Abstract
This study aimed to evaluate the prognostic value of plasma Epstein–Barr Virus DNA (EBV DNA) for local and regionally advanced nasopharyngeal carcinoma (NPC) patients treated with concurrent chemoradiotherapy in intensity-modulated radiotherapy (IMRT) era.
In this observational study, 404 nonmetastatic local and regionally advanced NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy were recruited. Blood samples were collected before treatment for examination of plasma EBV DNA levels. We evaluated the association of pretreatment plasma EBV DNA levels with progression-free survival rate (PFS), distant metastasis-free survival rate (DMFS), and overall survival rate (OS).
Compared to patients with an EBV DNA level <4000 copies/mL, patients with an EBV DNA ≥4000 copies/mL had a lower rate of 3-year PFS (76%, 95% CI [68–84]) versus (93%, 95% CI [90–96], P < 0.001), DMFS (83%, 95% CI [76–89]) versus (97%, 95% CI [94–99], P < 0.001), and OS (85%, 95% CI [78–92]) versus (98%, 95% CI [95–100], P < 0.001). Multivariate analysis showed that pretreatment EBV DNA levels (HR = 3.324, 95% CI, 1.80–6.138, P < 0.001) and clinical stage (HR = 1.878, 95% CI, 1.036–3.404, P = 0.038) were the only independent factor associated with PFS, pretreatment EBV DNA level was the only significant factor to predict DMFS (HR = 6.292, 95% CI, 2.647–14.956, P < 0.001), and pretreatment EBV DNA levels (HR = 3.753, 95% CI, 1.701–8.284, P < 0.001) and clinical stage (HR = 2.577, 95% CI, 1.252–5.050, P = 0.010) were significantly associated with OS. In subgroup analysis, higher plasma EBV DNA levels still predicted a worse PFS, DMFS, and OS for the patients stage III or stage IVa-b, compared with those with low EBV DNA levels.
Elevated plasma EBV DNA was still effective prognostic biomarker for local and regionally advanced NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy. Future ramdomized clinical trials are needed to further evaluate whether plasma EBV DNA levels could be applied to guide concurrent chemotherapy regimen for local and regionally advanced NPC patients.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an endemic in Southern China and Southeast Asia, with a peak incidence of 50 cases per 100,000.1 According to the data from GLOBOCAN on Cancer Research, there were >84,000 new NPC cases and 51,600 NPC-related deaths in 2012, with 80% of the cases located in Asia, especially China, and 5% in Europe and the USA.2 Radiotherapy is the primary treatment modality because of its high degree of radiosensitivity and inherent anatomic constraints, and concurrent chemoradiotherapy (CCRT) is currently considered as the standard treatment regimens of local and regionally advanced nasopharyngeal carcinoma.3–6 Recently, the quantification of pretreatment plasma Epstein–Barr virus (EBV) DNA was demonstrated a useful biomarker for the risk stratification, monitoring and prediction of the prognosis of NPC,7–11 but these findings were based on 2-dimensional radiotherapy (2D-CRT) or 3-dimensional radiotherapy (3D-CRT). Recently, compared with 2D-CRT, intensity-modulated radiotherapy (IMRT) has been demonstrated to gain superior locoregional control and improved long-term survival and was consider as the primary means of radiotherapy for NPC patients.12,13 The value of plasma EBV DNA for NPC patients treated with IMRT is still scarce. Chen14 have reported that the patients with a high pretreatment EBV DNA level had a worse disease-free survival (DFS) with short follow-up time, ∼2 years. Therefore, it is of interest to determine whether the plasma EBV DNA still has effective prognostic value, with a longer follow-up time, for local and regionally advanced patients treated with concurrent CCRT in IMRT era. Therefore, we conducted a retrospective cohort study to evaluate the prognostic role of plasma EBV DNA for local and regionally advanced NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy.
PATIENTS AND METHODS
Patients with biopsy-proven WHO type II or III and staged III–IVa-b locoregionally advanced nasopharyngeal carcinoma who were suitable for definitive chemoradiotherapy were eligible. In addition, patients were required to be >18 years old, with the value of Eastern Cooperative Oncology Group performance status (ECOG) of 0 or 1, and adequate hematologic, renal, and hepatic function. Patients were excluded if they are pregnant or lactating, or lost during the follow-up, or had a history of previous or synchronous malignant tumors, or previously received any anticancer therapy.
All patients were evaluated by complete physical examination, magnetic resonance imaging (MRI) of the head and neck, chest radiography, fiberoptic nasopharyngoscopy, abdominal sonography, and whole-body bone scan or18 FDG PET/CT. The following baseline data of sex, age, hereditary NPC, and smoking status was collected before treatment. The study was approved by Sun Yat-sen University's independent ethics committees and all patients provided written informed consent. According to the seventh American Joint Committee on Cancer (AJCC) TNM staging manual, all the patients were restaged, and finally 404 NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy were consecutively recruited from January 2008 to December 2012.
EBV DNA Measurement
As described in previous studies,11,15–20 patient plasma EBV DNA concentrations were measured by q-PCR before treatment. A cutoff level of 4000 copies/mL was chosen to define low and high EBV DNA levels because this threshold has previously been shown to be prognostic in previous NPC studies using the same measurement system.7,9,20 EBV-specific VCA/IgA antibodies and EBV-specific EA/IgA antibodies were measured using an immunoenzymic assay described previously.21 The cutoff value of VCA-IgA (≥1:80 vs <1:80) and EA-IgA (≥1:10 vs <1:10) was according to previous published literature.18,22
Chemotherapy and Radiation Therapy
All patients underwent CCRT, consisting of concurrent cisplatin (30–40 mg/m2) weekly or (80–100 mg/m2) chemotherapy on day1, 22, and 43 during radiation therapy.6,23 A total of 141 patients (34.9%) received weekly chemotherapy, and 263 patients (65.1%) received chemotherapy 2 or 3 cycles every 3 weeks. The design of IMRT plan was based on previous studies.12,24,25 All patients were treated according to the principles of treatment for NPC patients at Sun Yat-sen University Cancer Center, Guangzhou, China.
Outcome and Follow-Up
Our primary endpoint was progression-free survival (PFS), and distant metastasis-free survival (DMFS) and overall survival (OS) was included as secondary endpoints in this study. Progression-free survival was calculated from the date of initial treatment to the date of disease progression (local/regional recurrence or distant metastasis) or death from any cause or the censoring of the patient at the date of the last follow-up. DMFS was determined from the date of initial treatment to the date of distant relapse or death from any cause or patient censoring at the date of the last follow-up. OS was calculated from the date of initial treatment to the date of death from any cause or patient censoring at the date of the last follow-up. After treatment was completed, the patients were evaluated at 3-month intervals for the first 3 years and every 6 months thereafter. A complete physical examination and detailed history were performed at the time of each follow-up visits, and plasma EBV DNA, routine blood and biochemistry tests were carried out by collecting peripheral blood. MRI of the head and neck, nasopharyngescopy, chestradiography, abdominal sonography, a whole-body bone scan or PET/CT were routinely performed annually or at the time of the clinical suggestion of tumor recurrence.
Statistical Analysis
A Mann–Whitney test was used to detect differences of plasma EBV DNA for the subgroup patients with or without clinical events (progression, distant metastasis, or death). The Kaplan–Meier method was used to estimate the cumulative survival plot (<4000 or ≥4000 copies/mL) and compared using the log-rank test. Univariate analysis was performed for each of the variables, and variables with P value ≤0.05 were subjected to multivariate analysis. Multivariate analysis was performed using a Cox proportional hazards model, excluding insignificant variables by backward elimination. Factors were included in univariate analysis as follow: age (>45 years vs ≤45 years), sex (male vs female), clinical stage (IVa-b vs III), smoking status (yes vs no), family history of NPC (yes vs no), EBV DNA (≥4000 copies/mL vs <4000 copies/mL), VCA-IgA (≥1:80 vs <1:80), and EA-IgA (≥1:10 vs <1:10). All reported probability values were 2 tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient Characteristics and Distribution of Plasma EBV DNA Level in the Study Population
The pretreatment characteristics of NPC patients are listed in Table 1. The median follow-up time was 33.5 months (IQR: 24.2–51.8). Forty-eight patients emerged disease progression, 31 patients developed distant metastases (DM), and 31 patients dead at the date of the last follow-up. Patients who have disease progression had significantly higher EBV DNA levels before treatment than patients without disease progression during follow-up of this study: 7645 copies/mL (25th–75th percentile: 947–45,550 copies/mL) and 760.1 copies/mL (25th–75th percentile: 0–11,825 copies/mL), respectively (Figure 1A, P < 0.001). The median level of plasma EBV DNA was higher in patients with DM compared to patients without DM (Figure 1B, P <0.001), with median values of 9870 copies/mL (25th–75th percentile: 4360–57,800 copies/mL) and 776.9 copies/mL (25th–75th percentile: 0–12,500 copies/mL), respectively. Patients who was dead had significantly higher pretreatment EBV DNA levels than patients still alive during the follow-up time: 8840 copies/mL (25th–75th percentile: 947–45,550 copies/mL) and 856.4 copies/mL (25th–75th percentile: 0–11,825 copies/mL), respectively (Figure 1C, P <0.001).
TABLE 1.
Patient Demographics and Clinical Characteristics
FIGURE 1.
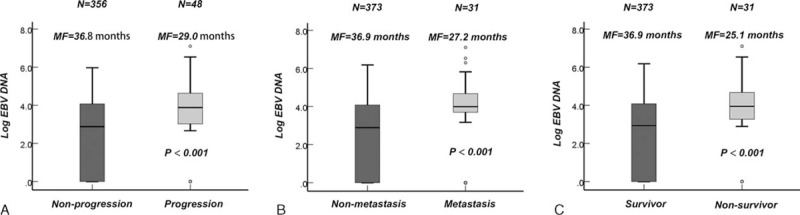
Log (EBV DNA) are expressed as the median and 5% to 95% percentile in patients (A) with/without progression, (B) with/without distant metastasis, and (C) survivor or deaths. P value was calculated by Wilcoxon rank-sum test.EBV DNA = Epstein–Barr Virus DNA, MF = median follow-up time.
The plasma EBV DNA level was higher in patients staged IVa-b than in patients with staged III (P < 0.001), with median values of 5105 copies/mL (25th–75th percentile: 0–38, 850 copies/mL) and 620.6 copies/mL (25th–75th percentile: 0–8505 copies/mL), respectively. A correlation analysis further proved that plasma EBV DNA levels correlated with ECOG performance, T stage, N stage, tumor TNM stage, and EA-IgA (Table 1).
Plasma EBV DNA Association With PFS, DMFS, and OS, and Multivariate Analyses of Pretreatment EBV DNA Levels as the Prognostic Factor
Compared to patients with a low pretreatment EBV DNA level, patients with a higher EBV DNA level had a lower rate 3-year of PFS, (76%, 95% CI [68–84]) versus (93%, 95% CI [90–96], P < 0.001, Figure 2A), DMFS (83%, 95% CI [76–89]) versus (97%, 95% CI [94–99], P < 0.001, Figure 2B), and OS (85%, 95% CI [78–92]) versus (98%, 95% CI [95–100], P < 0.001, Figure 2C). Univariate analysis indicated that age, clinical stage, and plasma EBV DNA was associated with treatment failure of NPC (Table 2).
FIGURE 2.
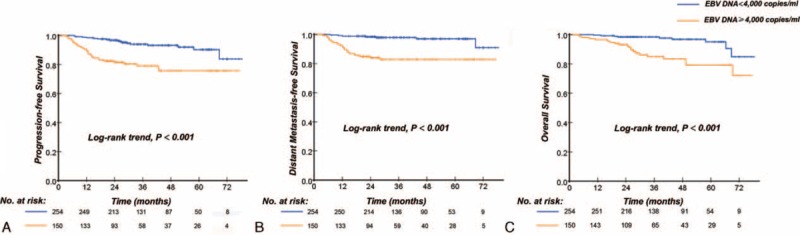
Kaplan–Meier curves of progression-free survival, distant metastasis-free survival, and overall survival according to the pretreatment EBV DNA levels (<4000 copies/mL vs ≥4000 copies/mL) for local and regionally advanced NPC patients: disease-free survival (A), distant metastasis-free survival (B), and overall survival (OS).EBV DNA = Epstein–Barr Virus DNA, NPC = nasopharyngeal carcinoma, OS = overall survival.
TABLE 2.
Univariate Cox Proportional Hazards Analysis
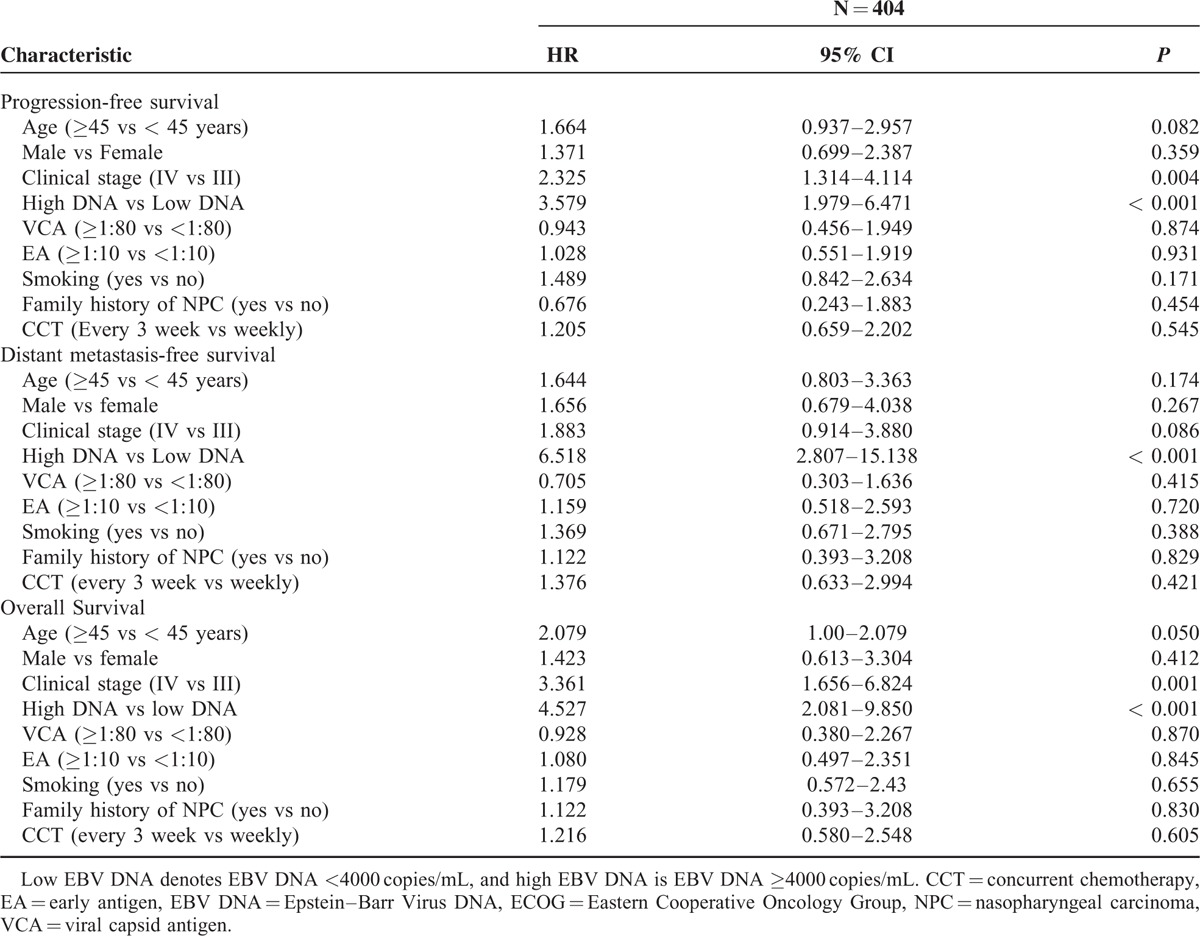
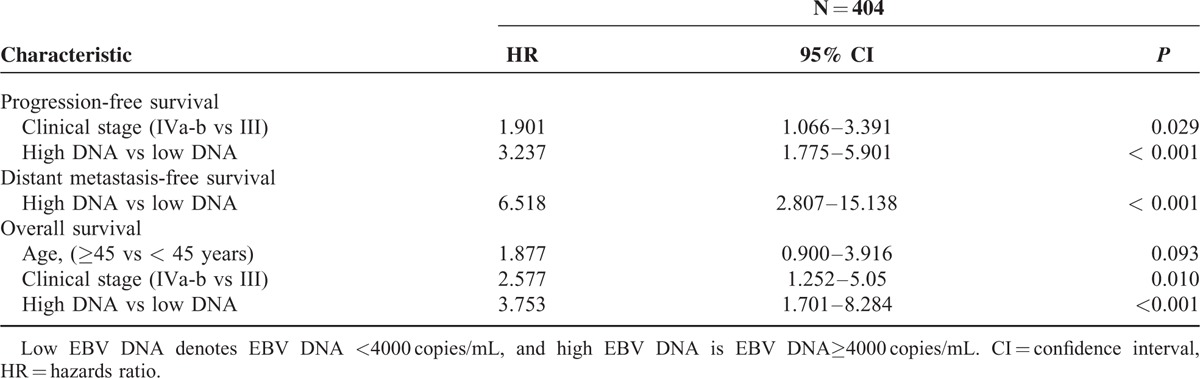
A multivariate Cox proportional-hazards model was generated that including the following variables: age, clinical stage, and plasma EBV DNA. The multivariate Cox regression analysis showed that the pretreatment EBV DNA levels (HR = 3.237, 95% CI, 1.775–5.901, P < 0.001) and clinical stage (HR = 1.901, 95% CI, 1.066–3.391, P = 0.029) were the only independent prognostic factor associated with PFS, pretreatment EBV DNA levels were the only significant factor to predict DMFS (HR = 6.518, 95% CI, 2.807 to 15.138, P < 0.001), and the pretreatment EBV DNA levels (HR = 3.753, 95% CI, 1.701–8.284, P < 0.001) and clinical stage (HR = 2.577, 95% CI, 1.252–5.050, P = 0.010) were significantly associated with OS (Table 3).
TABLE 3.
Multivariate Cox Proportional Hazards Analysis
Prognostic Significance of EBV DNA Level Within the Stage III and Stage IVa-b Classification
Given the independent prognostic significance of elevated EBV DNA levels in local and regionally advanced NPC patients, we evaluated the discrimination power of elevated EBV DNA levels in stage III and stage IVa-b patients. In subgroup analysis, for stage III patients, compared to patients with low EBV DNA levels, patients with elevated EBV DNA levels had lower rate 3 year of PFS (81%, 95% CI [72–89]) versus (95%, 95% CI [92–98], P < 0.001), DMFS (86%, 95% CI [79–93]) versus (97%, 95% CI [95–100], P < 0.001), and OS (88%, 95% CI [81–96]) versus (98%, 95% CI [97–100], P = 0.001) (Figure 3A–C). For staged IVa-b patients, the patients with high EBV DNA levels compared with those displaying low pretreatment EBV DNA levels, the 3-year PFS was 73% (95% CI, 61%–85%) and 88% (95% CI, 78%–98%), respectively (P = 0.051), the 3-year DMFS was 80% (95% CI, 69%–91%) and 100%, respectively (P = 0.014), and the 3-year OS was 79% (95% CI, 67%–92%) and 95% (95% CI, 88%–100%), respectively (P = 0.04) (Figure 3D-F).
FIGURE 3.
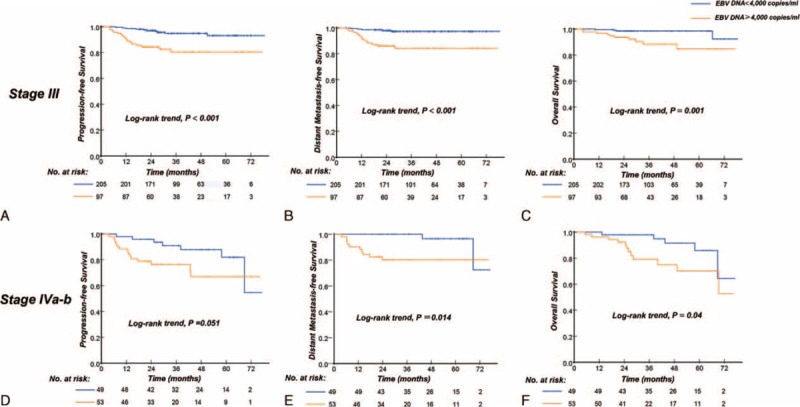
Kaplan–Meier curves of progression-free survival and distant metastasis-free survival according to the pretreatment EBV DNA levels (<4000 copies/mL vs ≥4000 copies/mL) for subgroup analysis. Progression-free survival (A), distant metastasis-free survival (B), and overall survival (C) for patients staged III; progression-free survival (D), distant metastasis-free survival (E), and overall survival (F) for patients staged IVa-b. EBV DNA = Epstein–Barr Virus DNA.
DISCUSSION
Previous studies,7,9–11,16 based on 2D-CRT or 3D-CRT, have examined the association between EBV DNA and NPC prognosis. Even in IMRT era, Chen14 also reported that the 2-year disease-free survival (DFS) rates in patients with positive and negative pretreatment plasma EBV-DNA were 94.4% and 80.8%, respectively. In this study we have confirmed that plasma EB DNA is still an effective biomarker for predicting disease progression and distant metastasis for advanced NPC patients treated with IMRT and concurrent chemotherapy with much longer follow-up time. The results of this study were similar to the report by Chen.14 In recent year, intensity-modulated radiotherapy has been proven to be effective in increasing the local control rate for NPC patients; however, distant metastasis was still the main treatment failure and become the greatest challenge for NPC patients.26 Currently, the tumor-node metastases (TNM) staging system has been widely used to predict and guide the treatment regimen of NPC patients. According to the NCCN guideline, all the local and regionally advanced NPC patients in this study were received concurrent chemoradiothrapy with similar regimen and radiation technique. However, we found that even these patients received similar treatment regimen, patients with ≥4000 copies/mL of EBV DNA display a >6-fold increased risk of distant metastasis compared with patients with <4000 copies/mL of EBV DNA. This indicated that it is not enough effective to used single drug of cisplatin for the high-risk local advance NPC patients to reduced distant metastasis, although cisplatin-based concurrent chemoradiotherapy was considered as the standard regimen for advanced NPC patients. Our result have demonstrated that pretreatment EBV DNA levels was the only effective factor to predict DMFS, regardless of patients staged III or stage IVa-b. The patients with EBV DNA ≥4000 copies/mL took a higher rate of 10% to develop distant metastasis, compared with those of EBV DNA <4000 copies/mL. Therefore, the high-risk group of advanced NPC patients, stratified by plasma EBVDNA, should require more intense treatment.
Recent studies have demonstrated that the adjuvant chemotherapy (ACT) did not provided any benefit for local advance stage NPC patients3,5,6 and the effect of NACT on NPC remains controversial. Thus, we assumed that cisplatin-based concurrent chemotherapy plus other drug such as paclitaxel, docetaxel, or gemcitabine may reduce micrometastasis lesion for high-risk advanced NPC patients. One prospective, ramdomized phase III clinical is going to be conducted to assess the benefit of concurrent chemotherapy regimen of cisplatin plus paclitaxel compared with cisplatin alone in high-risk local and regionally advanced NPC patients, identified with pretreatment plasma EBV DNA ≥4000 copies/mL.
For local and regionally advanced NPC patients with EBV DNA <4000 copies/mL, regardless of in the entire population or patients staged III or IV, the 3-year's PFS and DMFS was approximate to 90%, according to the founding of this study. The National Comprehensive Cancer Network (NCCN) recommends that CCRT with cisplatin (CDDP) be delivered at a high dose at 3-week intervals or at an intermediate dose weekly for stage II–IVB NPC. Many previous published literatures,27–30 such as Intergroup 0099 (INT-0099),27 NPC-9901,28 and NPC-9902 trial,29 have demonstrated the local and regionally advanced NPC patients benefit from cisplatin-based concurrent chemotherapy. However, the proportion of patients in the chemoradiation arm completed all 3 cycles of concurrent chemotherapy was 52% to 63% because of chemotherapy-induced toxicity. Therefore, avoidance of concurrent chemotherapy-related toxicity effects and unnecessary cost is important to improve long-term outcomes for advanced NPC patients that have excellent prognosis. Moreover, the results of combining analysis of the NPC-9901 and the NPC-9902 Trials,31 reported by Lee, proved that the 5-year local and regionally failure-free rate (FFR), for patients who received 0–1, 2, and 3 concurrent cycles were 79%, 88%, and 88%, and respectively; the corresponding distant-FFR by adjuvant cycles were 68%, 78%, and 77%, respectively. The difference of survival curve between 2 and 3 cycles was insignificant. Lee31 and Chan32 also considered that the Intergroup-0099 regimen could be refined by reducing from 3 to 2 concurrent cycles (ie, reducing the dose of concurrent cisplatin to 200 mg/m2) without affecting the efficacy. In addition, intensity-modulated radiotherapy (IMRT), with 5-year disease-specific survival (DSS) ∼85%, has gradually replaced 2-dimensional conventional radiotherapy as the primary radiotherapy modality for the treatment of NPC, due to its superior local and regionally control and improve the long-term survival of NPC patients.33,34 Given the reason mentioned above, plasma EBV DNA levels might be applied to guide concurrent chemotherapy regimen for local and regionally advanced NPC patients. Therefore, we make a hypothesis that the low-risk locoregionally advanced NPC patients received 2 cycles of chemotherapy may gain similar long-term survival as those received 3 cycles of chemotherapy, leading to less chemotherapy-induced toxicity. One prospective, phase II noninferiority, randomized controlled clinical trial is going to be designed to assess efficacy for low-risk patients, identified with pretreatment plasma EBV DNA <4000 copies/mL, received 2 cycle cisplatin-based chemotherapy compared with 3 cycle cisplatin-based chemotherapy. Therefore, combining the plasma EBV DNA might guide individualized concurrent chemoradiotherapy for advanced NPC patients in the future.
The major limitation of our study is that a single measurement of EBV DNA and the data were obtained exclusively at 1 center, and the measurement of plasma EBV DNA still needs to be globally standardized. The second limitation is that was that the time of follow-up was shorter in this study, and close monitoring and 5-year follow-up data are still required for these patients. The third shortcoming is that we still lacked of post-treatment EBV DNA level, future study need to continue to evaluate the prognostic value of post-treatment EBV DNA in the IMRT value.
In summary, elevated plasma EBV DNA was still an effective prognostic biomarker for local and regionally advanced NPC patients in the IMRT area. Future ramdomized clinical trials are needed to further evaluate whether plasma EBV DNA levels could be applied to guide individualized treatment for locoregionally advanced stage NPC patients.
Acknowledgements
The authors gratefully recognize the patients who participated in this study. They also thank Professor Qing Liu for statistical assistance.
Footnotes
Abbreviations: 2D-CRT = 2-dimensional radiotherapy,3D-CRT = 3-dimensional radiotherapy, AJCC = American Joint Committee on Cancer, CCRT = concurrent chemoradiotherapy, CT = computed tomography, DMFS = distant metastasis-free survival, EBV DNA = Epstein–Barr Virus DNA, IMRT = intensity-modulated radiation therapy, MRI = magnetic resonance imaging, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression metastasis-free survival.
W-HC, L-QT, and S-SG contributed equally to this study.
Funding: this study was partly supported by the 863 Project (No: 2012AA02A501), the Ministry of Science and Technology of China (No: 2011CB504304 and 2012CB967000), the National Natural Science Foundation of China (No:81230045, 81425018, 81201629, 30600755, and 81072226), the National Key Basic Research Program of China (No: 2013CB910304), the Sci-Tech Project Foundation of Guangdong Province (No: 2014A020212103 and 2011B031800161), the Special Support Plan of Guangdong Province (No:2014TX01R145), the Sun Yat-sen University Clinical Research 5010 Program and the Fundamental Research Funds for the Central Universities.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wee JT, Ha TC, Loong SL, et al. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer 2010; 29:517–526. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay JS, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. 2013; Lyon: International Agency for Research on Cancer, 2013–2012–2012, 2014–2001–2018. [Google Scholar]
- 3.Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 2004; 22:4604–4612. [DOI] [PubMed] [Google Scholar]
- 4.Baujat B, Audry H, Bourhis J, et al. Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma. Cochrane Database Syst Rev 2006; 4:CD004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol 2002; 25:219–223. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012; 13:163–171. [DOI] [PubMed] [Google Scholar]
- 7.Chan AT, Lo YM, Zee B, et al. Plasma Epstein–Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Nat Cancer Inst 2002; 94:1614–1619. [DOI] [PubMed] [Google Scholar]
- 8.Leung SF, Chan AT, Zee B, et al. Pretherapy quantitative measurement of circulating Epstein–Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 2003; 98:288–291. [DOI] [PubMed] [Google Scholar]
- 9.Leung SF, Zee B, Ma BB, et al. Plasma Epstein–Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24:5414–5418. [DOI] [PubMed] [Google Scholar]
- 10.Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004; 350:2461–2470. [DOI] [PubMed] [Google Scholar]
- 11.Lo YM, Chan LY, Lo KW, et al. Quantitative analysis of cell-free Epstein–Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999; 59:1188–1191. [PubMed] [Google Scholar]
- 12.Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011; 80:661–668. [DOI] [PubMed] [Google Scholar]
- 13.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012; 104:286–293. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Yin L, Wu J, et al. Impact of plasma Epstein–Barr virus-DNA and tumor volume on prognosis of locally advanced nasopharyngeal carcinoma. Biomed Res Int 2015; 2015:617949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein–Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 2004; 100:1162–1170. [DOI] [PubMed] [Google Scholar]
- 16.An X, Wang FH, Ding PR, et al. Plasma Epstein–Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer 2011; 117:3750–3757. [DOI] [PubMed] [Google Scholar]
- 17.Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein–Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013; 31:2861–2869. [DOI] [PubMed] [Google Scholar]
- 18.Tang LQ, Chen QY, Guo SS, et al. The impact of plasma Epstein–Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Brit J Cancer 2014; 111:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang LQ, Li CF, Chen QY, et al. High-sensitivity C-reactive protein complements plasma epstein-barr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. Int J Radiat Oncol Biol Phys 2015; 91:325–336. [DOI] [PubMed] [Google Scholar]
- 20.Chen WH, Tang LQ, Wang FW, et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer 2014; 14:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Huang Q, Liu W, et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer 2012; 131:406–416. [DOI] [PubMed] [Google Scholar]
- 22.Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol 2012; 13:633–641. [DOI] [PubMed] [Google Scholar]
- 23.Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011; 103:1761–1770. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014; 110:398–403. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Han F, Lu LX, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng 2004; 23 (11 Suppl):1532–1537. [PubMed] [Google Scholar]
- 26.Xiao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer 2011; 117:1874–1883. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998; 16:1310–1317. [DOI] [PubMed] [Google Scholar]
- 28.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005; 23:6966–6975. [DOI] [PubMed] [Google Scholar]
- 29.Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 66:142–151. [DOI] [PubMed] [Google Scholar]
- 30.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005; 23:6730–6738. [DOI] [PubMed] [Google Scholar]
- 31.Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011; 47:656–666. [DOI] [PubMed] [Google Scholar]
- 32.Loong HH, Chan AT. Controversies in the systemic treatment of nasopharyngeal carcinoma. Oral Oncol 2014; 50:785–790. [DOI] [PubMed] [Google Scholar]
- 33.Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014; 110:377–384. [DOI] [PubMed] [Google Scholar]
- 34.Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60:1440–1450. [DOI] [PubMed] [Google Scholar]


