Abstract
Several meta-analyses have shown no significant difference in stent thrombosis (ST) between sirolimus eluting stents (SES) and paclitaxel eluting stents (PES). However, other meta-analyses have found SES to be superior to PES. Therefore, to solve this issue, we aim to compare the clinical outcomes between SES and PES during a follow-up period of about 1 or more years.
We have searched Medline and EMBASE for randomized controlled trials (RCTs) comparing SES with PES. These RCTs have been carefully analyzed and then different types of ST including ST defined by the Academic Research Consortium (ARC), acute ST, late and very late ST have all been considered as the clinical endpoints in this study. A follow-up period of about 1 year, between 1 and 2 years as well as a longer follow-up period between 1 and 5 years have been considered. Data were retrieved and combined by means of a fixed-effect model because of a lower heterogeneity observed among the results. Odd ratios (OR) and 95% confidence intervals (CIs) were calculated and the pooled analyses were performed with RevMan 5.3 software.
Twenty-nine studies from 19 RCTs comprising of 16,724 patients (8115 patients in the SES group and 8609 patients in the PES group) satisfied the inclusion criteria and were included in this meta-analysis. No significant differences in ST have been observed between SES and PES. Results were as follow: definite ST with OR: 0.87; 95% CI: 0.64–1.18, P = 0.36; probable ST with OR:0.72; 95% CI: 0.42–1.21, P = 0.21; definite, probable and/or possible ST with OR: 0.94; 95% CI: 0.75–1.17, P = 0.57; acute ST with OR: 0.99; 95% CI: 0.38–2.56, P = 0.98; subacute ST with OR: 0.72; 95% CI: 0.41–1.25, P = 0.25; early ST with OR: 0.81; 95% CI: 0.53–1.25, P = 0.34; late ST with OR: 0.72; 95% CI: 0.39–1.34, P = 0.30; very late ST with OR: 1.02; 95% CI: 0.72–1.44, P = 0.92; and any ST with OR: 0.86; 95% CI: 0.69–1.07, P = 0.18. Long-term ST between 1 and 5 years with OR: 0.93; 95% CI: 0.71–1.22, P = 0.60 was also not significantly different.
No significant difference in ST has been observed between patients treated with either SES or PES. Hence SES and PES can both be considered almost equally effective.
INTRODUCTION
Stent thrombosis (ST) is a major concern for patients treated with drug eluting stents (DES). Sirolimus eluting stents (SES) and paclitaxel eluting stents (PES) have been the most commonly used first-generation DES.
Several meta-analyses comparing SES with PES showed no significant difference in ST between these 2 types of stents. For example, the meta-analysis conducted by Gurm et al1 in 2008, including 7455 patients, found no significant difference in ST between SES and PES. A similar result was reported in another meta-analysis of 6 randomized controlled trials (RCTs) including 1183 patients with type 2 diabetes mellitus.2 The result from the meta-analysis conducted by Zhang et al3 in 2010 also showed no significant difference in ST among the 1173 patients analyzed. Moreover, the meta-analysis conducted by Kastrati et al4 in 2005 including 3669 patients also showed a similar rate of ST between SES and PES in patients with coronary artery disease (CAD). Zhang et al's5 study which included both RCTs and observational studies, also did not find any significant difference in ST between these 2 groups among the patients from RCTs.
However, ST was not always similar between SES and PES. The meta-analysis conducted by Schömig et al6 in 2007 including 16 RCTs with 8695 patients surprisingly showed a significant reduction in ST with SES compared to PES. His study which included a large number of randomized patients could have had an effect on his results.
Therefore, in order to solve this issue, we aim to combine old studies comparing SES and PES with new ones and conduct a meta-analysis with an even larger number of randomized patients (a total of 16,724 patients) to confirm whether a significant difference in ST between the use of SES and PES really exists or not.
METHODS
Sources of Data and Search Strategy
We have searched Medline and EMBASE for RCTs by typing the words “drug eluting stents/DES and percutaneous coronary intervention/PCI,” and also replacing the word “DES” by “PES and/or SES” or their full form “paclitaxel eluting stents and sirolimus eluting stents.” PES and SES have also been replaced by Taxus and Cypher, respectively. All reference lists of related studies were also reviewed for relevant articles. No language restriction was applied.
Inclusion and Exclusion Criteria
Studies were included if:
They were RCTs.
They compared sirolimus eluting stents (SES) with paclitaxel eluting stents (PES).
ST was reported among the clinical endpoints.
Studies were excluded if:
They were non-RCTs (observational studies, reports, meta-analyses, letter to editors).
They did not compare SES with PES but instead, showed the effectiveness of SES and PES separately without comparison or compared SES or PES with another DES.
ST was not reported among the clinical endpoints.
Defining the Different Types of Stent Thromboses, Outcomes, and Follow-Up
Definite ST, probable ST, possible ST, acute, subacute, late and very late ST with a follow-up of 1 month, short-term follow-up (1–12 months) and long-term follow-up (>1 year) including a follow-up between 1 and 2 years, and 1 and 5 years were analyzed in this study. The different types of ST have been defined in Table 1. Table 2 shows the types of ST reported in each of the included trials.
TABLE 1.
Definitions of the Different Types of Stent Thrombosis Reported in This Study
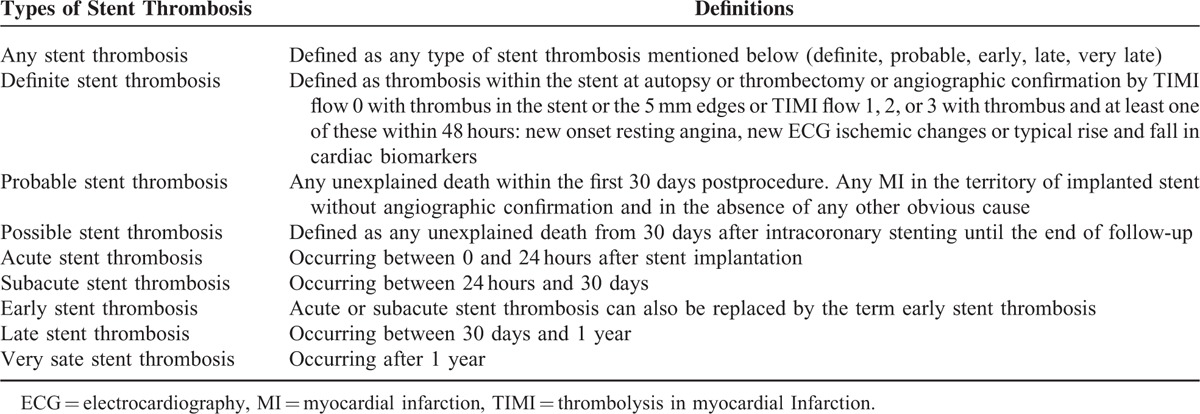
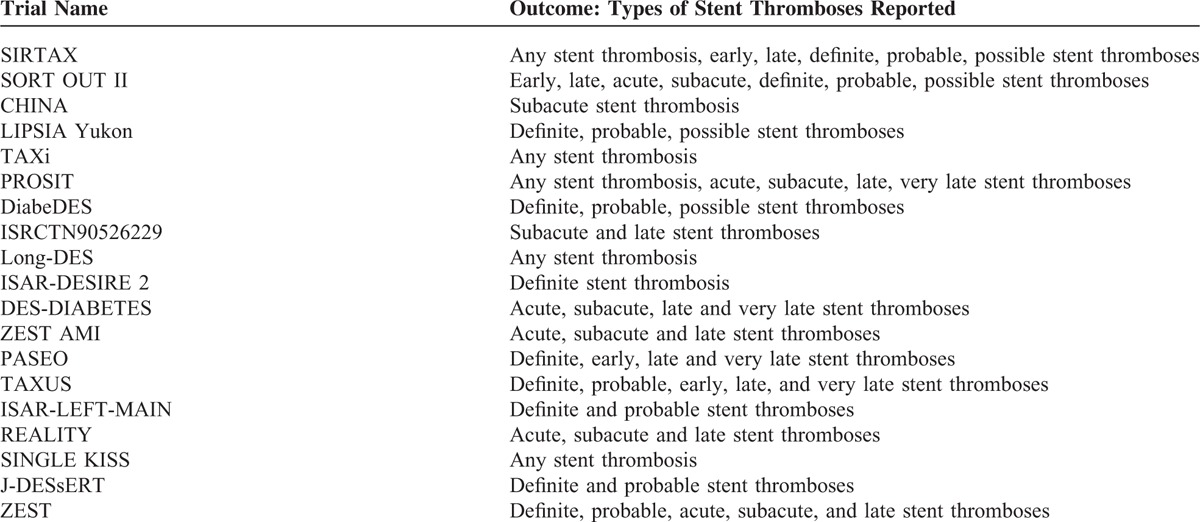
TABLE 2.
Types of Stent Thrombosis Reported Among the Trials
Data Collection and Analysis
Study Selection
All titles and abstracts were independently screened by 2 authors (PKB and ZW) and full papers of those studies which met the inclusion criteria were obtained for review. These studies were carefully checked. Disagreements were carefully discussed between these 2 authors, and if the authors could not reach a final decision, whether to include the study or not, disagreements were resolved by the help of the third author (MHC).
Data Extraction and Management
Data extraction was performed from full-text articles by the same 2 independent authors (PKB and ZW). Any disagreement was resolved through discussion and consensus between these 2 authors. The following data were extracted from each of the trials: author identification, year of patient enrollment, year of publication, language of publication, study design, study population, patient characteristics, intervention and outcomes reported as well as the follow-up periods.
Assessment of Risk of Bias
The bias risk of the included trials was assessed with the components recommended by the Cochrane Collaboration.7
Each of the included trials has been carefully assessed and a grade ranging from A to E has been allocated to specific trials depending on whether they satisfied all the components recommended by the Cochrane Collaboration. In other word, a grade between A to E was allocated to the trials depending on their risk of bias. Completely low risk of bias among all of these 6 components mentioned above corresponded to a grade A, whereas a grade E was given if this evaluation showed a high risk of bias among the data corresponding to these RCTs.
Except for 1 trial which have been allocated a grade C, all the other trials have been allocated a grade B even if a few were almost on a range between an A and a B.
Methodological Quality and Statistical Analysis
The selection of studies, collection and analysis of data, and reporting of the results obtained, followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The Cochrane Q-statistic and the I2-statistic tests were used to assess heterogeneity among the studies whereby P ≤ 0.05 was considered statistically significant and P > 0.05 was considered statistically insignificant. I2 described the total variation (due to heterogeneity rather than chance) across studies in terms of percentages whereby a value of 0% indicated no heterogeneity, and larger values especially from 50% and above indicated increasing heterogeneity. A fixed effect model was used if I2 was <50% and a random effect model was used if I2 was >50%. Funnel plots were assessed for publication bias. Odd ratios (OR) and 95% confidence intervals (CIs) were calculated for categorical variables. RevMan 5.3 software was used for the statistical analysis. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written. Ethical approval was not required since this study is a systematic review and meta-analysis of RCTs.
RESULTS
Figure 1 shows the flowchart of study selection. We identified 42 studies comparing SES with PES in patients with CAD. Thirteen studies were excluded (6 of them were meta-analysis and 7 were observational studies). Finally, 29 studies from 19 RCTs that met the inclusion criteria comprising of 16,724 patients (8115 patients in the SES group and 8609 patients in the PES group) were included in this systematic review and meta-analysis.
FIGURE 1.
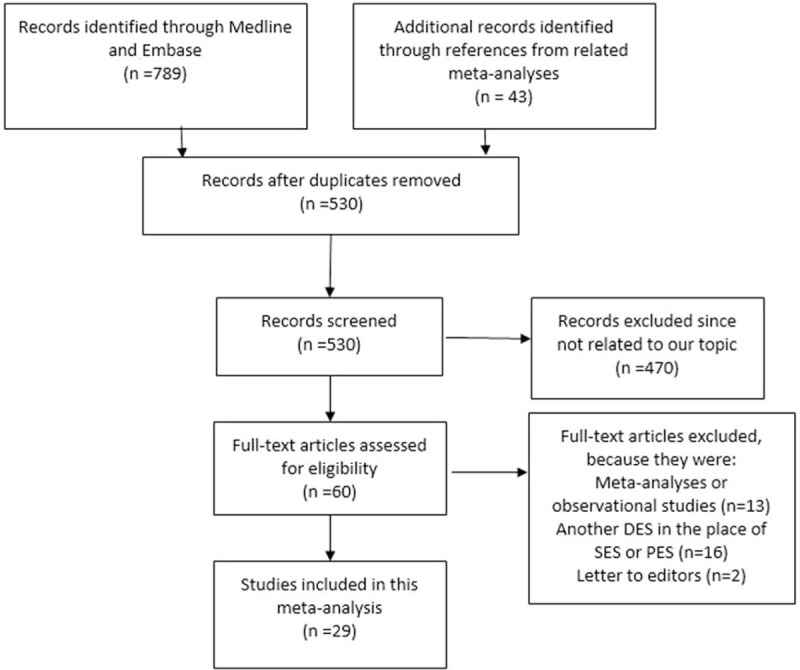
The flow diagram for study selection.
General Characteristics of Included Trials
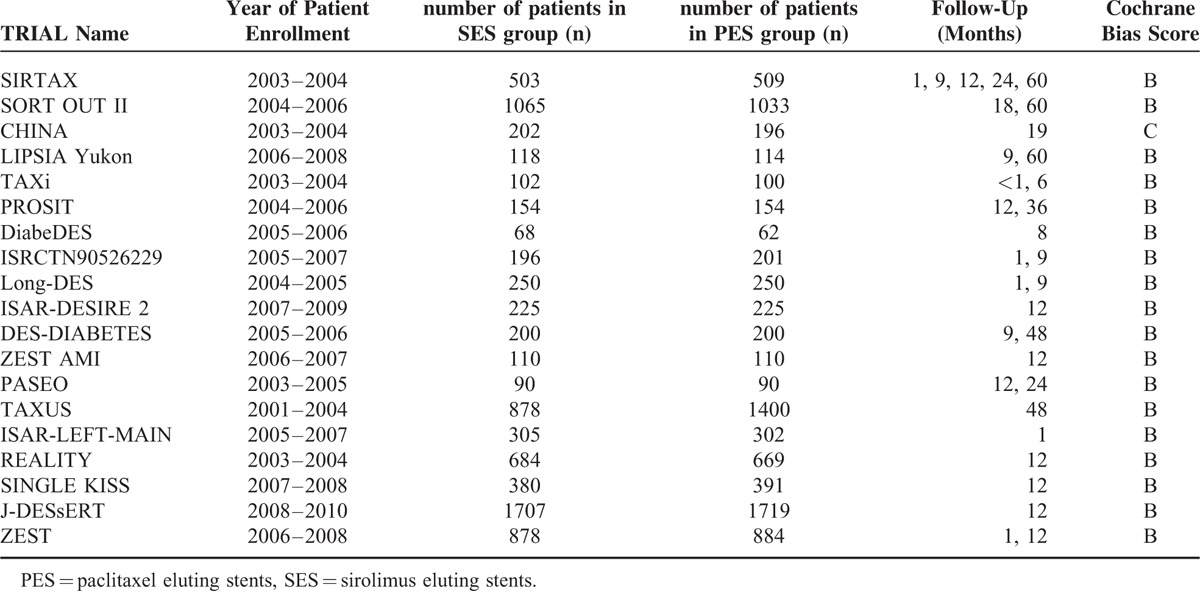
Table 3 reports the general features of all the 19 trials included in this meta-analysis. Features such as the number of participants involved in the SES group, number of participants involved in the PES group, the year of enrollment of patients for these trials, the follow-up periods as well as the Cochrane Bias Risk grade have been summarized in Table 3.
TABLE 3.
General Features of the Included Trials
Baseline Characteristics of the Included Trials
Table 4 reports the baseline features of each of the included studies. Data from each study have been reported. The mean age of the patients, the percentage of male patients, the percentage of patients with hypertension, those patients who are current smokers and the percentage of patients who suffer from type 2 diabetes mellitus have been listed in Table 4.
TABLE 4.
Baseline Features of the Included Studies
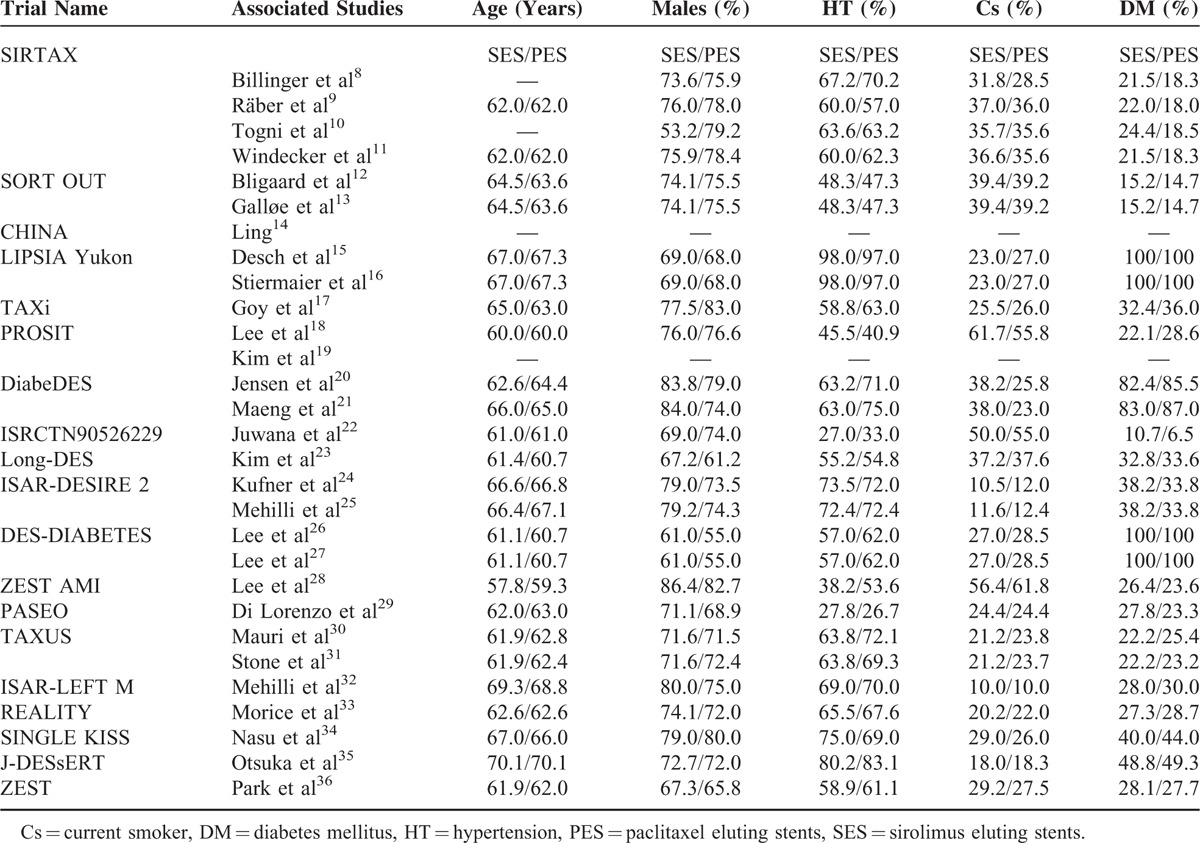
No significant difference in age has been observed in patients from both groups. Almost all the studies reported similar number of male patients. Percentage of patients suffering from hypertension, smoking, and diabetes mellitus was almost similar in both groups. Overall, no significant differences have been observed in the baseline characteristics between these 2 groups.
Baseline features for the studies14,19 have not been included since they were not made available by the authors in the original articles.
Main Results of This Meta-Analysis
The result for ST has been divided into several groups. Twelve thousand forty-five patients were analyzed for definite ST (5769 treated with SES and 6276 treated with PES), 11,415 patients were analyzed for probable ST (5454 treated with SES and 5961 treated with PES), 11,545 patients were analyzed for definite and probable/and or possible ST (5522 treated with SES and 6023 treated with PES), 6141 patients were analyzed for acute ST (3091 treated with SES and 3050 treated with PES), 6536 patients were analyzed for subacute ST (3289 treated with SES and 3247 treated with PES), 9931 patients were analyzed for early ST (4722 treated with SES and 5209 treated with PES), 8905 patients were analyzed for late ST (4204 treated with SES and 4696 treated with PES), 5788 patients were analyzed for very late ST (2646 treated with SES and 3142 treated with PES), and 16,724 patients were analyzed for any type of ST (8115 treated with SES and 8609 treated with PES).
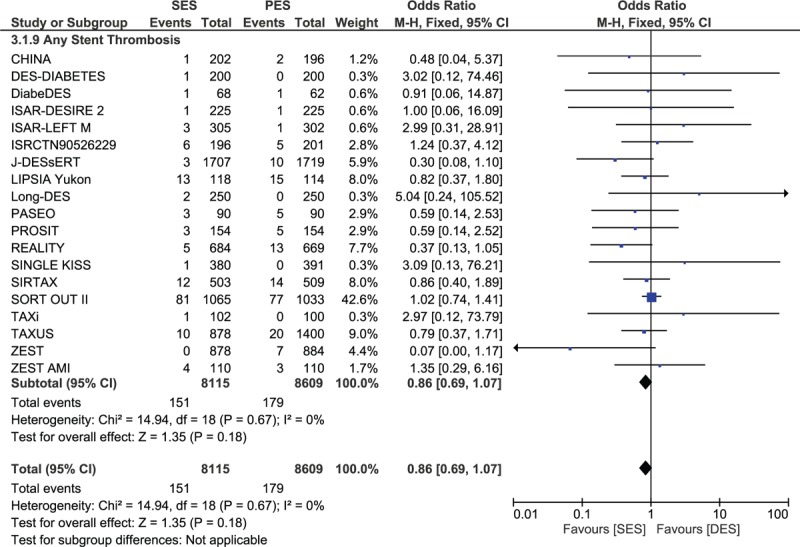
The pooled analysis showed no significant difference between SES and PES in any category of ST. Since a lower heterogeneity has been observed, a fixed effect model has been used for the analysis. The results were as follow: definite ST with OR: 0.87; 95% CI: 0.64–1.18, P = 0.36; probable ST with OR:0.72; 95% CI: 0.42–1.21, P = 0.21; definite, probable and/or possible ST with OR: 0.94; 95% CI: 0.75–1.17, P = 0.57; acute ST with OR: 0.99; 95% CI: 0.38–2.56, P = 0.98; subacute ST with OR: 0.72; 95% CI: 0.41–1.25, P = 0.25; early ST with OR: 0.81; 95% CI: 0.53–1.25, P = 0.34; late ST with OR: 0.72; 95% CI: 0.39–1.34, P = 0.30; very late ST with OR: 1.02; 95% CI: 0.72–1.44, P = 0.92; and any ST with OR: 0.86; 95% CI: 0.69–1.07, P = 0.18. Table 5 summarizes the main result of this study and Figure 2 A–C represents the detailed analysis of this meta-analysis.
TABLE 5.
Summary of the Main Results of This Study
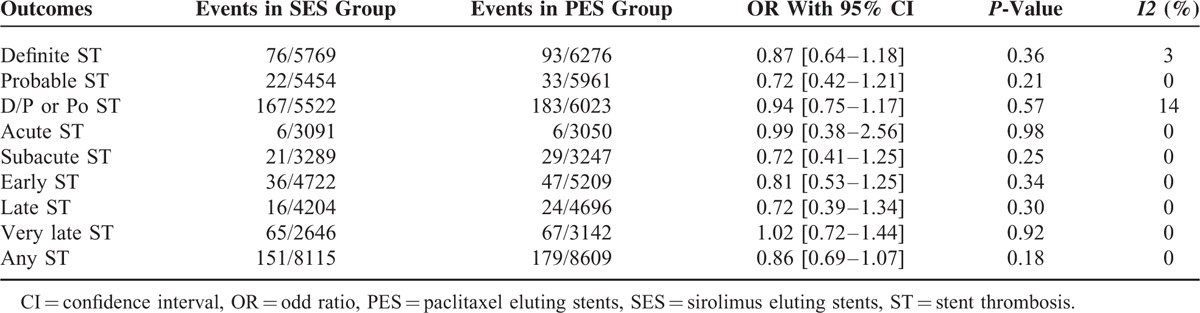
FIGURE 2.
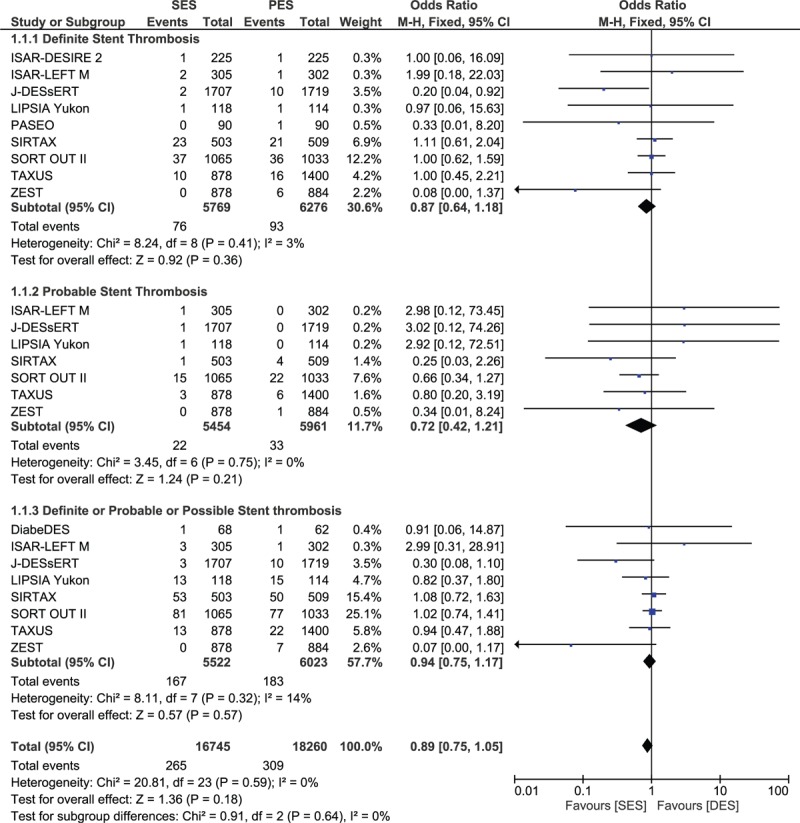
Forest plot comparing stent thrombosis between SES and PES.
Excluding the trials with a follow-up period of 1 year or less, another analysis was carried out considering trials with a follow-up period between 1 and 2 years, and between 1 and 5 years. However, even with these long-term follow-up periods, no significant differences in ST have been observed between SES and PES. Results were as follow: OR: 1.27; 95% CI: 0.83–1.94, P = 0.27 for a follow-up period between 1 and 2 years and OR: 0.93; 95% CI: 0.71–1.22, P = 0.60 for a follow-up period between 1 and 5 years. These results have been illustrated in Figure 3.
FIGURE 2 (Continued).

Forest plot comparing stent thrombosis between SES and PES.
FIGURE 3.
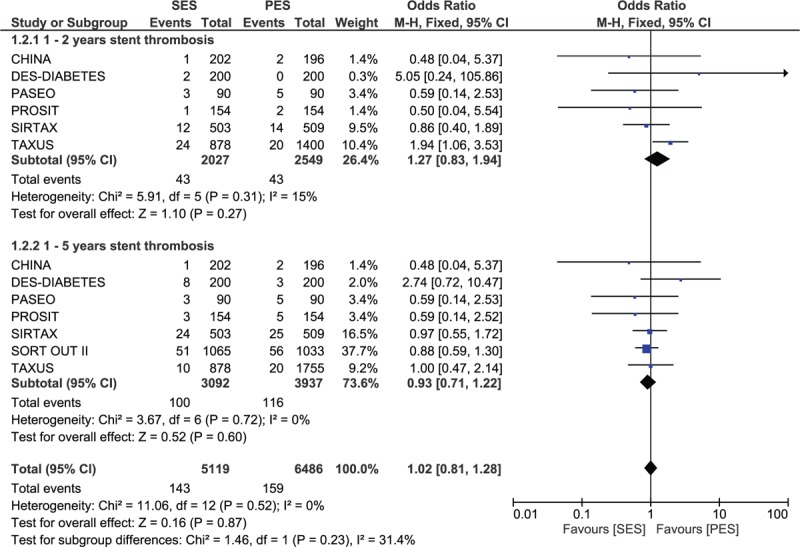
Forest plot comparing long-term (1–2 years and 1–5 years) stent thrombosis between SES and PES.
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plots, there has been no evidence of publication bias for the included studies that assessed these ST. The funnel plots have been illustrated in Figure 4A and B.
FIGURE 2 (Continued).
Forest plot comparing stent thrombosis between SES and PES.
FIGURE 4.
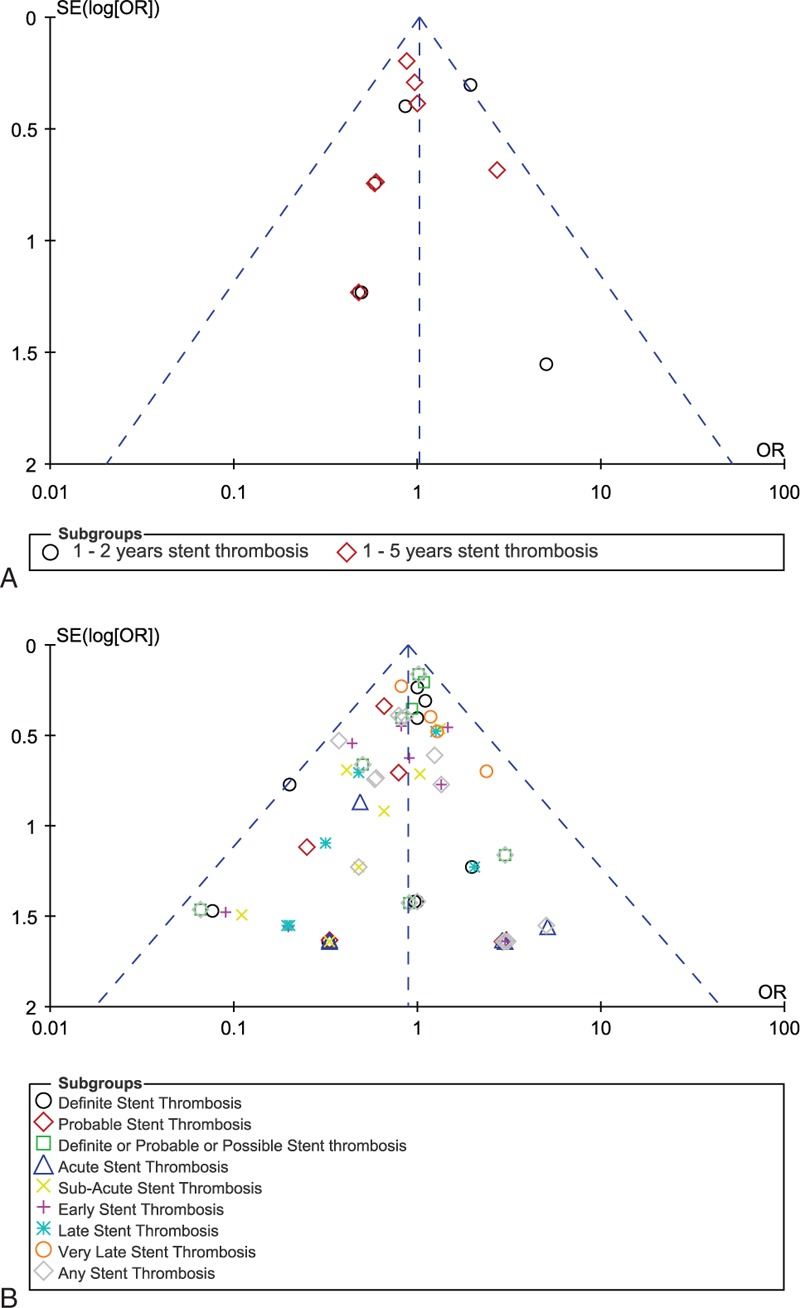
Funnel plots for sensitivity analyses.
DISCUSSION
Many recently published meta-analyses showed no differences in ST associated with SES and PES. However, the meta-analysis conducted by Schömig et al6 in 2007 surprisingly showed a significant reduction in ST with the use of SES compared to PES. Compared to many previous studies, his study included a larger number of randomized patients. Therefore, to confirm the existence or absence of any significant difference in ST between SES and PES, old and new studies were combined to conduct this meta-analysis.
Among the 48.5% patients treated with SES, and the 51.5% patients treated with PES, no significant difference in ST has been observed between these 2 groups. 1.9% of the patients in the SES group and 2.08% of the patients in the PES group suffered “any” ST (meaning any kind of ST). However, this result was not statistically significant. The result was still not statistically significant during a follow-up between 1 and 2 years as well as during a follow-up period between 1 and 5 years.
Despite of including a larger number of randomized patients in our study, our result was similar to the meta-analyses conducted by Gurm et al in 2008,1 Zhang et al in 2010 and 2014, Kufner et al in 2011 and Kastrati et al in 2005.2–5 These studies had a limited number of patients and 2 of these studies were conducted on patients with type 2 diabetes mellitus. Despite these differences, our study also showed similar results. Even many retrospective studies as well as the observational studies with a very large number of consecutive patients comparing SES with PES showed an equal rate of ST between these 2 groups.
In this current Era, which can be considered as a “winning” world for the DES, the use of bare-metal stents (BMS) is often only indicated when the use of DES is contraindicated. The use of DES clinically, has significantly lowered the incidence of several major adverse cardiovascular events as well as reduced the incidence of restenosis after PCI compared to the use of BMS. However, the use of DES is still associated with a higher rate of ST.
As shown in our results, and in the results of many other studies, the comparison of ST between the 2 first-generation DES has resulted in no significant difference in ST between SES and PES. However, it is a fact that most of the drugs which are currently approved to coat stents used in coronary angioplasty do not differentiate or simply do not recognize the difference between proliferative vascular smooth muscle cells (VSMCs) and endothelial cells (ECs).37 The specification of these antiproliferative drugs which is normally very important, is often lacking and therefore, the proliferation and migration of VSMCs and ECs in the vessel are affected. These antiproliferative drugs also cause the inhibition of neointimal hyperplasia and they also severely affect the endothelial regeneration which is very important for the healing of these injured vessels and finally resulting in an incomplete reendothelialization. Because of this insufficient reendothelialization, late/long-term ST often manifests.38–39
Moreover, p27 is a component expressed by arteries. When arteries are injured, p27 is rapidly downregulated due to mechanisms initiated by activated VSMC triggering intimal hyperplasia which then results in arterial restenosis. Overexpression of exogenous p27 in VSMC results in mechanisms that led to the significant reduction in neointimal formation. Studies have shown sirolimus, which is the coating material of certain DES, would inhibit the breakdown of p27, as well as induce the increased production of p27 thus preventing the proliferation and migration of VSMC and could therefore inhibit reendothelialization of vessels by this way.40–41 Also, overexpression of p27 without sparring ECs could prevent the normal physiological function of the injured blood vessels and this could further increase the risk of late ST among the several DES.
Even though the use of DES is associated with a higher rate of ST, our study showed a similar rate of ST between SES and PES. However, to further minimize ST in similar patients with CAD, other researches have introduce the microRNA-based strategy in coating stents.42 MicroRNA has been described previously.43 This microRNA-based strategy can strictly reduce restenosis by selectively inhibiting the proliferation and migration of VSMC without disturbing or inhibiting reendothelialization or causing impairment to the function of EC. Hence, incorporating the microRNA-based strategy in stents could increase the overexpression of p27 and at the same time protect ECs thus reducing ST in those patients treated with DES after PCI. Further studies should be conducted to confirm the effect of this microRNA-based strategy to make sure whether it truly protects ECs.42 This will be a revolution in the field of Interventional Cardiology.
Our results showed SES and PES to have a similar rate of ST even during the long-term follow-up. However, a few studies also showed results which were different from our study. For example, the meta-analysis including 16 RCTs comparing SES with PES in patients with CAD published by Schömig et al6 in 2007 showed a completely different result from ours. His study surprisingly showed ST to be significantly lower in the SES group compared to the PES group. However, in his study, ST was not reported according to the Academic Research Consortium (ARC) criteria which defined ST as definite and probable ST. Also, data which the author had used in his meta-analysis were completely different from that of our study because majority of his data were directly obtained from unpublished articles whose abstracts were represented and discussed in international meetings or from principal investigators. In our study, ST was reported according to ARC and we did not include data from unpublished articles.
The study by Bangalore et al44 also showed different results from ours. His study which compared the effectiveness and safety between BMS and DES, performed a mixed treatment comparison meta-analysis (MTC meta-analysis) commonly known as a network meta-analysis. “An MTC meta-analysis is slightly different in the way that it is an extension that allows data to be combined and compared directly and indirectly,” which, are supposedly not considered as randomized data, but are “observational findings across trials, which might result in more bias, for example due to confounding,” even if they included high-quality RCTs.7 However, in our study, all data were strictly obtained directly from RCTs.
Even if there is no novelty in our idea, our study satisfies all the requirements for a meta-analysis, in terms of low heterogeneity, absent publication bias, and sensitivity analysis, and provides robust scientific validity to our findings. Hence, it is believed to show better results.
LIMITATIONS
This study also has several limitations. First of all, as a general consideration, authors always consider their studies to have a limited number of patients. Hence, even if our study included more than 16,000 patients, it is always a fact that due to a small population of patients compared to other studies, this could have an effect on our results too. Most of the RCTs had a follow-up period of at least 1 year; however, several other RCTs had a follow-up period of less or more than 1 year. Therefore, combing different follow-up periods that are almost of the same length, but not exactly the same, could affect the results in one way or the other. Moreover, our study included patients from different categories of diseases. For example, a few studies were conducted on the general population suffering from CAD; however, other studies were conducted on patients with long coronary lesions, ST elevated myocardial infarction or on patients suffering from type 2 diabetes mellitus. These patients have other abnormalities, for example patients with type 2 diabetes mellitus have platelet dysfunction which expose them to a higher possibility of ST after PCI. So, this could also be a limitation in our study.
CONCLUSION
Similar to many meta-analyses and RCTs, no significant difference in ST has been observed between SES and PES. Hence, both SES and PES are expected to be equally effective.
Footnotes
Abbreviations: CAD = coronary artery disease, DES = drug eluting stents, PES = paclitaxel eluting stents, SES = sirolimus eluting stents, ST = stent thrombosis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Gurm HS, Boyden T, Welch KB. Comparative safety and efficacy of a sirolimus-eluting versus paclitaxel-eluting stent: a meta-analysis. Am Heart J 2008; 155:630–639. [DOI] [PubMed] [Google Scholar]
- 2.Kufner S, de Waha A, Tomai F, et al. A meta-analysis of specifically designed randomized trials of sirolimus-eluting versus paclitaxel-eluting stents in diabetic patients with coronary artery disease. Am Heart J 2011; 162:740–747. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Dong L, Ge J. Meta analysis of five randomized clinical trials comparing sirolimus- versus paclitaxel-eluting stents in patients with diabetes mellitus. Am J Cardiol 2010; 105:64–68. [DOI] [PubMed] [Google Scholar]
- 4.Kastrati A, Dibra A, Eberle S, et al. Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA 2005; 294:819–825. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Xie J, Li G, et al. Head-to-head comparison of sirolimus-eluting stents versus paclitaxel-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of 76 studies. PLoS ONE 2014; 9:e97934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schömig A, Dibra A, Windecker S, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol 2007; 50:1373–1380. [DOI] [PubMed] [Google Scholar]
- 7.Wiley, Higgins JPT, Altman DG. Higgins JPT, Green S. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Vol. 1 2008; 187–241. [Google Scholar]
- 8.Billinger M, Beutler J, Taghetchian KR, et al. Two-year clinical outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents in diabetic patients. Eur Heart J 2008; 29:718–725. [DOI] [PubMed] [Google Scholar]
- 9.Räber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation 2011; 123:2819–2828.6 p following 2828. [DOI] [PubMed] [Google Scholar]
- 10.Togni M, Eber S, Widmer J, et al. Impact of vessel size on outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents: a subgroup analysis of the SIRTAX trial. J Am Coll Cardiol 2007; 50:1123–1131. [DOI] [PubMed] [Google Scholar]
- 11.Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel eluting stents for coronary revascularization. N Engl J Med 2005; 353:653–662. [DOI] [PubMed] [Google Scholar]
- 12.Bligaard N, Thuesen L, Saunamäki K, et al. Similar five-year outcome with paclitaxel- and sirolimus-eluting coronary stents. Scand Cardiovasc J 2014; 48:148–155. [DOI] [PubMed] [Google Scholar]
- 13.Galløe AM, Thuesen L, Kelbaek H, et al. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA 2008; 299:409–416. [DOI] [PubMed] [Google Scholar]
- 14.Han YL, Wang XZ, Jing QM, et al. Comparison of Rapamycin and Paclitaxel eluting stent in patients with multi-vessel coronary disease. Zhonghua Xin Xue Guan Bing Za Zhi 2006; 34:123–126. [PubMed] [Google Scholar]
- 15.Desch S, Schloma D, Möbius-Winkler S, et al. Randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus: the LIPSIA Yukon trial. JACC Cardiovasc Interv 2011; 4:452–459. [DOI] [PubMed] [Google Scholar]
- 16.Stiermaier T, Heinz A, Schloma D, et al. Five-year clinical follow-up of a randomised comparison of a polymer-free sirolimus-eluting stent versus apolymer-based paclitaxel-eluting stent in patients with diabetes mellitus (LIPSIA Yukon trial). Catheter Cardiovasc Interv 2014; 83:418–424. [DOI] [PubMed] [Google Scholar]
- 17.Goy JJ, Stauffer JC, Siegenthaler M, et al. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J Am Coll Cardiol 2005; 45:308–311. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kim HS, Lee SW, et al. Prospective randomized comparison of sirolimus- versus paclitaxel-eluting stents for the treatment of acute ST-elevation myocardial infarction: pROSIT trial. Catheter Cardiovasc Interv 2008; 72:25–32. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Lee JH, Lee SW, et al. Long-term safety and efficacy of sirolimus- vs. paclitaxel-eluting stent implantation for acute ST-elevation myocardial infarction: 3-year follow-up of the PROSIT trial. Int J Cardiol 2011; 147:253–257. [DOI] [PubMed] [Google Scholar]
- 20.Jensen LO, Maeng M, Thayssen P, et al. Neointimal hyperplasia after sirolimus-eluting and paclitaxel-eluting stent implantation in diabetic patients: the Randomized Diabetes and Drug-Eluting Stent (DiabeDES) Intravascular Ultrasound Trial. Eur Heart J 2008; 29:2733–2741. [DOI] [PubMed] [Google Scholar]
- 21.Maeng M, Jensen LO, Galloe AM, et al. Comparison of the sirolimus-eluting versus paclitaxel-eluting coronary stent in patients with diabetes mellitus: the diabetes and drug-eluting stent (DiabeDES) randomized angiography trial. Am J Cardiol 2009; 103:345–349. [DOI] [PubMed] [Google Scholar]
- 22.Juwana YB, Suryapranata H, Ottervanger JP, et al. Comparison of rapamycin- and paclitaxel-eluting stents in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2009; 104:205–209. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Park SW, Lee SW, et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation 2006; 114:2148–2153. [DOI] [PubMed] [Google Scholar]
- 24.Kufner S, Byrne RA, de Waha A, et al. Sirolimus-eluting versus paclitaxel-eluting stents in diabetic and non-diabetic patients within sirolimus-eluting stent restenosis: results from the ISAR-DESIRE 2 trial. Cardiovasc Revasc Med 2014; 15:69–75. [DOI] [PubMed] [Google Scholar]
- 25.Mehilli J, Byrne RA, Tiroch K, et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol 2010; 55:2710–2716. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus Paclitaxel-eluting stent implantation in patients with diabetes mellitus. J Am Coll Cardiol 2008; 52:727–733. [DOI] [PubMed] [Google Scholar]
- 27.Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus: 4-year clinical outcomes of DES-DIABETES (drug-eluting stent in patients with DIABETES mellitus) trial. JACC Cardiovasc Interv 2011; 4:310–316. [DOI] [PubMed] [Google Scholar]
- 28.Lee SW, Park SW, Kim YH, et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus 2-year clinical outcomes of the DES-DIABETES trial. J Am Coll Cardiol 2009; 53:812–813. [DOI] [PubMed] [Google Scholar]
- 29.Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) Randomized Trial. JACC Cardiovasc Interv 2009; 2:515–523. [DOI] [PubMed] [Google Scholar]
- 30.Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029. [DOI] [PubMed] [Google Scholar]
- 31.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008. [DOI] [PubMed] [Google Scholar]
- 32.Mehilli J, Kastrati A, Byrne RA, et al. Paclitaxel- versus sirolimus-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol 2009; 53:1760–1768. [DOI] [PubMed] [Google Scholar]
- 33.Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA 2006; 295:895–904. [DOI] [PubMed] [Google Scholar]
- 34.Nasu K, Oikawa Y, Yoshikawa R, et al. A randomized comparison of sirolimus- vs. paclitaxel-eluting stents for treatment of bifurcation lesions by single stent and kissing balloon: results of the SINGLE KISS trial. Int J Cardiol 2013; 166:187–192. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka M, Yokoi H, Matsuyama Y, et al. Comparison of sirolimus- and paclitaxel-eluting stents in patients with moderate renal insufficiency: results from the J-DESsERT trial. Cardiovasc Revasc Med 2014; 15:323–328. [DOI] [PubMed] [Google Scholar]
- 36.Park DW, Kim YH, Yun SC, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol 2010; 56:1187–1195. [DOI] [PubMed] [Google Scholar]
- 37.Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 2012; 5:12–20. [DOI] [PubMed] [Google Scholar]
- 38.Liu HT, Li F, Wang WY, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells. Tex Heart Inst J 2010; 37:194–201. [PMC free article] [PubMed] [Google Scholar]
- 39.Cassese S, Kastrati A. New-generation drug-eluting stents for patients with myocardial infarction. JAMA 2012; 308:814–815. [DOI] [PubMed] [Google Scholar]
- 40.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 2011; 4:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Marx SO, Chen HJ, et al. Role for p27 (Kip1) in vascular smooth muscle cell migration. Circulation 2001; 103:2967–2972. [DOI] [PubMed] [Google Scholar]
- 42.Santulli G, Wronska A, Uryu K, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest 2014; 124:4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santulli G, Totary-Jain H. Tailoring mTOR-based therapy: molecular evidence and clinical challenges. Pharmacogenomics 2013; 14:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012; 125:2873–2891. [DOI] [PubMed] [Google Scholar]


