Abstract
Previous studies suggest that an association between restless legs syndrome (RLS) and migraine exists. However, population-based data are unavailable in Asian cohorts. Our study thus aims to evaluate the association between migraine and RLS in a nationwide, population-based cohort in Taiwan and to examine the effects of age, sex, migraine subtype, and comorbidities on RLS development.
Data from the Taiwan National Health Insurance Research Database were used. Patients aged 20 years or older with newly diagnosed migraine from 2000 to 2008 were included; 23,641 patients with newly diagnosed migraine and 94,564 subjects without migraine were randomly selected and followed until RLS development, withdrawal from the National Health Insurance, or until the end of 2011. A multivariate Cox proportional hazards regression model was used to explore the risk of RLS in patients with migraine after adjustment for demographic characteristics and comorbidities.
Both cohorts were followed for a mean of 7.38 years. After adjustment for covariates, the risk of RLS was 1.42-fold higher (95% confidence interval = 1.13–1.79) in the migraine cohort than in the nonmigraine cohort (7.19 versus 3.42 years per 10,000 person-years). The increased risk was more prominent in males in the migraine cohort (1.87-fold increased risk, 95% confidence interval 1.22–2.85). Neither comorbidity status nor migraine subtype influenced the RLS risk.
This population-based study demonstrated that migraine is associated with an increased risk of RLS compared with those without migraine, particularly in male patients with migraine and regardless of the comorbidity status.
INTRODUCTION
Migraine is a prevalent primary headache disorder that affects approximately 10% to 20% of the general population, predominantly females (female:male incidence = 2–3:1).1,2 It is characterized by recurrent headache attacks and is associated with the hypersensitivity of various central nervous functional systems.1 Accumulating evidence suggests that multiple disorders are associated with migraine, including cardiovascular disease, anxiety and depressive disorders, epilepsy, and pain disorders.3–6 In addition, earlier studies have suggested that sleep disorders, particularly restless legs syndrome (RLS), are possible comorbidities in patients with migraine.7,8
Restless legs syndrome is a sleep-related sensorimotor disorder first described in 1945 by the Swedish neurologist Ekbom. It has a prevalence of 4% to 29% in the general population, and similar to migraine, it predominantly affects females (female:male incidence = 2:1).9–11 Restless legs syndrome is characterized by uncomfortable sensations in the legs, an overwhelming urge to move the legs, especially when at rest and during nighttime; the symptoms are relieved only upon moving the legs (eg, standing and walking).12,13 Restless legs syndrome may be idiopathic, or, as accumulating evidence suggests, may develop secondary to a variety of medical conditions, such as diabetes, iron deficiency anemia, depression, anxiety, sleep disorder, Parkinson disease, and renal disease.14–16 Moreover, pathophysiological mechanisms common to both migraine and RLS were proposed,17 involving dopaminergic dysfunction, abnormalities of iron metabolism, the endogenous opioid system, and common genetic factors.18,19
Previous studies have demonstrated a higher prevalence of RLS in patients with migraine as compared with individuals without migraine.13,20–28 Similarly, a higher prevalence of migraine in patients with RLS was also reported.29–31 Furthermore, results from a prior systematic review suggest that migraine is associated with increased odds for RLS.32 However, among these studies, only 2 were large-scale and population-based studies: Schürks et al13 (Women's Health Study) and Winter et al27 (Physicians’ Health Study). These studies have their limitations; their cohorts were restricted to health-care professionals and a single sex (female and male, respectively). Furthermore, Winter et al employed a cross-sectional design rather than a longitudinal design. Nevertheless, both studies identified a higher RLS prevalence in patients with migraine compared with controls.13,27
To confirm these results in a broader population sample and to identify the potential confounders, such as age, migraine subtype, and comorbidities, we conducted a nationwide, large-scale, population-based study using the Taiwan National Health Insurance Research Database (NHIRD).
METHODS
Data Source
The National Health Insurance (NHI) program, implemented in 1995, is a compulsory single-payer healthcare system covering 99.9% of Taiwan's population at the end of 2014. The NHIRD is managed by the Taiwanese National Health Research Institutes and published by the Bureau of NHI of Taiwan. In this study, we used the Longitudinal Health Insurance Database 2000 (LHID 2000), a subset of the NHIRD. The LHID 2000 includes the 1996 to 2011 medical claims of 1 million beneficiaries randomly selected from the 23.75 million NHI beneficiaries. The LHID 2000 contains comprehensive outpatient and inpatient information, including demographic data, clinical visit details, prescription details, and the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes. To ensure patient confidentiality, the National Health Research Institutes scrambled the patient identification number, which is necessary to link the data with a patient identity. Our study was approved by the Ethics Review Board of China Medical University (CMU-REC-101–012).
Study Population
We conducted a population-based retrospective cohort study by selecting participants aged 20 years and older who were newly diagnosed with migraine (ICD-9-CM 346) and without a previous diagnosis of RLS (ICD-9-CM 333.90 and 333.99) during 2000 to 2008, as the migraine cohort. Migraine diagnoses were identified according to the International Classification of Headache Disorders, Second Edition criteria.33 The date of the first migraine diagnosis was used as the index date. In addition, we divided the migraine cohort into 4 subcohorts: migraine with aura (ICD-9-CM 346.0), migraine without aura (ICD-9-CM 346.1), unspecified migraine (ICD-9-CM 346.9), and other migraine (ICD-9-CM 346.2 and 346.8) cohorts. For each patient with migraine, 4 insured individuals without migraine were randomly selected from the NHIRD, and frequency-matched for sex, age (5-year span), and the index year using the same inclusion criteria as that of the migraine cohort.
Outcome and Comorbidities
The primary outcome was RLS (ICD-9-CM 333.90 and 333.99). Restless legs syndrome diagnoses complied with the recommendations of the International RLS Study Group.12 All participants were followed up from the index date to RLS onset, withdrawal from the NHI, or December 31, 2011. The medical comorbidities, including diabetes (ICD-9-CM 250), iron deficiency anemia ([IDA], ICD-9-CM 280), depression (ICD-9-CM 296.2, 296.3, 300.4, and 311), anxiety (ICD-9-CM 300.00), sleep disorder (ICD-9-CM 307.4 and 780.5), Parkinson disease (ICD-9-CM 332), and renal disease (ICD-9-CM 580–589), were defined as diseases diagnosed before the index date.
Statistical Analyses
The distributions of the baseline characteristics (sex, age group, and comorbidities) were compared between the migraine and nonmigraine cohorts. A χ2 test was used to examine the categorical variables, and a Student t test was used to examine the continuous variables. The incidence density rates (per 10,000 person-years) of RLS stratified by sex, age, and comorbidity were calculated for the migraine and nonmigraine cohorts. We assessed the cumulative RLS incidences in the migraine and nonmigraine cohorts by using the Kaplan–Meier method, and tested the differences by using a log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to assess the risk of RLS and the RLS-associated risk factors. The multivariate Cox proportional hazards regression model was adjusted for sex, age, and comorbidities of diabetes, IDA, depression, anxiety, sleep disorder, Parkinson disease, and renal disease. We also used Cox proportional hazards regression models to calculate the risk of RLS and migraine stratified by sex, age, and comorbidities. We used SAS 9.4 software (SAS Institute, Cary, NC) to manage and analyze the data. Two-sided P < 0.05 was considered significant.
RESULTS
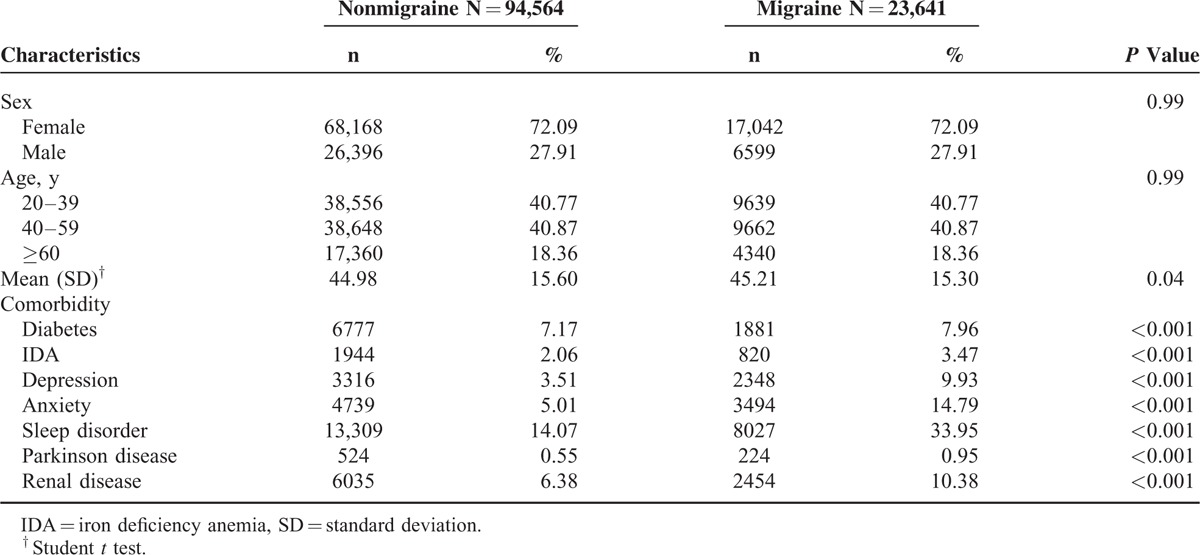
We included 23,641 patients with migraine and 94,564 subjects without migraine. In the migraine cohort, 81.64% were younger than 60 years and 72.09% were females. The mean ages in the migraine and nonmigraine cohorts were 45.21 (standard deviation = 15.30) and 44.98 years (standard deviation = 15.60), respectively. Compared with the nonmigraine cohort, the comorbidities of diabetes (7.96% versus 7.17%), IDA (3.47% versus 2.06%), depression (9.93% versus 3.51%), anxiety (14.79% versus 5.01%), sleep disorder (33.95% versus 14.07%), Parkinson disease (0.95% versus 0.55%), and renal disease (10.38% versus 6.38%) were more prevalent in the migraine cohort (Table 1).
TABLE 1.
Baseline Demographic Factors and Comorbidity of Study Participants According to Migraine Status
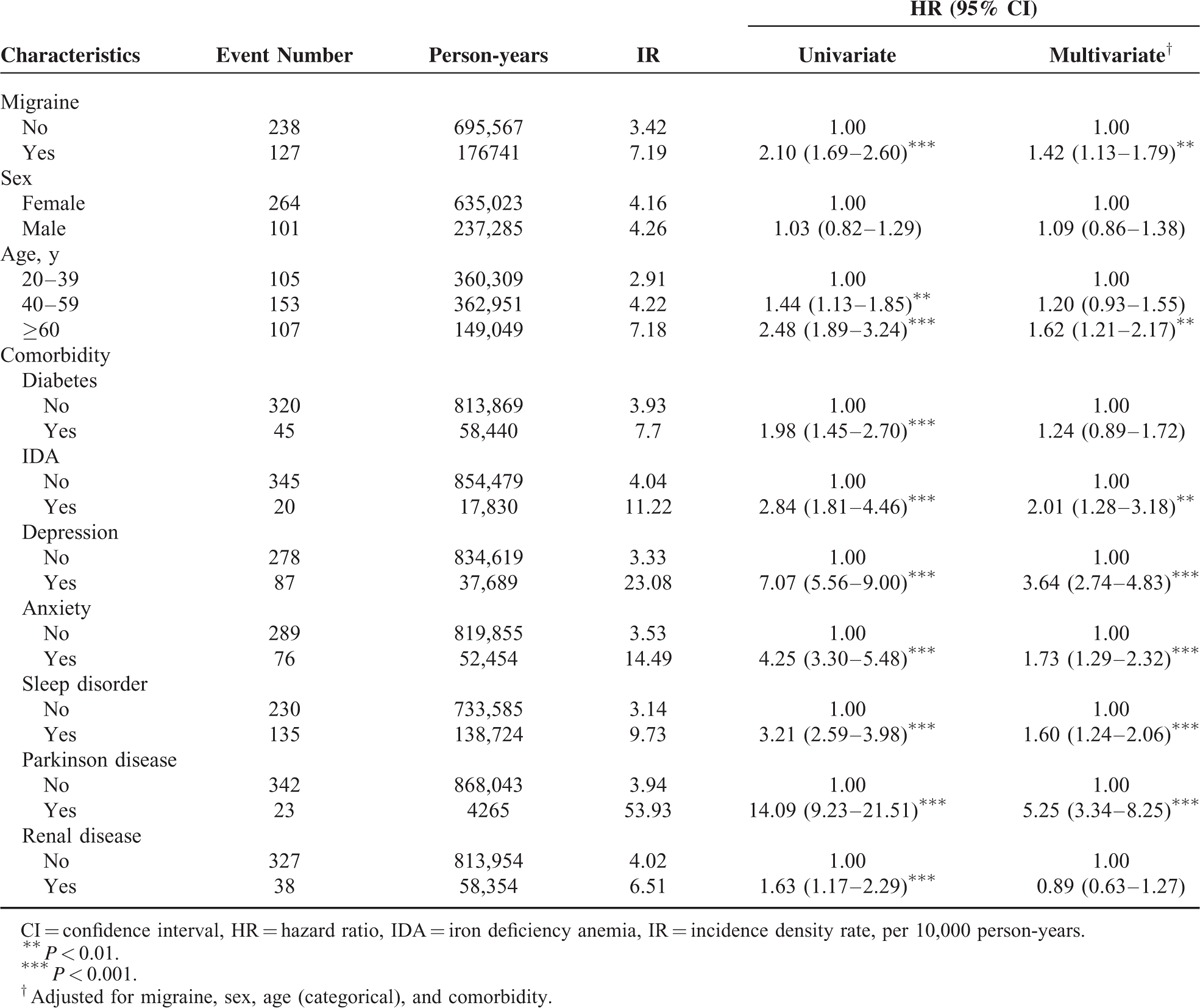
The cumulative incidence curves of RLS for migraine status are illustrated in Figure 1. We used the log-rank test to examine the cumulative incidence of RLS in the migraine and nonmigraine cohorts and discovered that the cumulative incidence of RLS was significantly higher in the patients with migraine than in the participants without migraine (P < 0.001). During an average follow-up period of 7.38 years, we observed a higher incidence density rate of RLS in the migraine cohort than in the nonmigraine cohort (7.19 versus 3.42 years per 10,000 person-years). After adjustment for sex, age, and comorbidities of diabetes, IDA, depression, anxiety, sleep disorder, Parkinson disease, and renal disease, the adjusted hazard ratio (aHR) of RLS was 1.42-fold higher (95% confidence interval [CI] = 1.13–1.79) for the migraine cohort than for the nonmigraine cohort (Table 2). Compared with younger patients, the risk of RLS was 1.62-fold higher (95% CI = 1.21–2.17) in patients >60 years, suggesting that RLS risk increases with age. In the multivariable model, the risk of RLS was higher for the patients with comorbidities of IDA (aHR = 2.01, 95% CI = 1.28–3.18), depression (aHR = 3.6, 95% CI = 2.7–4.83), anxiety (aHR = 1.73, 95% CI = 1.29–2.32), sleep disorder (aHR = 1.60, 95% CI = 1.2–2.06), and Parkinson disease (aHR = 5.25, 95% CI = 3.3–8.25).
FIGURE 1.
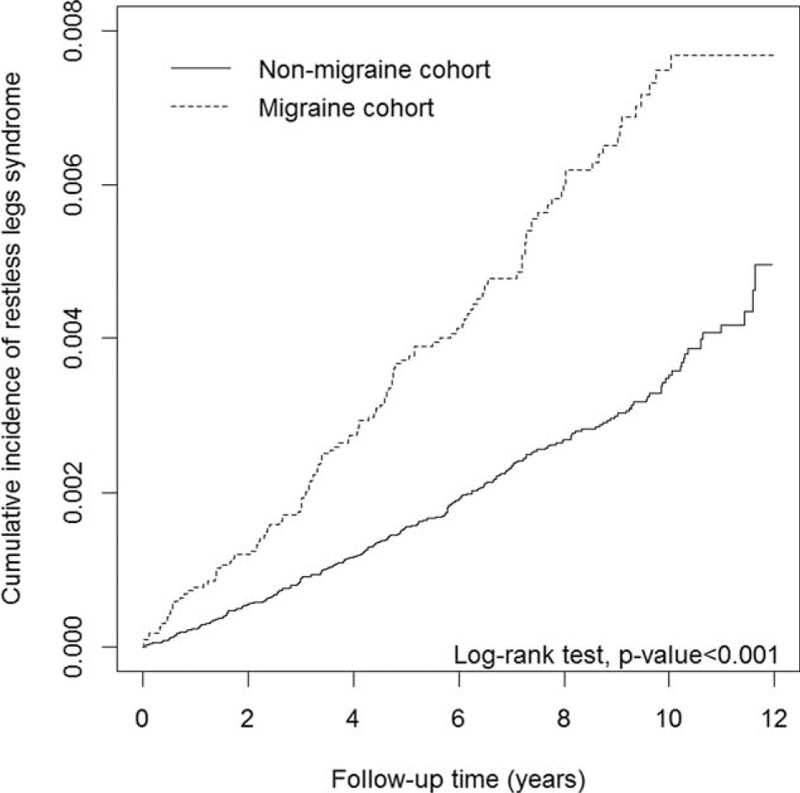
Cumulative incidence curves of restless legs syndrome for cohorts with and without migraine.
TABLE 2.
Cox Model Measured Hazard Ratios and 95% Confidence Interval of Restless Legs Syndrome Associated With Migraine and Covariates
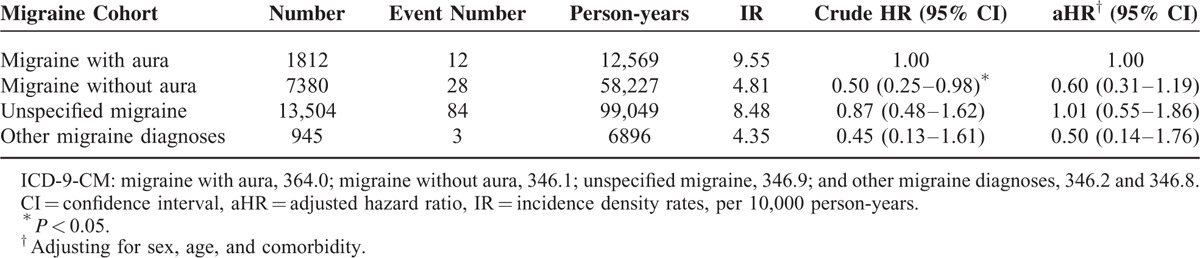
The sex-specific analysis showed that the incidence density rates of RLS in females and males with migraine were 6.77 and 8.31 years per 10,000 person-years, respectively; higher than those in the nonmigraine cohort (3.49 and 3.23 years per 10,000 person-years, respectively). In addition, male patients with migraine exhibited a significantly higher risk of RLS (aHR = 1.87, 95% CI = 1.22–2.85) than did the male patients without migraine. The incidence density rate of RLS increased with the age in both cohorts; however, patients with migraine were not associated with the risk of RLS compared with individuals without migraine in all age groups. The migraine cohort exhibited a higher risk of RLS than did the nonmigraine cohort both for patients without any comorbidity (aHR = 1.59, 95% CI = 1.07–2.36) and with comorbidity (aHR = 1.59, 95% CI = 1.22–2.08) (Table 3). Among patients with migraine, the risks of RLS in the migraine without aura, unspecified migraine, and other migraine cohorts were not significantly higher than that for the migraine with aura cohort (Table 4).
TABLE 3.
Incidence Density Rates and Hazard Ratios of Restless Legs Syndrome According to Migraine Status Stratified by Sex, Age, and Comorbidity
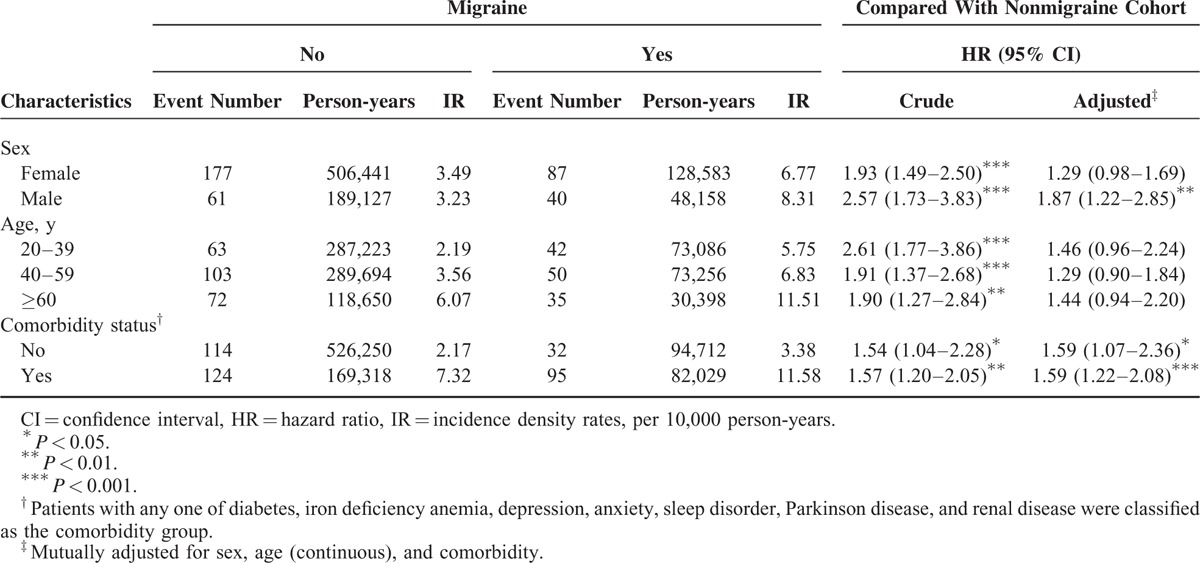
TABLE 4.
Incidence Density Rates and Hazard Ratios of Restless Legs Syndrome Among Different Type of Migraine Patients
DISCUSSION
To the best of our knowledge, this is the first nationwide, population-based longitudinal study that demonstrates an increased risk of RLS in patients with migraine. We demonstrated that the risk of subsequent development of RLS in patients with migraine was 1.42-fold higher than in the nonmigraine cohort, after adjustment for age, sex, and medical comorbidities. We discovered that male patients with migraine had a high risk (1.87-fold) of RLS. Moreover, regardless of the comorbidity status or migraine subtype, the migraine cohort exhibited a higher risk of RLS than did the nonmigraine cohort.
Our results were consistent with those of the previous 2 large-scale, population-based studies, although some methodological dissimilarities must be considered.13,27 Our study included participants of diverse age (20–60-year old) and the cohorts were of mixed sex. In addition, we considered a longitudinal study design, unlike that of Winter et al.27 The longitudinal study design enables the consideration of possible confounders (risk factors) for the development of RLS and is more generalizable.
In the current study, consistent with previous observations,3–6 the possible comorbidities of migraine, such as diabetes, IDA, depression, anxiety, sleep disorders, Parkinson disease, and renal disease were more prevalent in the migraine cohort than in the comparison cohort. Although the patients with migraine in this study exhibited a different prevalence of comorbidities associated with the RLS development compared with the nonmigraine cohort, multivariate Cox analyses demonstrated that migraine was an independent factor for predicting the RLS risk.
Our cohort study results should be interpreted cautiously and with recognition of its inherent ethnic and methodological differences. For example, our control cohort's annual RLS incidence rate (3.42/10,000 person-years) was lower than that of a German cohort (9–22/1,000 person-years).34 Moreover, studies have reported that Asian controls show a lower RLS prevalence than the European and North American (ie, non-Asian) controls.23,35 Furthermore, in our study cohorts, the RLS occurrence showed no clear sex-dependent predisposition (Table 2), unlike the many studies where a higher RLS prevalence was reported in females than in males.9–11 Therefore, our results should be interpreted cautiously, and consideration should be given to the effects of differences in cohort ethnicities, methodologies, and clinical settings. Notably, our data showed a high aHR for RLS in elderly patients (>60 years) and patients with the comorbidity of IDA, depression, anxiety, sleep disorders, or Parkinson disease (Table 2). This result is in agreement with earlier reports of these conditions as potential risk factors for RLS.14,15,36
The migraine subcohort analysis revealed that the male patients with migraine exhibited a significantly higher risk of RLS than did the patients without migraine, which is inconsistent with the results of previous population-based studies.13,27 However, interpretation of the discrepancy warrants caution because of the limited number of population-based studies and possible ethnic differences. We identified that the higher risk of RLS in the patients from the migraine cohort was not influenced by the age after adjustment for multivariable effects; this risk was high regardless of the comorbidity status. These results reinforce our finding that migraine is independently associated with RLS. Finally, no differences were observed in RLS occurrence among the patients with different migraine subtypes (ie, migraine with or without aura). This finding is consistent with that of the previous studies demonstrating that the odds of RLS in patients with migraine did not differ in the presence or absence of auras.13,28
Two key mechanisms linking migraine and RLS are currently being explored. First, the neurotransmitter dopamine is suspected in the migraine pathophysiology because dopamine-mediated behaviors appear before the migraine attacks (eg, yawning, food craving, and gastrointestinal disorders).1 In addition, dopamine agonists can aggravate the symptoms, whereas dopamine antagonists are effective in treatment regimens.37 Studies on rats have shown that dopamine directly inhibits nociception in the trigeminocervical complex.38,39 The association of dopamine and RLS is more tenuous, although central dopaminergic hypofunction of hypothalamic neurons (A11) targeting the spinal cord have been implicated in the RLS pathophysiology.19 This hypothesis is supported by the substantial improvement in RLS symptoms seen following the initiation of dopamine agonist treatment.40 Moreover, the dopamine link between migraine and RLS is supported by Cologno et al,41 who observed a higher prevalence of premonitory symptoms in patients with migraine and RLS than in patients with migraine and no RLS.
The second hypothesis relates to iron metabolism. Iron deposition in the brain is considered to be involved in migraine pathogenesis, and increased iron accumulation appears to be associated with repeated migraine attacks.42 Iron deficiency also appears to play a role in the etiology and pathogenesis of RLS.43 Therefore, deregulated iron metabolism (eg, inappropriate deposition leading to reduced bioavailable iron) may contribute to the pathophysiology of (and a link between) these disorders. Furthermore, since iron is a cofactor of tyrosine hydroxylase, an enzyme required for dopamine synthesis, evidence suggesting that disruptions in brain iron trafficking lead to disturbances in striatal dopamine neurotransmission, which may be related to RLS pathophysiology, is mounting.44 Therefore, the link between iron deficiency and dopamine metabolic theories may be considered in conjunction with dopamine deregulation. Finally, previous studies have pointed to a common genetic origin for RLS and migraine located on chromosome 14q21.45,46 It has also been reported that families with several members of successive generations are affected by migraine with aura and RLS.47 Furthermore, a recent study demonstrated that the Meis homeobox 1 gene was associated with a significantly higher susceptibility to RLS in patients with migraine.48 These findings support the hypothesis of a shared genetic etiology of RLS and migraine. Collectively, these evidences suggest that the relationship between migraine and RLS may be one of common pathogenetic substrates.
Our findings have several clinical implications. Migraine should be regarded a potential risk factor for RLS, and patients with migraine should be evaluated for RLS both initially and at follow-up. In addition, given the differences between our study and previous studies,27,41 additional research is required to evaluate the influence of sex or hormones on the migraine–RLS relationship.
The major strengths of this study lie in its large (nationwide) sample size and longitudinal design, which provide sufficient power to delineate the differences between the 2 study cohorts and across various covariates. Moreover, in this study, the diagnoses of “migraine” and “RLS” were based on the ICD-9 codes determined by qualified clinical physicians (not always neurologists) for the strictly audited reimbursement process. Migraine diagnoses were identified according to the International Classification of Headache Disorders, Second Edition criteria,33 and RLS diagnoses complied with the recommendations of the International RLS Study Group.12 In addition, NHIRD covers a highly representative sample of Taiwan's general population because the reimbursement policy is universal and operated by a single buyer, the government in Taiwan. All insurance claims should be scrutinized and coded by medical reimbursement specialists and peer reviewed according to the standard criteria for diagnoses of migraine and RLS in the study. Therefore, the diagnoses and coding in this study were highly reliable. Finally, our longitudinal study afforded an assessment of the temporal relation between RLS and migraine, which helps comprehend the direction of the association between these 2 disorders to provide a potential bidirectional relationship suggesting comorbidity.
The study had several limitations. First, the NHIRD dataset is derived from an administrative database that lacks detailed clinical data, such as duration and frequency of migraine occurrences, RLS severity, neuroimaging, polysomnograms, suggested immobilization tests, and other laboratory results. Therefore, the precise diagnosis of idiopathic or secondary RLS was not available in this database. Second, the NHIRD-based cohort only included patients with migraine seeking medical treatment throughout the study period. Patients with migraine who did not seek medical attention may have been grouped incorrectly into the control cohort, leading to the underestimation of the RLS risk in patients with migraine. Finally, we could not establish an association with the causalities in this study, an aspect that can be considered in further research.
In conclusion, this large-scale, cohort, nationwide population-based longitudinal study demonstrated an association between migraine and the risk of RLS, particularly in male patients with migraine, regardless of comorbidities or migraine subtype. These findings lay the foundation for deeper understanding of the underlying mechanisms for both disorders and provide direction to clinicians to improve their diagnoses and therapies.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BNHI = Bureau of National Health Insurance, CI = confidence interval, LHID 2000 = Longitudinal Health Insurance Database 2000, NERD = nonerosive reflux disease, NHI = National Health Insurance, NHIA = National Health Insurance Administration, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, RLS = restless legs syndrome.
H-HC and C-HK contribute equally to this article.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212–113019); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM10501010037); NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol 2006; 5:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain 2010; 11:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache 2006; 46:1327–1333. [DOI] [PubMed] [Google Scholar]
- 4.Tietjen GE, Brandes JL, Peterlin BL, et al. Allodynia in migraine: association with comorbid pain conditions. Headache 2009; 49:1333–1344. [DOI] [PubMed] [Google Scholar]
- 5.Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. Br Med J 2009; 339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol 2010; 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon PR, Larner AJ. Migraine and restless legs syndrome: is there an association? J Headache Pain 2011; 12:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cevoli S, Giannini G, Favoni V, et al. Migraine and sleep disorders. Neurol Sci 2012; 33:43–46. [DOI] [PubMed] [Google Scholar]
- 9.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005; 165:1286–1292. [DOI] [PubMed] [Google Scholar]
- 10.Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med 2011; 12:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura T, Inoue Y, Kusumi M, et al. Prevalence of restless legs syndrome in a rural community in Japan. Mov Disord 2008; 23:2363–2369. [DOI] [PubMed] [Google Scholar]
- 12.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003; 4:101–119. [DOI] [PubMed] [Google Scholar]
- 13.Schürks M, Winter AC, Berger K, et al. Migraine and restless legs syndrome in women. Cephalalgia 2012; 32:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szentkirályi A, Völzke H, Hoffmann W, et al. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology 2014; 82:2026–2033. [DOI] [PubMed] [Google Scholar]
- 15.Peeraully T, Tan EK. Linking restless legs syndrome with Parkinson's disease: clinical, imaging and genetic evidence. Transl Neurodegener 2012; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med 2009; 266:419–431. [DOI] [PubMed] [Google Scholar]
- 17.Sabayan B, Bagheri M, Borhani Haghighi A. Possible joint origin of restless leg syndrome (RLS) and migraine. Med Hypotheses 2007; 69:64–66. [DOI] [PubMed] [Google Scholar]
- 18.Akerman S, Goadsby PJ. Dopamine and migraine: biology and clinical implications. Cephalalgia 2007; 27:1308–1314. [DOI] [PubMed] [Google Scholar]
- 19.Paulus W, Dowling P, Rijsman R, et al. Pathophysiological concepts of restless legs syndrome. Mov Disord 2007; 22:1451–1456. [DOI] [PubMed] [Google Scholar]
- 20.Young WB, Piovesan EJ, Biglan KM. Restless legs syndrome and drug-induced akathisia in headache patients. CNS Spectr 2003; 8:450–456. [DOI] [PubMed] [Google Scholar]
- 21.Rhode AM, Hösing VG, Happe S, et al. Comorbidity of migraine and restless legs syndrome: a case-control study. Cephalalgia 2007; 27:1255–1260. [DOI] [PubMed] [Google Scholar]
- 22.d’Onofrio F, Bussone G, Cologno D, et al. Restless legs syndrome and primary headaches: a clinical study. Neurol Sci 2008; 29:S169–S172. [DOI] [PubMed] [Google Scholar]
- 23.Chen PK, Fuh JL, Chen SP, et al. Association between restless legs syndrome and migraine. J Neurol Neurosurg Psychiatry 2010; 81:524–528. [DOI] [PubMed] [Google Scholar]
- 24.Lucchesi C, Bonanni E, Maestri M, et al. Evidence of increased restless legs syndrome occurrence in chronic and highly disabling migraine. Funct Neurol 2012; 27:91–94. [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Suzuki K, Miyamoto M, et al. Evaluation of contributing factors to restless legs syndrome in migraine patients. J Neurol 2011; 258:2026–2035. [DOI] [PubMed] [Google Scholar]
- 26.Seidel S, Böck A, Schlegel W, et al. Increased RLS prevalence in children and adolescents with migraine: a case-control study. Cephalalgia 2012; 32:693–699. [DOI] [PubMed] [Google Scholar]
- 27.Winter AC, Schurks M, Berger K, et al. Migraine and restless legs syndrome in men. Cephalalgia 2013; 33:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanigni S, Giannini G, Melotti R, et al. Association between restless legs syndrome and migraine: a population-based study. Eur J Neurol 2014; 21:1205–1210. [DOI] [PubMed] [Google Scholar]
- 29.Gozubatik-Celik G, Benbir G, Tan F, et al. The prevalence of migraine in restless legs syndrome. Headache 2014; 54:872–877. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Lahan V, Goel D. Primary headaches in restless legs syndrome patients. Ann Indian Acad Neurol 2012; 15:S104–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Matarrubia M, Cuadrado ML, Sánchez-Barros CM, et al. Prevalence of migraine in patients with restless legs syndrome: a case-control study. Headache 2014; 54:1337–1346. [DOI] [PubMed] [Google Scholar]
- 32.Schürks M, Winter A, Berger K, et al. Migraine and restless legs syndrome: a systematic review. Cephalalgia 2014; 34:777–794. [DOI] [PubMed] [Google Scholar]
- 33.Headache Classification Subcommittee of the International Headache Society. International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24:1–160. [DOI] [PubMed] [Google Scholar]
- 34.Szentkiralyi A, Fendrich K, Hoffmann W, et al. Incidence of restless legs syndrome in two population-based cohort studies in Germany. Sleep Med 2011; 12:815–820. [DOI] [PubMed] [Google Scholar]
- 35.Cho YW, Shin WC, Yun CH, et al. Epidemiology of restless legs syndrome in Korean adults. Sleep 2008; 31:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadjigeorgiou GM, Stefanidis I, Dardiotis E, et al. Low RLS prevalence and awareness in central Greece: an epidemiological survey. Eur J Neurol 2007; 14:1275–1280. [DOI] [PubMed] [Google Scholar]
- 37.Silberstein SD, Young WB, Mendizabal JE, et al. Acute migraine treatment with droperidol: a randomized, double-blind, placebo-controlled trial. Neurology 2003; 60:315–321. [DOI] [PubMed] [Google Scholar]
- 38.Bergerot A, Storer RJ, Goadsby PJ. Dopamine inhibits trigeminovascular transmission in the rat. Ann Neurol 2007; 61:251–262. [DOI] [PubMed] [Google Scholar]
- 39.Charbit AR, Akerman S, Goadsby PJ. Dopamine: what's new in migraine? Curr Opin Neurol 2010; 23:275–281. [DOI] [PubMed] [Google Scholar]
- 40.Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med 2008; 9:874–881. [DOI] [PubMed] [Google Scholar]
- 41.Cologno D, Cicarelli G, Petretta V, et al. High prevalence of dopaminergic premonitory symptoms in migraine patients with restless legs syndrome: a pathogenetic link? Neurol Sci 2008; 29:S166–S168. [DOI] [PubMed] [Google Scholar]
- 42.Kruit MC, Launer LJ, Overbosch J, et al. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia 2009; 29:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connor JR, Ponnuru P, Wang X-S, et al. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011; 134:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earley CJ, Connor J, Garcia-Borreguero D, et al. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis-Ekbom Disease). Sleep Med 2014; 15:1288–1301. [DOI] [PubMed] [Google Scholar]
- 45.Soragna D, Vettori A, Carraro G, et al. A locus for migraine without aura maps on chromosome 14q21.2-q22.3. Am J Hum Genet 2003; 72:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonati MT, Ferini-Strambi L, Aridon P, et al. Autosomal dominant restless legs syndrome maps on chromosome 14q. Brain 2003; 126:1485–1492. [DOI] [PubMed] [Google Scholar]
- 47.Tartarotti S, Kallweit U, Bassetti CL. Association of restless legs syndrome, chronic motor tic disorder and migraine with aura: a case of a single family. J Neurol 2010; 257:1043–1044. [DOI] [PubMed] [Google Scholar]
- 48.Fuh JL, Chung MY, Yao SC. Susceptible genes of restless legs syndrome in migraine. Cephalalgia 2015; pii: 0333102415620907. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


