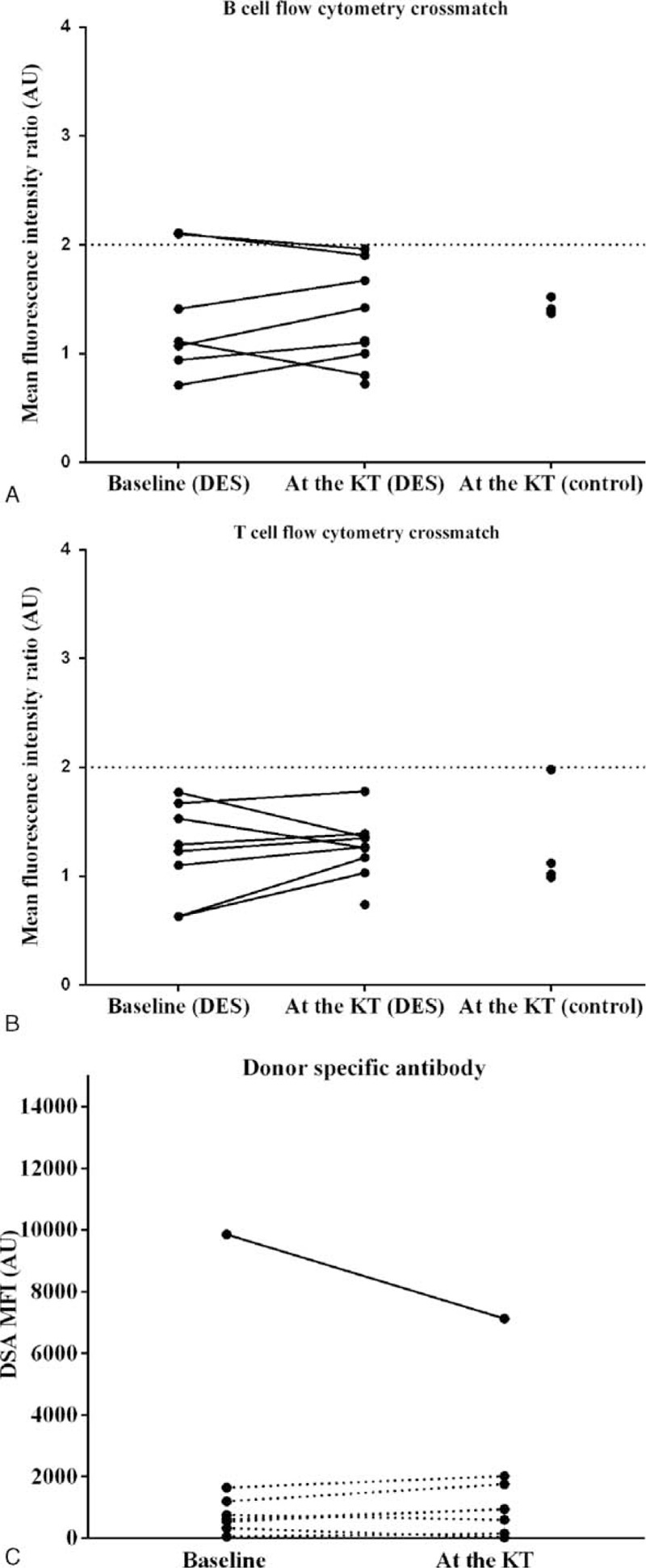
Changes in intensity of flow cytometry crossmatch results (A-B) and levels of DSA (C) after desensitization. The positivity of flow cytometry crossmatch results were interpreted based on the MFI ratio. Each horizontal line of graph (A) and (B) indicates the cutoff value for the B- and T-cell flow cytometry crossmatch, respectively. In graph (C), each dot represents the summation of donor-specific antibodies. Solid line represents the case whose baseline DSA value was above 2000 MFI. Dashed line represents the cases whose baseline DSA values were below 2000 MFI. Desensitization reduced DSA level only in 1 case where baseline DSA was above 2000 MFI. AU = arbitrary unit, DES = desensitization, DSA = donor-specific antibody, KT = kidney transplantation, MFI = mean fluorescence intensity.
Effects of More Liberal Acceptable Strategies
To illustrate the benefit of desensitization, post hoc analysis was performed. We virtually broadened acceptable mismatch criteria, and included cases with MFI ratios between 2.0 and 3.0 in FC-XM, who lost the opportunity for DDKT because of the strict crossmatch criteria. In previous studies, acceptable mismatch criteria used a mean channel shift of 250, which was associated with low levels of antihuman globulin-enhanced CDC-XM positive results.4–6,21 In our center, a MFI ratio of 3.0 in FC-XM is approximately equivalent to a low degree of antihuman globulin-enhanced CDC-XM positive results. When the criteria of acceptable mismatch were changed to a MFI ratio below 3.0 instead of 2.0, the virtual transplantation rate was increased in both the desensitization and control groups, and the benefit of desensitization was still present (P = 0.002, Figure 6A). In this scenario, desensitization increased the virtual transplantation rate more significantly (HR, 29.67; 95% CI, 5.05–174.38; P < 0.001). When we analyzed the DSA levels of virtual transplantation cases, desensitization reduced DSA levels in transplant patients whose DSA levels were above 2000 MFI before desensitization (P = 0.04, Wilcoxon signed-rank sum test, Figure 6B).
FIGURE 6.
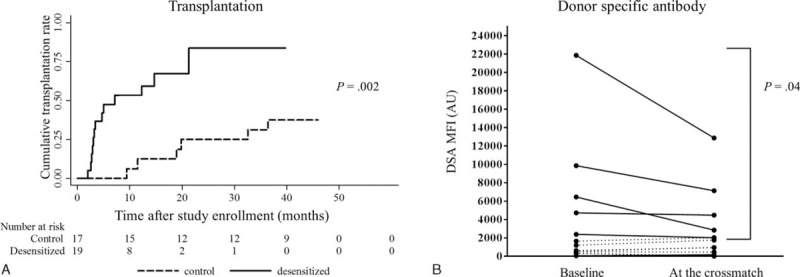
Post hoc analysis of transplantation and DSA reduction after desensitization using more liberal acceptable mismatch criteria. A, Post hoc analysis results of transplantation when acceptable mismatch criteria were set at more flexible crossmatch criteria (MFI ratio <3.0, approximate to 250 of the mean channel shift value) in both T- and B-cell flow cytometry crossmatch. Transplantation rates increased in both the desensitization and the control groups compared with Figure 2A, and desensitization increased probability of transplantation significantly (P = 0.002, log-rank test). Kaplan–Meier curves represent cumulative incidence of transplantation from the desensitization in the desensitization group or from the study enrollment in the control group. B, Virtual results of changes in DSA levels after desensitization according to the same criteria. Desensitization significantly decreased DSA levels in patients with baseline DSA levels above 2000 (P = 0.04, signed Wilcoxon signed-rank sum test), whereas there was no significant change in patients with baseline DSA levels below 2000. DSA = donor-specific antibody; MFI = mean fluorescence intensity.
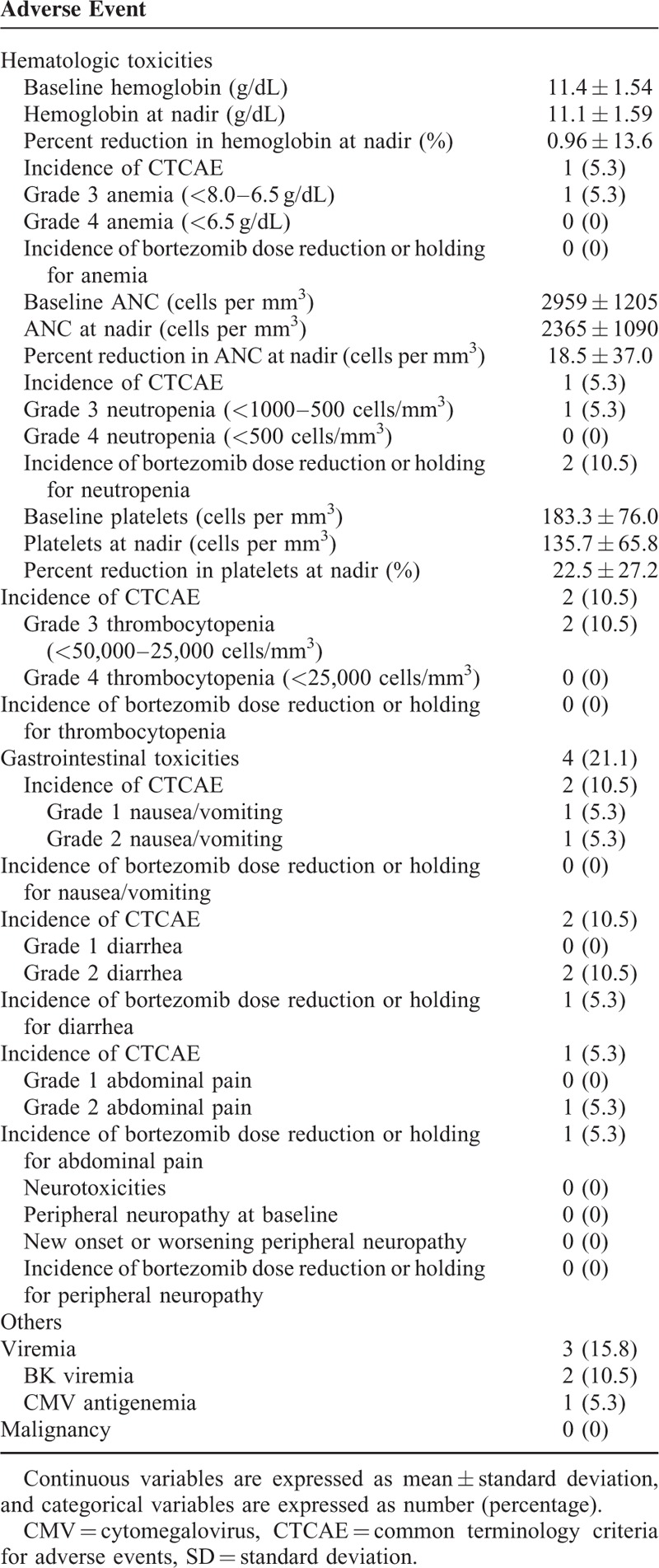
The Desensitization Regimen Was Well Tolerated
Among the 19 patients who received desensitization, 18 patients completed the full course of the desensitization regimen. One patient received only 2 doses of bortezomib because of neutropenia and abdominal pain after the second dose of bortezomib. According to CTCAE, gastrointestinal toxicity was the most common adverse event (21%), followed by opportunistic infection (15.8%), and thrombocytopenia (10.5%) (Table 5). Among opportunistic infection, BK viremia appeared after transplantation in 2 patients in the desensitization group. In the control group, there was no BK viremia after transplantation. (P = 0.51) Cytomegalovirus viremia occurred before transplantation in 1 desensitized patient, but it spontaneously resolved without causing cytomegalovirus disease (Table 5). No malignancy occurred in the desensitization group.
TABLE 5.
Profiles of Adverse Events
Posttransplant Outcomes of Desensitization
After DDKT, there was no graft loss in the desensitization group after a median follow-up period of 23 months (range, 1–48 months). Borderline rejection occurred in 4 (50%) patients in the desensitization group. All 4 patients were detected in the protocol biopsy; 2 patients were detected in the D10 biopsy, and the other 2 patients in the 1-year protocol biopsy. In the control group, which had a median follow-up period of 33 months (range 3.1–46 months), 2 patients experienced (50%) acute rejection. One patient experienced acute T cell-mediated rejection (grade II A). The other patient experienced both acute antibody-mediated rejection (grade II) and mixed rejection, and lost the graft. The same patient died of fibrosing cholestatic hepatitis because of a new hepatitis C virus infection (Table 6). De novo DSA was not detected in either the desensitization group or the control group.
TABLE 6.
Posttransplant Allograft Outcomes of Kidney Transplant Recipients
DISCUSSION
We demonstrated that a desensitization regimen, including bortezomib, high-dose IVIG, and rituximab significantly increased the probability of receiving DDKT in highly sensitized wait-listed patients. The desensitization decreased percent and peak MFI values of PRA class I at 2 months by 15.5% and 20.8%, respectively. The beneficial effects of desensitization on DSA, however, were observed only for patients with significant baseline DSA levels. When more liberal criteria for acceptable mismatch were applied, desensitization virtually decreased DSA levels significantly. The desensitization was well tolerated, and resulted in good graft outcomes.
To find out whether the increased transplant conversion resulted from the antibody reduction effect of desensitization, we further analyzed the change in anti-HLA antibody levels. Desensitization reduced percent and peak MFI values of class I PRA within 2 months. There, however, was no change in cPRA values. Because cPRA value calculations are based on the frequency of HLA antigen in the general population, the degree of reduction of common HLA antigens can heavily affect cPRA values. Therefore, the cPRA value seems to be a more conservative index as an assessment tool for desensitization than plain PRA percent value or peak MFI value. Interestingly, the PRA reduction was effective for only class I antibodies, not for class II antibodies, including public epitope,22 and the mechanisms need to be further analyzed. Despite an early reduction in PRA after desensitization, PRA levels rebounded within 3 to 6 months, and indeed most of DDKT occurred within 6 months of desensitization. This rebound despite bortezomib therapy, might be explained by escape mechanisms of plasma cells against bortezomib and recovery of antibody production after discontinuation of bortezomib. Plasma cells can be protected in the niche of bone marrow environment, and drug efficacy against plasma cells can be decreased during the inactive stage of antibody production.23,24 To bypass rebound problems or extend the immediate benefit for antibody reduction, it is possible to repeat the current desensitization regimen or apply desensitization to patients when allocation can be offered within a brief period (eg, patients with a long waiting period and a recent history of offer).
In regard to DSA, most transplant patients with negative FC-XM results did have a very low DSA at baseline even before desensitization, which might mask the benefits of desensitization. This phenomenon could have been because of the strict crossmatch criteria in our program. As shown above, desensitization reduced PRA values to some degree, but if the goal was set too high, it could not be achieved. To reveal the masked effects of desensitization on DSA reduction, we performed virtual exploratory analysis by changing the acceptable mismatch criteria to levels in other studies.4–6 Using the more liberal criteria, desensitization more significantly increased the transplant conversion rate compared with the control. Furthermore, desensitization reduced DSA levels more clearly. Therefore, to apply desensitization program to wait-listed patients to enhance access to transplantation, setting proper criteria for acceptable mismatch is critical. On the contrary, the increased transplant conversion for the desensitization group under the strict crossmatch criteria could have been caused not by DSA reduction, but by “study bias” in this open trial.9 That is, desensitized patients could be more compliant to a doctor's advice, more easily communicate with transplant coordinators, and have a more active attitude toward an allocation offer, although the allocation priority of KONOS was not affected by this study.
Patients’ compliance to the current protocol was generally good. Desensitized patients, however, tended to have more viremia, although this difference did not reach statistical significance. Application of multiple cycles of desensitization might increase the risk of viral infection. Active viral replication in desensitized patients can be explained by a few mechanisms. B-cell depletion can weaken the role of B cells as antigen-presenting cells, and bortezomib can suppress T-cell machinery directly.25,26 Therefore, careful monitoring of viral infection is important.
Acute rejection rates were 37% to 50% at follow-up of 1 to 2 years in DDKT patients after desensitization with high-dose IVIG.4–6 When patients with a positive CDC-XM response received living donor kidney transplantation after desensitization using plasmapheresis, acute antibody-mediated rejection occurred in 61.5% of patients at a 5-year follow-up assessment.27 Overt acute rejection, however, did not occur in DDKT patients after desensitization at a median follow-up period of 23 months in the current study. Consistent with our results, a recent desensitization study that used bortezomib, rituximab, and plasmapheresis showed a low acute rejection rate of 18.8% at a 6-month follow-up assessment.15 The good outcomes in the current study might be attributed to the more powerful desensitizing regimen, including bortezomib, or to the strict acceptance criteria. Long-term follow-up of the transplanted patients is warranted.
Because the current study was a small-sized, open-labeled, nonrandomized trial, further multicenter, large-scale, randomized trials are needed to confirm our results. Nevertheless, the current study is the first to demonstrate the impact of desensitization using bortezomib with high-dose IVIG/rituximab on DDKT conversion for highly sensitized, wait-listed patients. Outcomes of desensitization in regions with longer waiting times for DDKT patients (ie, nations outside North America or Europe) are also informative.
In conclusion, a desensitization protocol using bortezomib, high-dose IVIG, and rituximab, increased the opportunity for DDKT and decreased waiting time for highly sensitized, wait-listed patients. Anti-HLA antibody was reduced to 3 months. The desensitization regimen was generally well tolerated, and posttransplant outcomes were acceptable.
Acknowledgments
The authors would like to thank Han Ro for his advice in clinical trial conductance and article review.
Footnotes
Abbreviations: AHG = antihuman globulin-enhanced, CDC-XM = complement-dependent cytotoxicity crossmatch, CMV = cytomegalovirus, cPRA = calculated panel reactive antibody, CTCAE = Common Terminology Criteria for Adverse Events, DDKT = deceased donor kidney transplantation, DSA = donor-specific antibody, FC-XM = flow cytometry crossmatch, HS = highly sensitised, IVIG = intravenous immunoglobulin, KONOS = Korean network for organ sharing, MFI = mean fluorescence intensity.
Both JCJ and EJ equally contributed to this work as coprimary authors.
Clinical Trial Notation: Registration number: NCT01502267 at Clinicaltrials.gov.
Our work was supported by a grant from the National Strategic Coordinating Center for Clinical Research (A102065).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Korean Network for Organ Sharing. Annual report. http://www.konos.go.kr Accessed 20 May 2015. [Google Scholar]
- 2.Kim MG, Ro H, Kim YJ, et al. Management of patients on the waiting list for deceased donor kidney transplantation. Transplant Proc 2012; 44:66–71. [DOI] [PubMed] [Google Scholar]
- 3.Shin YJHJ, Kim SJ, Park MH. Analysis of panel reactive antibody in patients awaiting renal transplantation. J Korean Soc Transplant 2009; 2009:28. [Google Scholar]
- 4.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 2008; 359:242–251. [DOI] [PubMed] [Google Scholar]
- 5.Vo AA, Choi J, Cisneros K, et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation 2014; 98:312–319. [DOI] [PubMed] [Google Scholar]
- 6.Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation 2010; 89:1095–1102. [DOI] [PubMed] [Google Scholar]
- 7.Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation 2013; 95:852–858. [DOI] [PubMed] [Google Scholar]
- 8.Marfo K, Ling M, Bao Y, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation 2012; 94:345–351. [DOI] [PubMed] [Google Scholar]
- 9.Alachkar N, Lonze BE, Zachary AA, et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation 2012; 94:165–171. [DOI] [PubMed] [Google Scholar]
- 10.Lobashevsky AL, Higgins NG, Rosner KM, et al. Analysis of anti-HLA antibodies in sensitized kidney transplant candidates subjected to desensitization with intravenous immunoglobulin and rituximab. Transplantation 2013; 96:182–190. [DOI] [PubMed] [Google Scholar]
- 11.Ramos EJ, Pollinger HS, Stegall MD, et al. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant 2007; 7:402–407. [DOI] [PubMed] [Google Scholar]
- 12.Perry DK, Pollinger HS, Burns JM, et al. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant 2008; 8:133–143. [DOI] [PubMed] [Google Scholar]
- 13.Jackson AM, Kraus ES, Orandi BJ, et al. A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int 2015; 87:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant 2009; 9:201–209. [DOI] [PubMed] [Google Scholar]
- 15.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant 2015; 15:101–118. [DOI] [PubMed] [Google Scholar]
- 16.Trotti A, Colevas A, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13:176–181. [DOI] [PubMed] [Google Scholar]
- 17.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8:753–760. [DOI] [PubMed] [Google Scholar]
- 18.Organ Procurement and Transplantation Network. cPRA calculator. http://optn.transplant.hrsa.gov/converge/resources/allocationcalculators.asp?index=78 Accessed 20 March 2015. [Google Scholar]
- 19.Ro H, Hong J, Kim BS, et al. Roles of toll-like receptors in allogeneic islet transplantation. Transplantation 2012; 94:1005–1012. [DOI] [PubMed] [Google Scholar]
- 20.Dekker FW, de Mutsert R, van Dijk PC, et al. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int 2008; 74:994–997. [DOI] [PubMed] [Google Scholar]
- 21.Leonard GR, Shike H, Uemura T, et al. Liver transplantation with a strongly positive crossmatch: case study and literature review. Liver Transpl 2013; 19:1001–1010. [DOI] [PubMed] [Google Scholar]
- 22.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol 2005; 66:364–370. [DOI] [PubMed] [Google Scholar]
- 23.Redfield RR, Lou Y, Rodriguez E, et al. Sustained reduction of alloantibody secreting plasma cells and donor specific antibody with proteasome inhibition in mice. Transpl Immunol 2013; 29:11–16. [DOI] [PubMed] [Google Scholar]
- 24.Guthoff M, Schmid-Horch B, Weisel KC, et al. Proteasome inhibition by bortezomib: effect on HLA-antibody levels and specificity in sensitized patients awaiting renal allograft transplantation. Transpl Immunol 2012; 26:171–175. [DOI] [PubMed] [Google Scholar]
- 25.Sharif A, Racusen L, Montgomery R, et al. Risk for BK viremia and nephropathy after desensitization. Transplantation 2014; 98:e7–8. [DOI] [PubMed] [Google Scholar]
- 26.Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol 2012; 7:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riella LV, Safa K, Yagan J, et al. Long-term outcomes of kidney transplantation across a positive complement-dependent cytotoxicity crossmatch. Transplantation 2014; 97:1247–1252. [DOI] [PubMed] [Google Scholar]


