Supplemental Digital Content is available in the text
Abstract
The overexpression of phosphorylated signal transducer and activator of transcription 3 (p-stat3) was detected in a variety of human tumors. The published studies on p-stat3 expression among gastric carcinoma patients remain controversial.
In order to clarify the prognosis value of p-stat3 with overall survival and its association with clinicopathological characteristics in gastric carcinoma, we performed a systematic review and meta-analysis.
Eligible studies were retrieved by searching PubMed, Embase, Cochrane library, and Chinese biomedical literature service system databases.
Studies described the association between p-stat3 expression and clinicopathological characteristics and overall survival in gastric carcinoma patients; p-stat3 expression was detected by immunohistochemistry (IHC).
Odds ratio (OR) and hazard ratio (HR) were considered as a measure of evaluating the association in meta-analysis; I2 was used to assess the heterogeneity across studies; publication bias was assessed with funnel plot, Egger test, and Begg test.
Twenty-three studies including 2872 patients which evaluated the p-stat3 expression by IHC in gastric carcinoma were included. The pooled HR (HR = 2.02, 95% CI: 1.49–2.73, P < 0.00001) indicated that the increased p-stat3 expression was significantly associated with poor overall survival. In addition, when we investigated the association between p-stat3 overexpression and clinicopathological characteristics of gastric carcinoma, we found that the increased p-stat3 expression was significantly associated with tumor differentiation (poorly vs well-moderately: OR = 3.70, 95% CI: 1.98–6.93, P < 0.0001) and lymph node metastasis (present vs absent: OR = 2.40, 95% CI: 1.28–4.50, P = 0.007).
The different type of primary antibody was used; the assessment methods of p-stat3 positive expression were defined differently; the locations of p-stat3 expression were different; the method of extrapolating HR from Kaplan–Meier survival curves did seem to be less reliable than when HR was extracted directly from literatures; sample sizes, the age of patients, and the follow-up durations are different.
In conclusion, our meta-analysis indicates that the increased p-stat3 expression may be not only predict poor prognosis, but also be associated with worse tumor differentiation and lymph node metastasis in patients with gastric carcinoma.
INTRODUCTION
Gastric cancer is now the fifth most common cancer and the third most common cause of cancer death in the world. It was estimated from GLOBOCAN 2012 showed that 951,594 new gastric cancer cases and 723,027 deaths occurred globally in 2012.1 Almost 3 quarters of the new cases occurred in Asia, and more than two-fifths occurred in China. The 5-year relative survival rates of gastric cancer only changed from 25.2% (1993–2003) to 29% (2004–2010), despite the developments in diagnosed and therapeutic techniques.2 Gastric cancer is the result of accumulated genomic damage that involves activation of oncogenes and inactivation of tumor suppressor genes. Some oncogenes are preferentially altered in gastric cancer, such as human epidermal growth factor receptor (HER2), epidermal growth factor receptor (EGFR), COX-2, and K-ras. Overexpression of HER2, EGFR, COX-2, and K-ras may be one of the molecular abnormalities linked to the development of gastric cancer, with a negative impact on prognosis.3–5 As such, molecular biological factors may serve as suitable predictors of clinical outcome and reveal novel therapeutic targets in gastric carcinoma patients.
Signal transducer and activator of transcription (STAT) proteins were originally discovered in 1993 by Darnell6 which are latent transcription factors. Of the STAT family members, STAT3, controls numerous physiological processes including proliferation, differentiation, survival, development, inflammation7 and is abnormally expressed in pathological conditions such as a wide variety of human cancers.8 Tyrosine-705 phosphorylation is the major mechanism of STAT3 activation. Phosphorylated STAT3 (p-stat3) monomers combine to form dimers and translocate into the nucleus followed by its binding to the specific DNA elements for initiation of transcription. Then stat3 proteins are inactivated by tyrosine dephosphorylation and return to the cytoplasm.9 P-stat3 overexpression were detected in a wide variety of human cancer cell lines and primary tumors including prostate,10 renal,11 breast,12 head and neck,13 ovary,14 lung,15 cervical,16 colorectal,17 and gastric18 cancers.
A recent study suggested that high p-stat3 expression might serve as a strong predictor of poor prognosis among patients with nonsmall cell lung cancer. However the prognosis value of p-stat3 with overall survival and its association with clinicopathological characteristics remains controversial in gastric carcinoma. To address this question, we performed this systematic review of the published with meta-analysis and clarified the role of p-stat3 as prognostic factor and clinicopathological characteristics in gastric carcinoma.
METHODS
Search Strategy
We searched PubMed, Embase, Cochrane library, and Chinese biomedical literature service system (SinoMed) databases for studies describing the expression of p-stat3 in gastric carcinoma. The following search terms were used19: (stomach neoplasms OR gastric cancer OR gastric neoplasms OR gastric carcinoma OR gastric tumor OR stomach cancer OR stomach carcinoma OR stomach tumor) AND (STAT3 transcription factor OR STAT3 OR signal transducer and activator of transcription 3) AND phosphorylated. The references of eligible studies were manually searched for additional studies. The search was updated to October 18, 2015. Since all analyses were based on previous published studies, ethics approval was not required for this systematic review.
Inclusion Criteria
The studies describing the association between the p-stat3 expression and clinicopathological characteristics as well as overall survival (OS) in gastric carcinoma were included in this systematic review.
To be eligible studies for inclusion need to meet the following criteria:
Patients were diagnosed with gastric carcinoma by pathologist;
P-stat3 expression was detected by immunohistochemistry (IHC) in gastric carcinoma specimen;
Study provided the expression of p-stat3 status on clinicopathological characteristics; clinicopathological characteristics included tumor differentiation, tumor node metastasis (TNM) stage, lymph node metastasis, histological type according to the Lauren classification.
Study gave us enough data to extract hazard ratio (HR) and 95% confidence interval (CI) for overall survival according to p-stat3 expression status;
P-stat3 expression status should be classified into positive/negative or high/low;
Full text studies were published by English or Chinese;
If the same patient population by the same author or group were published more than once, only the most complete or the recently published one was included.
Conference reports, animal studies, cell studies, and reviews were excluded. Studies were also excluded if only the stat3 expression status was reported. Two authors (KJ and MZ) screened all studies and determined the eligible study independently. Disagreements were resolved by discussion with a third author (QC) if consensus was not achieved by 2 authors.
Data Extraction
All data were extracted independently by 2 authors (KJ and XL) with using a pre-designed form. Data extraction included first author's name, country, publication year, journal, language of publication, the source of the patients, number of patients, age, gender, detection method, source of the antibody and concentration, location of p-stat3 expression, cut-off value, the percent of p-stat3 positive/negative or high/low expression in gastric carcinoma tissues, clinicopathological characteristics (including tumor differentiation, TNM stage, lymph node metastasis, histological type according to the Lauren classification), follow-up period and survival data. Disagreements were resolved by discussion with a third author (LZ) if consensus was not achieved by 2 authors.
Quality Assessment
Two authors (KJ and WW) read each study and performed the quality assessment independently according to the quality scale for biological prognostic factors designed by the European Lung Cancer Working Party (ELCWP).20 This scale was grouped into 4 main categories: scientific design; laboratory methodology; generalizability; results analysis. Each category had a maximum score was 10 points, so the total maximum score was 40 points. The scores were compared and a consensus value for each category was reached during a meeting. The final scores were expressed as percentages, ranging from 0% to 100%, higher values meant a better methodological quality.
Statistical Analysis
The odds ratio (OR) with 95% CI was calculated to evaluate the association between the p-stat3 overexpression and clinicopathological characteristics in gastric carcinoma patients. Clinicopathological characteristics included tumor differentiation, TNM stage, lymph node metastasis, histological type according to the Lauren classification, and gender. In some analyses, data were combined, such as TNM stage III and IV versus I and II, poorly differentiated versus well and moderately differentiated. The HR with 95% CI was pooled to estimate the impact of p-stat3 expression on overall survival. If the HR and 95% CI had been reported in the studies, we will extract the data directly. If the HR and 95% CI was not reported directly, we will calculate from the available numerical data according to the methods reported by Parmar.21 Otherwise, if the Kaplan–Meier survival curves were given, we will read the data using the software Engauge Digitizer (version: 4.1, http://sourceforge.net/projects/digitizer/), and calculate the HR and 95% CI using the program files supplied by Jayne F Tierney22 (http://www.biomedcentral.com/content/supplementary/1745-6215-8-16-S1.xls). Heterogeneity among studies was assessed using I2 statistics, If I2 > 50%, it represented obvious heterogeneity between studies, we will use a random effects model, otherwise, a fixed effects model will be used.23 We conducted subgroup analyses to explore the potential heterogeneity among studies and the difference between subgroups was detected by meta-regression analysis. Sensitivity analysis was performed to investigate the sources of heterogeneity and stability of results. When there are at least 10 eligible studies included in the meta-analysis, we will examine the potential publication bias with funnel plot and assessed the funnel plot asymmetry by using Egger test and Begg test. If the publication bias was detected, the trim and fill method was used to test and adjust for potential publication bias. The meta-analysis was performed with Review Manager 5 (version: 5.2, Cochrane Informatics and Knowledge Management Department, http://tech.cochrane.org/revman/download). STATA (version 12.0, StataCorp, College Station, TX) was used to assess the funnel plot asymmetry, trim and fill method, and meta-regression analysis. Mann–Whitney U test was applied to compare the quality scores difference between subgroups by using the software of SPSS version 14.0 (SPSS, Inc., Chicago, IL). All tests were 2 sided with a significance level of 0.05.
RESULTS
Study Selection
The electronic search strategy identified 780 potentially relevant articles by using our defined criteria. After removing duplications, 643 unique articles remained. By carefully reading the titles and abstracts, 602 studies were excluded, because they do not meet the inclusion criteria. Reviewing the full text of remaining 41 studies, 18 studies were excluded for the following reasons: the same study had been reported in 2 studies (n = 1), the expression of p-STAT3 was detected by mRNA (n = 1), data were not provided about overall survival or clinicopathological characteristics (n = 16). All included studies investigated the association between p-stat3 expression and clinicopathological parameters of gastric carcinoma patients, but 11 studies investigated the association between p-stat3 expression and overall survival. Finally 23 studies18,24–45 were included in the systematic review. Flow diagram showing the selection of studies is present in Figure 1.
FIGURE 1.
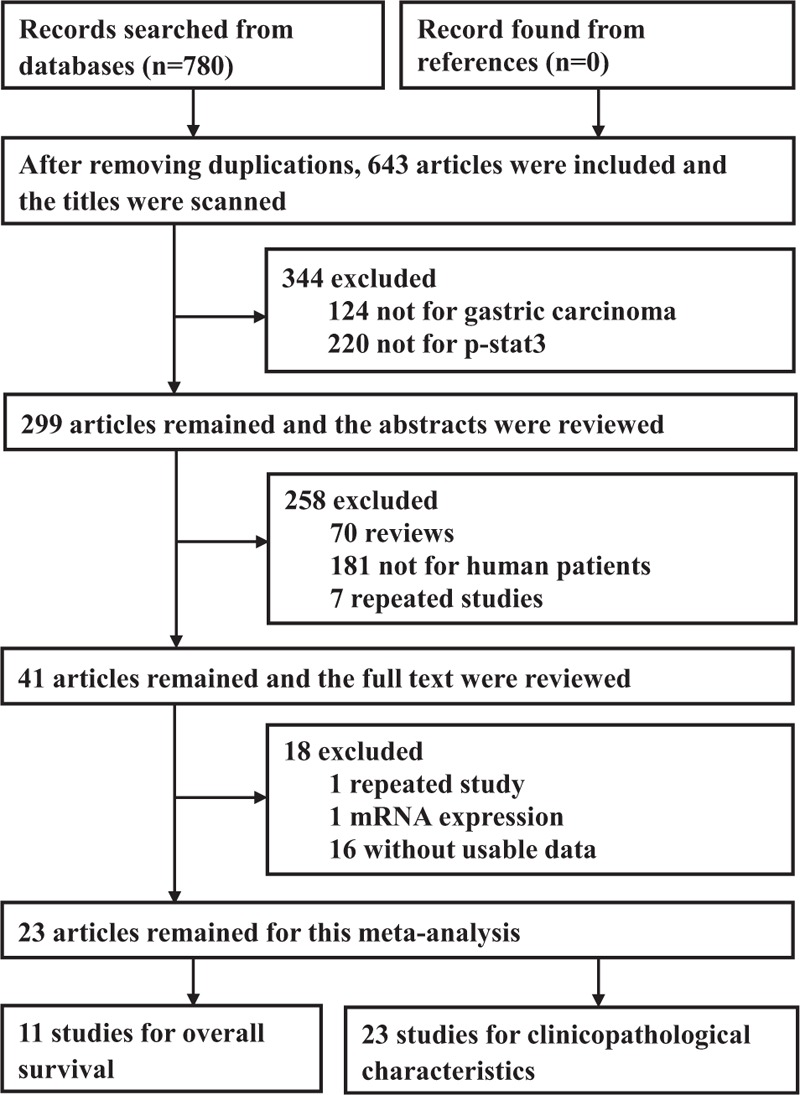
Flow diagram of included studies.
Study Characteristics
The basic characteristics of the 23 studies are summarized in Table 1. These studies were published between 2004 and 2015. In all included studies, 1 study27 was conducted in America populations, and others were conducted in Asian populations, including 3 studies from Japan,24,26,38 4 from Korean,28–29,35,41 and 15 from China.18,25,30–34,36–37,39–40,42–45 The total number of patients was 2872, with a median number of 98 patients each study (range: 50–343). P-stat3 expression was detected by IHC in gastric carcinoma tissues. In 11 included studies,25,27,33,36–37,39–40,42–45 p-stat3 expression was quantified according to the percentage of positive cells and staining intensity. In other 12 included studies,18,24,26,28–32,34–35,38,41 p-stat3 expression was quantified according to the percentage of positive cells alone. In most studies, the positive staining of p-stat3 was located in nucleus, others located in nucleus and cytoplasm26,36,40,30,33,44 All included studies reported the association between p-stat3 expression and clinicopathological characteristics, and 11 studies reported data on the effect of p-stat3 expression on overall survival. HRs and 95% CI were obtained directly from 8 studies,18,24,27–28,30–33 in other 3 studies25,26,29 HR were estimated from Kaplan–Meier curves.
TABLE 1.
Main Characteristics and Results of Included Studies

Quality Assessment
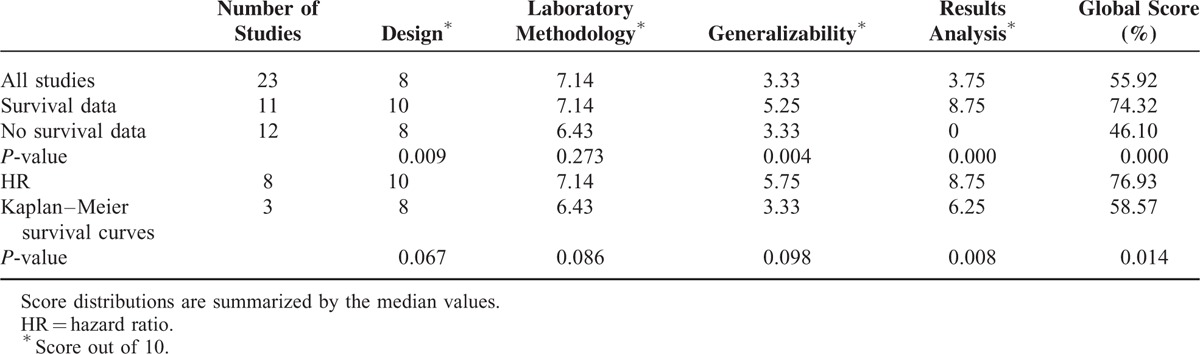
To evaluate the quality of included studies in the meta-analysis, we performed a qualitative assessment for each study according to the ELCWP quality scale. In all studies, 9 studies36–41,43–45 could not be scored in the category of “results analysis,” because they did not provide survival data. The global quality score ranged 34.82% to 84.44%, with a median of 55.92% (Table 2). When we compared global scores of studies that provided extractable survival data (n = 11) with those not provided survival data (n = 12), a significant difference was found between 2 groups (median of 74.32% vs 46.10%, P < 0.001 by the Mann–Whitney U test). And when we compared the 4 main categories of 2 groups, the scientific design and generalizability in extractable survival data group had significant higher scores (P values were 0.009 and 0.004, respectively). When we compared the 2 groups that extracting HR by directly or by Kaplan–Meier survival curves indirectly, significant difference was found in the results analysis (P = 0.008) and global quality scores (P = 0.014).
TABLE 2.
Quality Assessment According to the ELCWP Scale
Meta-Analysis Results
Association Between p-stat3 Expression and Overall Survival in Gastric Carcinoma Patients
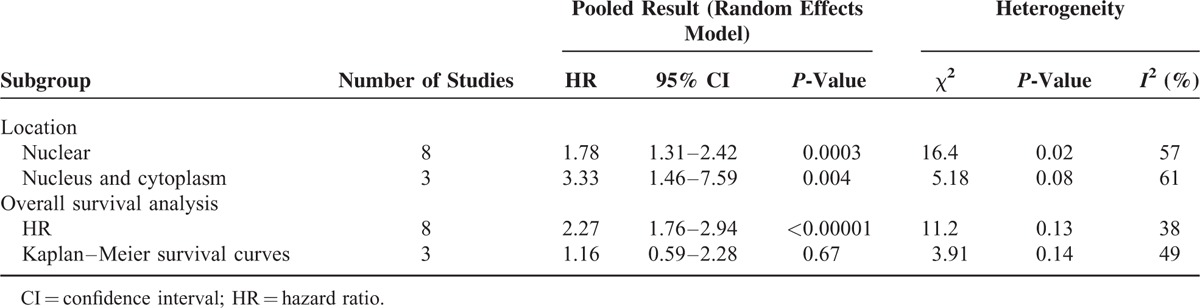
We investigated the association of p-stat3 expression with the overall survival in gastric carcinoma patients. For this purpose, 11 studies with a total of 1579 patients were included in the final analysis. The pooled HR of overall survival was 2.02 (95% CI: 1.49–2.73, z = 4.13, P < 0.00001) by a random effects model demonstrated that increased p-stat3 expression showed statistically significant association with poor overall survival in gastric carcinoma patients, and a significant heterogeneity was observed (I2 = 61%, P = 0.004) (Figure 2). To explore the sources of potential heterogeneity, we performed subgroup analyses according to the locations of p-stat3 and HR estimation (Table 3). The pooled HRs of overall survival were 1.78 (1.31–2.42) in nucleus p-stat3 expression group and 3.33 (1.46–7.59) in nucleus and cytoplasm p-stat3 expression group, meta-regression analysis showed that no statistical significance difference was found between subgroups (P = 0.16). When the HRs directly extracted from studies were pooled, combined HR was 2.27 (1.76–2.94), which demonstrated that increased p-stat3 expression was significant associated with poor overall survival in gastric carcinoma patients (P < 0.00001); when the HRs obtained from the Kaplan–Meier curves were pooled, combined HR was 1.16 (0.59–2.28), which was not significantly associated with poor overall survival (P = 0.67); meta-regression analysis suggested that the difference in results between the 2 subgroups was statistically significant (P = 0.04). These subgroups analysis results indicated that HR estimation might contribute to the heterogeneity.
FIGURE 2.
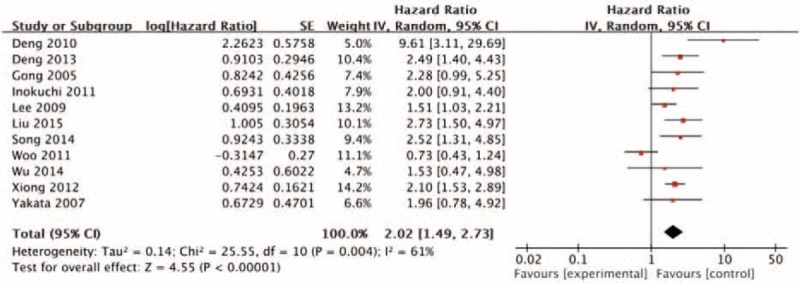
Forest plot for the association of p-stat3 expression with overall survival in gastric carcinoma patients.
TABLE 3.
Subgroup Analysis of p-stat3 Expression With Overall Survival in Gastric Carcinoma Patients
In the sensitivity analysis, we compared the fixed effects model and random effects model, but no significant difference was found in the pooled HR between 2 models (fixed effects model HR = 1.83, 95% CI: 1.54–2.16). In addition, when we excluded a single study without adjusted any variables and yielded a pooled HR for the remaining studies (Table S1). The results indicated that the stability of our results supporting the hypothesis that p-stat3 as a prognostic factor in gastric carcinoma patients were not influenced by any single study. But when we excluded the study by Woo et al29 and yielded a pooled HR of 2.19 (95% CI: 1.75–2.73), no heterogeneity was detected with I2 = 22%.
Association Between p-stat3 Expression and Clinicopathological Characteristics in Gastric Carcinoma Patients
To further understand the role of p-stat3 as biological marker, we investigated the association between p-stat3 overexpression and clinicopathological characteristics of gastric carcinoma by using a random effects model. As shown in Figure 3, increased p-stat3 expression was significantly associated with tumor differentiation (poorly vs well-moderately: OR = 3.70, 95% CI: 1.98–6.93, P < 0.0001), and lymph node metastasis (present vs absent: OR = 2.40, 95% CI: 1.28–4.50, P = 0.007), but not significantly associated with gender (female vs male: OR = 1.10, 95% CI: 0.92–1.33, P = 0.29), TNM stage (III–IV vs I–II: OR = 1.63, 95% CI: 0.96–2.76, P = 0.07) and the type of Lauren (diffuse vs intestinal: OR = 0.86, 95% CI: 0.68–1.11, P = 0.24) (Figure S1).
FIGURE 3.
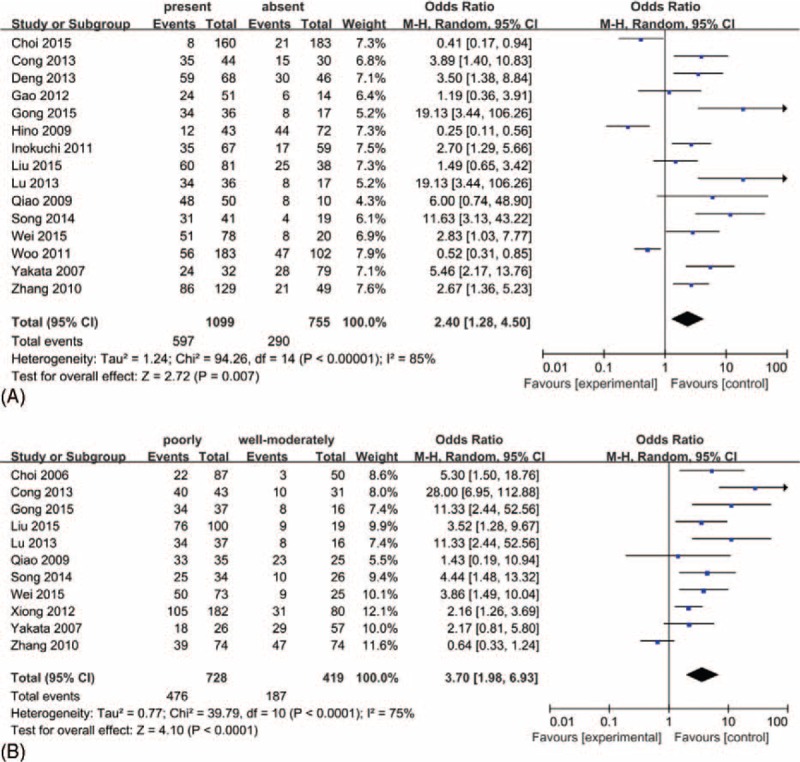
Forest plots of odds ratios (OR). (A) OR for the association of p-stat3 expression and lymph node metastasis status in gastric carcinoma patients; (B) OR for the association of p-stat3 expression and tumor differentiation in gastric carcinoma patients.
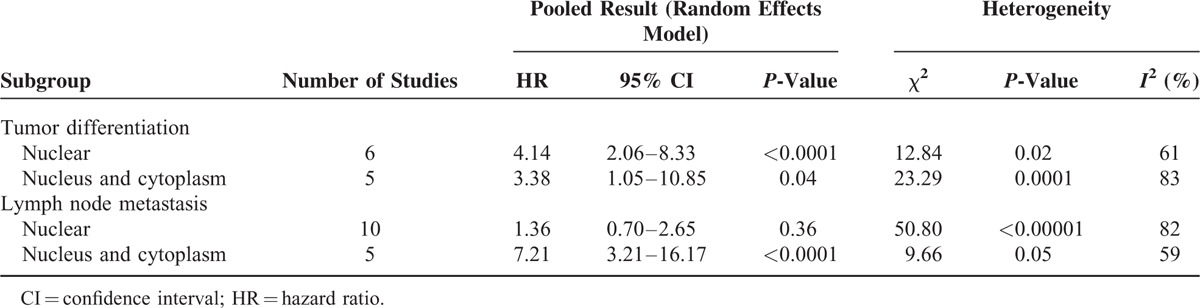
However significant heterogeneity was observed in the meta-analysis of the correlation between p-stat3 expression and tumor differentiation (I2 = 75%), lymph node metastasis (I2 = 85%), TNM stage (I2 = 81%). And no significant heterogeneity was observed in gender (I2 = 0%) and in Lauren classification (I2 = 0%). To explore the sources of potential heterogeneity, we performed the subgroup analyses according to the location of p-stat3 (nucleus or nucleus and cytoplasm). In subgroup analysis of tumor differentiation, the pooled ORs of the increased p-stat3 expression in nucleus was 4.14 (95% CI: 2.06–8.33) and was 3.38 (95% CI: 1.05–10.85) in nucleus and cytoplasm subgroup, and meta-regression analysis showed that the difference in results between the 2 subgroups was no statistical significant (P = 0.67). In subgroup analysis of lymph node metastasis, the pooled ORs of the increased p-stat3 expression in nucleus was 1.36 (95% CI: 0.70–2.65) and was 7.21 (95% CI: 3.21–16.17) in nucleus and cytoplasm subgroup, and meta-regression analysis suggested that obvious statistical difference was found between subgroups (P = 0.01) (Table 4). Thus, the different locations of the p-stat3 expression may be one of the sources of heterogeneity when we evaluated the relationship between the p-stat3 expression and lymph node metastasis. In addition, we performed the sensitivity analysis by excluding 1 study in turn, none of the individual study significantly influenced the pooled ORs (Table S2–S3).
TABLE 4.
Subgroup Analysis of p-stat3 Expression With Lymph Node Metastasis or Tumor Differentiation According to the Location of p-stat3 in Gastric Carcinoma Patients
Publication Bias
Funnel plot, Egger test, and Begg test were used to assess the publication bias. The funnel plot did not show obvious asymmetry among the studies investigating p-stat3 expression on overall survival (Figure 4), Egger test and Begg test also indicated that there was no evidence of publication bias (P = 0.375 and 1.000, respectively). In the analysis of evaluating the association between p-stat3 expression and lymph node metastasis or tumor differentiation, visual inspection of the funnel plot, Egger test, and Begg test suggested the probable evidence of publication bias (lymph node metastasis, P = 0.012 and 0.042 respectively; tumor differentiation, P = 0.032 and 0.036, respectively) (Figure 5).
FIGURE 4.

Funnel plot for the p-stat3 expression with overall survival in gastric carcinoma patients.
FIGURE 5.

Funnel plot for the p-stat3 expression with lymph node metastasis or tumor differentiation in gastric carcinoma patients.
In order to assess the impact of potential publication bias, trim, and fill analysis46 was performed with the random effects model. The corrected OR regarding the association between p-stat3 expression and tumor differentiation was 2.410 (95% CI: 1.28–4.53, P = 0.006), which showed a significantly positive association between p-stat3 expression and tumor differentiation (Figure S2 A). The corrected OR regarding the association between p-stat3 expression and lymph node metastasis was 1.545 (95% CI: 0.84–2.83, P = 0.160), which showed that the correction for potential publication bias regarding the association between p-stat3 expression and lymph node metastasis had an influence on the stability of the result (Figure S2 B). Omitting studies one by one was used to further explore the source of potential publication bias among studies investigating p-stat3 expression and lymph node metastasis. When the study by Woo et al29 was excluded, the funnel plot did not show obvious asymmetry, Egger test and Begg test indicated that there was no obvious evidence of publication bias (P = 0.083 and 0.090, respectively). But when we excluded any other study, the publication bias was obvious (Table S4).
DISCUSSION
Stat3 as an oncogene, played an essential role in the progression of a wide variety of cancers. In many human cancers, stat3 is persistently activated. Phosphorylation of specific tyrosine residue is an essential step for stat activation. Once activated, p-stat3 can induce expression of a variety of genes involved in cell survival and proliferation. This brought out numerous studies which investigated the expression of p-stat3 in malignant tumors. However, some of published papers present conflicting data especially with respect to the prognosis, even if they were performed in the same tumor entity. As a result, it is important to combine these data by meta-analysis technology and evaluate the association between p-stat3 and clinicopathological characteristics as well as prognosis in cancer patients. Previous studies have demonstrated that high p-stat3 expression is a strong predictor of poor prognosis among patients with nonsmall-cell lung cancer47; however, no data have been reported in gastric carcinoma patients.
In order to clarify the association between p-stat3 expression and prognosis as well as clinicopathological characteristics in gastric carcinoma patients, we conducted a meta-analysis. In this meta-analysis, we summarized 23 eligible studies including 2872 patients to evaluate the association between p-stat3 expression and overall survival or clinicopathological characteristics in gastric carcinoma patients. GLOBOCAN 20121 shows that new cases of gastric carcinoma mainly occurred in Asia, by coincidence, all included studies except one come from Asia in our meta-analysis. In all studies, p-stat3 expression was detected by IHC in gastric carcinoma tissues.
We assessed the prognostic significance of p-stat3 expression in gastric carcinoma patients, the pooled results suggested that increased p-stat3 expression was statistically and significantly related to poor overall survival. These findings indicated that increasing p-stat3 expression predicted a worse clinical prognosis than those with decreasing p-stat3 expression. At present, it had been reported that many gene expression was associated with poor prognosis of gastric carcinoma patients, such as p53,48 c-erbB-2,49 ERCC1,50 CD24,51 SPARC,52 MMP-7,53 CD44,54 and survivin.55
In the present study, a significant heterogeneity was observed among the studies. Therefore, we performed subgroup analyses according to location of p-stat3 and HR estimation. The results of subgroup analyses and meta-regression analyses suggested that the HR estimation may be significant variable associated with heterogeneity among studies. In order to further explore the sources of heterogeneity and the stability of our pooled results, we conducted sensitivity analysis by excluding a single study. The results showed that the stability of our results was not influenced by any single study. Moreover, we found when we excluded the study by Woo et al29 and pooled the HR, no significant heterogeneity was detected (I2 = 22%). This result indicated that the heterogeneity was also significantly influenced by excluding Woo's study. When we further analyzed the study, through the Kaplan–Meier curves, we found that patients with p-stat3 expression showed a significantly better survival rate than those without its expression. However in all the other included studies, patients without p-stat3 expression had a better survival rate than those with its expression. We speculated that this discrepancy in the study of Woo et al, might stem from the lower positive expression rates of p-stat3 (the positive expression rates of p-stat3 was 36.14%, the median positive expression rate was 49.55%), the numbers of tumor cases, the distribution of patients or the antibody used in IHC.29 To examine the publication bias among the studies investigating p-stat3 expression on overall survival, we conducted funnel plot and performed Egger test and Begg test, no obvious asymmetry and publication bias were found.
We also assessed the associations between p-stat3 expression and clinicopathological characteristics in gastric carcinoma patients by meta-analysis. We found that increased p-stat3 expression was significantly associated with tumor differentiation and lymph node metastasis. There were also some previous studies indicating that the expression of certain genes was associated with clinicopathological characteristics of gastric carcinoma patients, for instance, Bcl-2 expression was significantly associated with TNM stage, the depth of invasion, and lymph node metastasis56; MicroRNA-21 expression was associated with tumor differentiation, lymph node metastasis, and TNM stage57; HER2-expression was associated with Bormann type, Lauren classification, tumor differentiation, lymph node status, venous invasion, and lymph vascular invasion58; Survivin expression was associated with metastatic lymph node status.59
Obvious heterogeneity was detected when we compared the expression of p-STAT3 with tumor differentiation or lymph node metastasis. To explore the sources of heterogeneity, we conducted subgroup analyses according to locations of p-stat3, we found that the different locations of the p-stat3 expression may be one of the sources of heterogeneity between the p-stat3 expression and lymph node metastasis. In subgroup analysis of tumor differentiation according to locations of p-stat3, meta-regression analysis suggested that no statistical significance was detected between subgroups. Furthermore, we analyzed that possible sources of heterogeneity between the p-stat3 expression and tumor differentiation may be derived from the differences in the cut-off of positive/high p-stat3 expression, the antibody, the scoring method of p-stat3 positive expression, and sample size. Because of limited information provided in included studies, we did not have more detail to further explore the sources of heterogeneity. In the test of sensitivity analysis, the pooled ORs of tumor differentiation and lymph node metastasis for p-stat3 expression were not influenced by leave-one-out analyses. The trim and fill method was used to assess the impact of potential publication bias, and the result showed a significantly positive association between p-stat3 expression and tumor differentiation. The correction for potential publication bias concerning the association between p-stat3 expression and lymph node metastasis had an effect on the stability of the result. To explore the source of publication bias we performed the analysis by omitting studies one by one, and we found the study by Woo et al which might contribute to the publication bias.
Some limitations should be pointed out. Firstly, the expression of p-stat3 in all included studies was detected by IHC. The results of IHC depended on types of primary antibody. However, it was impossible to conduct subgroup analyses by different types of antibodies to explore the potential influence on our pooled results. Secondly, the assessment methods of p-stat3 positive expression were defined differently. Some studies were according to the percentage of positive/high cells and staining intensity, others were according to the percentage of positive cells alone. Even using the same kind of assessment method, the cut-off value might be different. To date, there is no uniform standard to define the assessment methods of p-stat3 positive/high expression worldwide. Thirdly, the locations of p-stat3 expression were different in all included studies, of which some studies were defined in the nucleus; other studies were defined in the nucleus and cytoplasm. This could induce the difference of p-stat3 positive/high expression level across studies. Fourthly, in overall survival analysis, if the HR was not reported, it would be calculated from the data included in the study or extrapolated from the Kaplan–Meier survival curves. In fact, the method of extrapolating HR from Kaplan–Meier survival curves did seem to be less reliable than when HR was extracted directly from literatures because this strategy did not completely eliminate inaccuracy in the extracted survival rates. Furthermore, when we conducted the quality assessment, the scores of extracting HR directly were significantly higher than extracting HR by Kaplan–Meier survival curves indirectly. This indicated that the extraction method of HR might affect our pooled results. Fifthly, in all included studies, sample sizes were different, ranged from 50 to 343; the onset ages of patients were different in the available data, ranged from 22 to 89; the follow-up durations could be extracted are also different, ranged 72 to 135 months; postoperative treatments of patients were reported in most studies; all included studies were from the Asian populations except one. Finally, publication bias was found in the tumor differentiation and lymph node metastasis groups. The bias possibly resulted from negative results which are difficult to be published in some journals. Although the publication bias exists, sensitivity analyses demonstrated the reliability of our meta-analysis.
In conclusion, this is the first meta-analysis to systematically evaluate the association between p-stat3 expression and prognosis as well as clinicopathological characteristics in gastric carcinoma patients. Our findings indicate that the increased p-stat3 expression may be not only predict poor prognosis, but also be associated with worse tumor differentiation and positive lymph node metastasis in patients with gastric carcinoma. Therefore p-stat3 probably becomes a useful biomarker to predict prognosis for gastric carcinoma patients.
Supplementary Material
Footnotes
Abbreviations: CD24 = cluster of differentiation 24, CD44 = cluster of differentiation 44, CI = confidence interval, COX2 = cyclo-oxygen-ase 2, EGFR = epidermal growth factor receptor, ERCC1 = excision repair cross-complementation group 1, HER2 = human epidermal growth factor receptor, HR = hazard ratio, IHC = immunohistochemistry, MMP-7 = matrix metalloproteinase-7, OR = odds ratio, OS = overall survival, SPARC = secreted protein acidic and rich in cysteine, Stat3 = signal transducer and activator of transcription 3, TNM = tumor node metastasis.
This paper was supported by Program for Liaoning Excellent Talents in University (LJQ2014113).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. 2013; Lyon, France: International Agency for Research on Cancer, Available at. http://globocan.iarc.fr, Accessed on 18/2/2014. [Google Scholar]
- 2.Cancer Statistics Review. 1975–2011. Available at http://seer.cancer.gov/archive/csr/1975_2011/results_merged/sect_24_stomach.pdf [Google Scholar]
- 3.Duraes C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch 2014; 464:367–378. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Yang JM, Hu TT, et al. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res 2013; 44:380–389. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Liu W, Zhu YF, et al. Correlation of COX-2 and K-ras expression to clinical outcome in gastric cancer. Acta Oncol 2006; 45:1115–1119. [DOI] [PubMed] [Google Scholar]
- 6.Shuai K, Stark GR, Kerr IM, et al. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 1993; 261:1744–1746. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000; 19:2548–2556. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell 1999; 98:295–303. [DOI] [PubMed] [Google Scholar]
- 9.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 2014; 1845:136–154. [DOI] [PubMed] [Google Scholar]
- 10.Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 2002; 62:6659–6666. [PubMed] [Google Scholar]
- 11.Guo C, Yang G, Khun K, et al. Activation of Stat3 in renal tumors. Am J Transl Res 2009; 1:283–290. [PMC free article] [PubMed] [Google Scholar]
- 12.Sheen-Chen SM, Huang CC, Tang RP, et al. Prognostic value of signal transducers and activators of transcription 3 in breast cancer. Cancer Epidemiol Biomarkers Prev 2008; 17:2286–2290. [DOI] [PubMed] [Google Scholar]
- 13.Bourguignon LY, Earle C, Wong G, et al. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012; 31:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anglesio MS, George J, Kulbe H, et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res 2011; 17:2538–2548. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrow MG, Song LJ, Altiok S, et al. Curcumin: a novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur J Cancer Prev 2012; 21:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takemoto S, Ushijima K, Kawano K, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer 2009; 101:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchiyama T, Takahashi H, Endo H, et al. Role of the long form leptin receptor and of the STAT3 signaling pathway in colorectal cancer progression. Int J Oncol 2011; 39:935–940. [DOI] [PubMed] [Google Scholar]
- 18.Xiong H, Du W, Wang JL, et al. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012; 90:1037–1046. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Liu M, Li J, et al. Impact of V-ets erythroblastosis virus E26 oncogene homolog 1 gene polymorphisms upon susceptibility to autoimmune diseases: a meta-analysis. Medicine (Baltimore) 2015; 94:e923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001; 18:705–719. [DOI] [PubMed] [Google Scholar]
- 21.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 22.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Huang S, Mo S, et al. Susceptibility of autoimmune diseases in three polymorphisms of infection-associated gene IRAK1. J Infect Dev Ctries 2015; 9:614–623. [DOI] [PubMed] [Google Scholar]
- 24.Inokuchi M, Murayama T, Hayashi M, et al. Prognostic value of co-expression of STAT3, mTOR and EGFR in gastric cancer. Exp Ther Med 2011; 2:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu LJ, Li HX, Luo XT, et al. STAT3 activation in tumor cell-free lymph nodes predicts a poor prognosis for gastric cancer. Int J Clin Exp Pathol 2014; 7:1140–1146. [PMC free article] [PubMed] [Google Scholar]
- 26.Yakata Y, Nakayama T, Yoshizaki A, et al. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol 2007; 30:437–442. [PubMed] [Google Scholar]
- 27.Gong W, Wang L, Yao JC, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res 2005; 11:1386–1393. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Kang WK, Park JO, et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS 2009; 117:598–606. [DOI] [PubMed] [Google Scholar]
- 29.Woo S, Lee BL, Yoon J, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with better prognosis, cell proliferation and hypoxia-inducible factor-1alpha in human gastric cancer. Pathobiology 2011; 78:295–301. [DOI] [PubMed] [Google Scholar]
- 30.Deng JY, Sun D, Liu XY, et al. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol 2010; 16:5380–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng J, Liang H, Zhang R, et al. STAT3 is associated with lymph node metastasis in gastric cancer. Tumour Biol 2013; 34:2791–2800. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Deng J, Luo X, et al. Overexpression of SMYD3 was associated with increased STAT3 activation in gastric cancer. Med Oncol 2015; 32:404. [DOI] [PubMed] [Google Scholar]
- 33.Song YY, Sun LD, Liu ML, et al. STAT3, p-STAT3 and HIF-1alpha are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma. Oncol Lett 2014; 8:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu LF, Zhu YB, Qiao MM, et al. Constitutive activation and clinical significance of Stat3 in human gastric cancer tissues and cell lines. Zhonghua Yi Xue Za Zhi 2004; 84:2064–2069. [PubMed] [Google Scholar]
- 35.Choi JH, Ahn MJ, Park CK, et al. Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. APMIS 2006; 114:619–625. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JG, Zhao J, Xin Y. Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol 2010; 16:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao F, Lv Y, Zhu Y, et al. Correlation of epigenetic aberrance with STAT3 signaling pathway in gastric carcinogenesis. Dig Dis Sci 2012; 57:2055–2062. [DOI] [PubMed] [Google Scholar]
- 38.Hino R, Uozaki H, Murakami N, et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res 2009; 69:2766–2774. [DOI] [PubMed] [Google Scholar]
- 39.Xiong H, Du W, Sun TT, et al. A positive feedback loop between STAT3 and cyclooxygenase-2 gene may contribute to Helicobacter pylori-associated human gastric tumorigenesis. Int J Cancer 2014; 134:2030–2040. [DOI] [PubMed] [Google Scholar]
- 40.Gong DP, Zhang ZQ, Lu SM, et al. Relationship between Helicobacter pylori infection and expressions of p-stat3, Bcl-2, Mcl-1 in gastric carcinoma. Med Philos 2015; 36:70–73. [Google Scholar]
- 41.Choi E, Byeon SJ, Kim SH, et al. Implication of leptin-signaling proteins and Epstein-Barr virus in gastric carcinomas. PLoS ONE 2015; 10:e0130839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei ZW, Xia GK, Wu Y, et al. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett 2015; 359:335–343. [DOI] [PubMed] [Google Scholar]
- 43.Qiao YY, Jia MZ, Ma XM, et al. Expression of JAK1, p-stat3 and bcl-2 in gastric cancer and their significance. Cancer Res Prevent Treat 2009; 36:657–661. [Google Scholar]
- 44.Lu SM, Chen MR, Liu LN, et al. Relationship between STAT3 activation and epithelialmesenchymal transition in gastric carcinoma. World Chin J Digestol 2013; 21:2748–2753. [Google Scholar]
- 45.Cong L, Tao L, Zhao J, et al. Active level of p-stat3 and expression of p53 and C-erb-B2 in gastric cancer. Chin J Lab Diagn 2013; 17:896–899. [Google Scholar]
- 46.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 47.Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol 2014; 40:311–317. [DOI] [PubMed] [Google Scholar]
- 48.Yildirim M, Kaya V, Demirpence O, et al. Prognostic significance of p53 in gastric cancer: a meta- analysis. Asian Pac J Cancer Prev 2015; 16:327–332. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZQ, Sun BJ. c-erbB-2 expression and prognosis of gastric cancer: a meta-analysis. Genet Mol Res 2015; 14:1782–1787. [DOI] [PubMed] [Google Scholar]
- 50.Song P, Yin Q, Lu M, et al. Prognostic value of excision repair cross-complementation group 1 expression in gastric cancer: a meta-analysis. Exp Ther Med 2015; 9:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu JX, Zhao YY, Wu X, et al. Clinicopathological and prognostic significance of CD24 overexpression in patients with gastric cancer: a meta-analysis. PLoS ONE 2014; 9:e114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Hao B, Yang Y, et al. Prognostic role of SPARC expression in gastric cancer: a meta-analysis. Arch Med Sci 2014; 10:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soleyman-Jahi S, Nedjat S, Abdirad A, et al. Prognostic significance of matrix metalloproteinase-7 in gastric cancer survival: a meta-analysis. PLoS ONE 2014; 10:e0122316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Fu Z, Xu S, et al. The prognostic value of CD44 expression in gastric cancer: a meta-analysis. Biomed Pharmacother 2014; 68:693–697. [DOI] [PubMed] [Google Scholar]
- 55.Liu JL, Gao W, Kang QM, et al. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS ONE 2013; 8:e71930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng H, Wang X, Li T, et al. Bcl-2 expression and patient survival in gastric cancer: a systematic review of the literature with meta-analysis. Med Oncol 2015; 32:389. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Cai Q, Jiang Z, et al. Prognostic role of MicroRNA-21 in gastric cancer: a meta-analysis. Med Sci Monit 2014; 20:1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang JW, Zhang JJ, Zhang T, et al. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol 2014; 35:4849–4858. [DOI] [PubMed] [Google Scholar]
- 59.Krieg A, Baseras B, Tomczak M, et al. Role of survivin as prognostic and clinicopathological marker in gastric cancer: a meta-analysis. Mol Biol Rep 2013; 40:5501–5511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


