Abstract
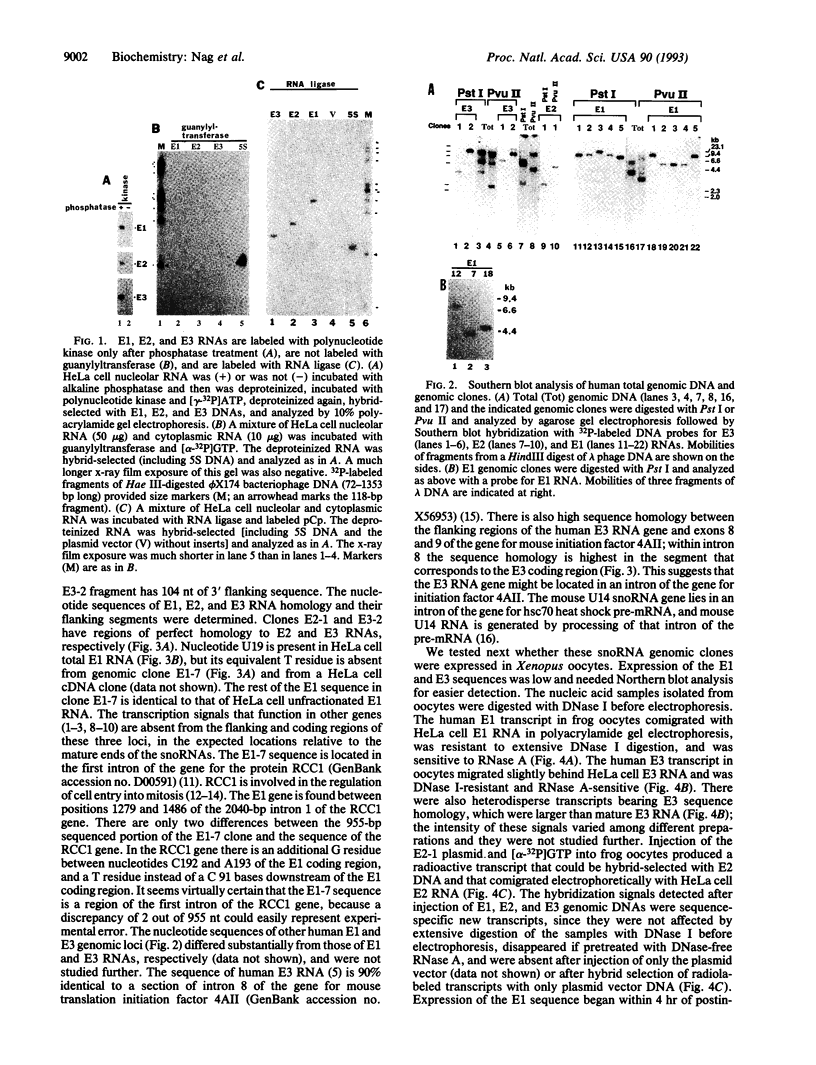
We have found earlier three small nucleolar RNA (snoRNA) species, named E1, E2, and E3, that have unique nucleotide sequences and may participate in ribosome formation. The present report shows that there is a monophosphate at the 5' end of each of these three snoRNAs, suggesting that their 5' termini are formed by RNA processing. E1, E2, and E3 human genomic sequences were isolated. Apparently, the E2 and E3 loci are genes for the main E2 and E3 RNA species, based on their full homology, while the E1 locus is a gene for an E1 RNA sequence variant in HeLa cells. These loci do not have any of the intragenic or flanking sequences known to be functional in other genes. The E1 gene is located within the first intron of the gene for RCC1, a protein that regulates onset of mitosis. There is substantial sequence homology between the human E3 gene and flanking regions, and intron 8 and neighboring exons of the gene for mouse translation initiation factor 4AII. Injection of the human E1, E2, and E3 genes into Xenopus oocytes generated sequence-specific transcripts of the approximate sizes of the respective snoRNAs. We discuss why the available results are compatible with specific transcription and processing occurring in frog oocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Maxwell E. S. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993 Apr;18(4):131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Furuno N., Nakagawa K., Eguchi U., Ohtsubo M., Nishimoto T., Soeda E., Ohtubo M. Complete nucleotide sequence of the human RCC1 gene involved in coupling between DNA replication and mitosis. Genomics. 1991 Oct;11(2):459–461. doi: 10.1016/0888-7543(91)90156-9. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Kazmaier M., Tebb G., Mattaj I. W. Functional characterization of X. laevis U5 snRNA genes. EMBO J. 1987 Oct;6(10):3071–3078. doi: 10.1002/j.1460-2075.1987.tb02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R. RNA polymerase III transcription of genes that lack internal control regions. Biochim Biophys Acta. 1991 Jan 17;1088(1):1–9. doi: 10.1016/0167-4781(91)90146-d. [DOI] [PubMed] [Google Scholar]
- Leverette R. D., Andrews M. T., Maxwell E. S. Mouse U14 snRNA is a processed intron of the cognate hsc70 heat shock pre-messenger RNA. Cell. 1992 Dec 24;71(7):1215–1221. doi: 10.1016/s0092-8674(05)80069-8. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T. Transcription of cloned Xenopus laevis ribosomal DNA microinjected into Xenopus oocytes, and the identification of an RNA polymerase I promoter. Cell. 1982 Oct;30(3):835–842. doi: 10.1016/0092-8674(82)90288-4. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Uzawa S., Schlegel R. Mitotic checkpoints. Curr Opin Cell Biol. 1992 Apr;4(2):174–179. doi: 10.1016/0955-0674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Rimoldi O. J., Raghu B., Nag M. K., Eliceiri G. L. Three new small nucleolar RNAs that are psoralen cross-linked in vivo to unique regions of pre-rRNA. Mol Cell Biol. 1993 Jul;13(7):4382–4390. doi: 10.1128/mcb.13.7.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M. Checkpoint controls that couple mitosis to completion of DNA replication. Trends Cell Biol. 1992 Sep;2(9):277–281. doi: 10.1016/0962-8924(92)90201-w. [DOI] [PubMed] [Google Scholar]
- Ruff E. A., Rimoldi O. J., Raghu B., Eliceiri G. L. Three small nucleolar RNAs of unique nucleotide sequences. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):635–638. doi: 10.1073/pnas.90.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]








