Supplemental Digital Content is available in the text
Abstract
Cytokine-induced killer (CIK) cell therapy has recently been used as an adjuvant setting following resection of hepatocellular carcinoma (HCC), while its benefit remains unclear. This study aimed to evaluate the efficacy of adjuvant CIK application in solitary HCC patients undergoing curative resection with stratification of microvascular invasion (MVI).
In total, specimens and data from 307 solitary HCC patients undergoing curative resection between January 2007 and December 2010 were included. Of these, 102 patients received CIK treatment after surgery (CIK group), whereas 205 patients did not (control group). Pathological evaluation was used to retrospectively determine MVI status. The CIK group had 60 MVI-negative and 42 MVI-positive patients, while the numbers in control group were 124 and 81. Kaplan-Meier and Cox regression analyses were used to validate possible effects of CIK treatment on disease free survival (DFS) and overall survival (OS) as appropriate.
For all patients, the CIK group exhibited significantly higher OS than the control group (log-rank test; PDFS = 0.055, POS = 0.020). Further analysis based on MVI stratification showed that for patients with MVI, DFS and OS did not differ between the 2 groups (PDFS = 0.439, POS = 0.374). For patients without MVI, the CIK group exhibited better DFS and OS than the control group (PDFS = 0.042, POS = 0.007), and multivariate analyses demonstrated that CIK treatment was an independent prognostic factor both for DFS and OS.
For solitary HCC, CIK cell therapy after curative resection improves DFS and OS for patients without MVI, but has no statistically significant survival benefit for patients with MVI.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the third highest cause of cancer-related death.1 To date, liver resection is considered to be the most valuable therapeutic approach for HCC;2 however, HCC recurs frequently after resection3 and is also notoriously insensitive to chemotherapy and radiotherapy.4 Recent work has suggested that immunosuppression in HCC patients is an important contributor to the high rates of recurrence and metastasis in HCC.5–8 Therefore, cytokine-induced killer (CIK) cell immunotherapy has been applied recently as a new strategy to improve the prognosis of HCC patients.
Briefly, CIK cells are a type of histocompatibility complex-unrestricted, anti-tumor T cell characterized by the co-expression of the T-cell marker, CD3, and the natural killer (NK) cell marker, CD56. CIKs can be easily generated by expanding human peripheral blood mononuclear cells (PBMCs) in vitro, in the presence of anti-CD3 antibody, interleukin (IL)-2, IL-1, and interferon-γ.9,10 Compared with lymphokine-activated killer cells and NK cells, CIK cells have higher proliferation rates and stronger cytolytic activities,11,12 while also imparting minimal damage to normal cells and the host immune system.13,14 Recently, autologous CIK cells also have been found to have a highly efficient antitumor cytotoxic activity against HCC cells in vitro and in a murine model.15
To date, the efficacy of CIK cell therapy combined with conventional HCC approaches has been investigated in some clinical studies.16–22 Some studies indicated that CIK cell transfusion decreased HCC recurrence after radiofrequency ablation therapy (RFA) and improved overall survival (OS) and progression-free survival after transarterial chemoembolization (TACE) and sequential therapy of TACE and RFA.16–18 In addition, our latest randomized controlled trial (RCT) also demonstrated that adjuvant CIK therapy could prolong the median time to recurrence in HCC patients after curative resection.19 Meanwhile, other studies also suggested that CIK transfusion may be beneficial after curative resection.20–22 However, we noted the results of these surgery-related CIK studies were not conclusive. In the studies of Takayama et al20 and Hui et al,21 CIK cell therapy could lower the recurrence rate and increase disease-free survival (DFS) of postsurgical HCC patients, but failed to affect OS. While the study of Pan et al showed that CIK cell therapy improved OS, but did not report on DFS.22 Another limitation of the studies by Hui et al21 and Pan et al 22 is that their inclusion criteria did not allow for distinction between microvascular-invasion (MVI)-positive and -negative patients, though the cohorts consisted exclusively of solitary HCC patients. As is known that MVI is an independent predictor of prognosis in HCC patients after curative resection,23 but most CIK-related studies involving HCC patients have not taken this important variable into consideration. Therefore, we hypothesized that analyzing the efficacy of CIK therapy without incorporating stratification of MVI status may impart bias and lead to improper conclusions. Thus, here, we conducted a retrospective study to evaluate the efficacy of CIK cell therapy comprehensively for MVI-positive versus MVI-negative, solitary HCC patients after curative resection.
METHODS
Patient Cohort and Selection Criteria
From January 2007 to December 2010, a total of 1533 patients received hepatic resection at Sun Yat-sen University Cancer Center (SYSUCC). From this population pool, 307 eligible patients were selected based on the following inclusion criteria: (1) pathologically confirmed HCC after surgery, (2) hepatic resection as the only primary treatment, (3) confirmation of a solitary tumor by preoperative imaging (computed tomography [CT] scan or magnetic resonance imaging [MRI]) and intraoperative ultrasound, (4) curative resection (with microscopically free resection margin >1 cm), (5) UICC TNM stage T1N0M0 or T2N0M0 (Union for International Cancer Control, 7th version); (6) adequate liver function (Child-Pugh class A), (7) no tumor fracture and hemorrhage before and during resection, and (8) no preoperative blood transfusion.
Our exclusion criteria eliminated patients who had (1) any other anticancer treatment before surgery, (2) postoperative anticancer treatment before recurrence (except for CIK transfusion), (3) tumor stage beyond T2N0M0, (4) Child-Pugh class B or C, (5) the presence of previous or simultaneous malignant diseases, (6) severe complications or severe adverse events after surgery within 1 month, to rule out possible nonspecific influences caused by the surgical procedure, and (7) postoperative death/recurrence and follow-up ≤3 months.
Curative resection for all enrolled patients was performed by the same group of experienced surgeons. After surgery, 102 patients commenced CIK cell transfusion within 1 month (CIK group), whereas the other 205 patients never received the therapy (control group). Before accepting transfusion, all of the cohort patients were fully informed as to the nature and the costs of CIK therapy, and then they chose to receive or refuse this adjuvant therapy entirely voluntarily without any specific selection criteria. Worrying about the possible side effects, doubting the therapeutic efficacy, and financial difficulty were the 3 main reasons for their refusals of CIK treatment in control group. Informed consent was obtained from all of the enrolled patients. The study protocol was approved by the institutional ethics committee of SYSUCC and in accord with the principles of the Declaration of Helsinki.
Preparation and Treatment of CIK Cells
CIK cells were prepared as described in our previous studies.18,22 Briefly, 2 weeks after surgery, 50 mL of peripheral blood was collected from each patient in the CIK group. Then, peripheral blood mononuclear cells from the blood sample were incubated with 1000 U/mL interferon-γ (ShangClone, Shanghai, China) under appropriate humidity with 5% CO2 in the atmosphere and at 37°C. The cell density in the medium was maintained at 2 × 106 cells/mL. After 24 hours, 100 ng/mL of mouse antihuman CD3 monoclonal antibody (R&D Systems, Shanghai, China), 100 U/mL of recombinant human IL-1a (Life Technologies, Guangzhou, China), and 1000 U/mL of recombinant human IL-2 (rhIL-2; Beijing Sihuan, Beijing, China) were added to the medium. After another 14 days, the CIK cell population was collected, which then contained >70% CD3+ cells, >30% CD3+/CD56+ cells, and >40% CD8+ cells calculated by manual hemacytometer cell counts. During the 14-day incubation, CIK cells were monitored 3 times for viability and presence of contamination. Lastly, before being transferred into the patient, CIK cells were washed and resuspended 3 times with normal saline. All of the preparation and administration of CIK cells was performed in the State Key Laboratory of Oncology in South China. 4 weeks after resection, patients underwent their first cycle of CIK transfusion, during which 1.0 to 1.5 × 1010 CIK cells were intravenously transfused back into the patient. Each patient in the CIK group received at least 4 cycles of transfusion with an interval of 2 weeks.
Follow-Up
Each patient in the cohort received regular examinations at our outpatient department or follow-up center every 2 to 3 months for the initial 2 years of post surgery. Thereafter, every patient was followed over an interval of every 3 to 4 months from the third year until December 31, 2014 (the date of study conclusion). The median follow-up time of the entire cohort was 57.4 months (4.5–95.9 months). The median follow-up time of CIK group and control group were 57.1 months (13.2–95.9 months) and 57.2 months (4.5–95.9 months), respectively. No patient dropped out from either group. At each examination, information was collected with respect to serum liver tests, alpha-fetoprotein tumor marker, chest X-ray, abdominal ultrasonography, and CT/MRI scan. When recurrence/metastasis was suspected, hepatic angiography, positron emission tomography-computed tomography, or ultrasound-guided biopsy methods were employed to confirm the diagnosis, if necessary. For patients who suffered tumor recurrence or metastasis, a second resection or locoregional therapy (TACE, RFA, PMCT and PEI) was performed as appropriate according to the specific situation of each individual and determined by a multidisciplinary team in our center (which consisted of surgeons, immunologists, and oncologists). All of the patients undergoing recurrence were treated equally, which indicates that no more treatment priority and special attention were given to the CIK group patients.
Confirmation of MVI
All hematoxylin and eosin-stained sections from eligible patients’ tumor specimens were retrospectively collected from the Pathology Department of SYSUCC. To determine each patient's MVI status, 2 professional pathologists from our center independently evaluated each hematoxylin and eosin section. If there was a discrepancy in the status for a given patient, the pathologists would revisit the specimens and come to a consensus. Of patients with confirmed MVI, 42 were in the CIK group and 81 were in the control group. Of patients that were MVI-negative, 60 were in the CIK group and 124 were in the control group.
Lymphocyte Subset Distribution Characterization
Peripheral blood was collected from each patient 1 week before resection to test for the distribution of their lymphocyte subsets. The monoclonal antibodies used (all from BD Biosciences) for flow cytometry (Beckman, FC500) were anti-CD3-cytochrome, anti-CD4-FITC, anti-CD8-FITC, anti-CD19-FITC, anti-CD25-PE, and anti-CD56-PE. The ratio of each lymphocyte subset was computed based on the cell density of each gate. The results were incorporated with each patient's Immunophenotyping Report from the Flow Cytometry Laboratory of SYSUCC. Because of the retrospective nature of this study, not all patients underwent this preoperative test. By reviewing the medical records from our computerized database, we were able to identify a total of 116 patients, including 71 MVI-negative patients (41 in the CIK group and 30 in the control group) and 45 MVI-positive patients (27 in the CIK group and 18 in the control group) who received the preoperative flow cytometry characterization.
Statistical Analysis
For comparison between the 2 groups, Student t test, the Mann–Whitney U test, and the χ2 test were used as appropriate. The rates of DFS and OS were calculated by the Kaplan-Meier method, and the log-rank test was used to identify significance. The Cox proportional regression hazards model was used in the univariate analysis to investigate the correlation of each variable with DFS and OS. All variables with P < 0.05 were subsequently subjected to the multivariate Cox regression model to determine the hazards ratios and the independence of effects. In addition, to better control the potential confounding effect, the variables that were not statistically comparable between the 2 groups would also be got included in the multivariate analysis model as a further correction. A P value (2-tailed) <0.05 was considered statistically significant. SPSS IBM 20.0 (SPSS Inc, Chicago, IL) was used to analyze all data.
RESULTS
General Description
In total, samples and data from 307 eligible solitary HCC patients undergoing curative resection were included in this study. Of these patients, 268 had hepatitis B virus (HBV)-related HCC, 11 had HCV (hepatitis C virus) infection, and 4 had co-infection of HBV combined HCV; for the other 24 patients with no hepatitis background, chronic alcohol abuse, HCC family history, and dietary exposure to the fungal hepatocarcinogen aflatoxin B1 were the main causes of HCC. Baseline characteristics between the 2 groups are presented in Table 1. Variables including sex, HBsAg, tumor size, pathological grade (histologically classified into well, moderate, and poor differentiation based on the Edmondson classification24), degree of tumor encapsulation, MVI, situation of liver cirrhosis (by imaging check and histologic detection of nontumorous liver tissue in resected specimens), alpha-fetoprotein, alanine aminotransferase, aspartate aminotransaminase, serum albumin, serum total bilirubin, and prothrombin time were statistically similar between the 2 groups (P > 0.05). For age, it was statistically comparable between the 2 groups when divided into 5 consecutive intervals. During the follow-up, 45.0% (138/307) and 24.4% (75/307) of the patients suffered recurrence and death, respectively. The 1-, 3-, and 5-year DFS rate for the study cohort were 82.3%, 61.0%, and 54.4%, respectively, whereas the respective OS rates were 96.1%, 84.3%, and 76.3%, respectively (Figure 1).
TABLE 1.
Baseline characteristics of study cohort
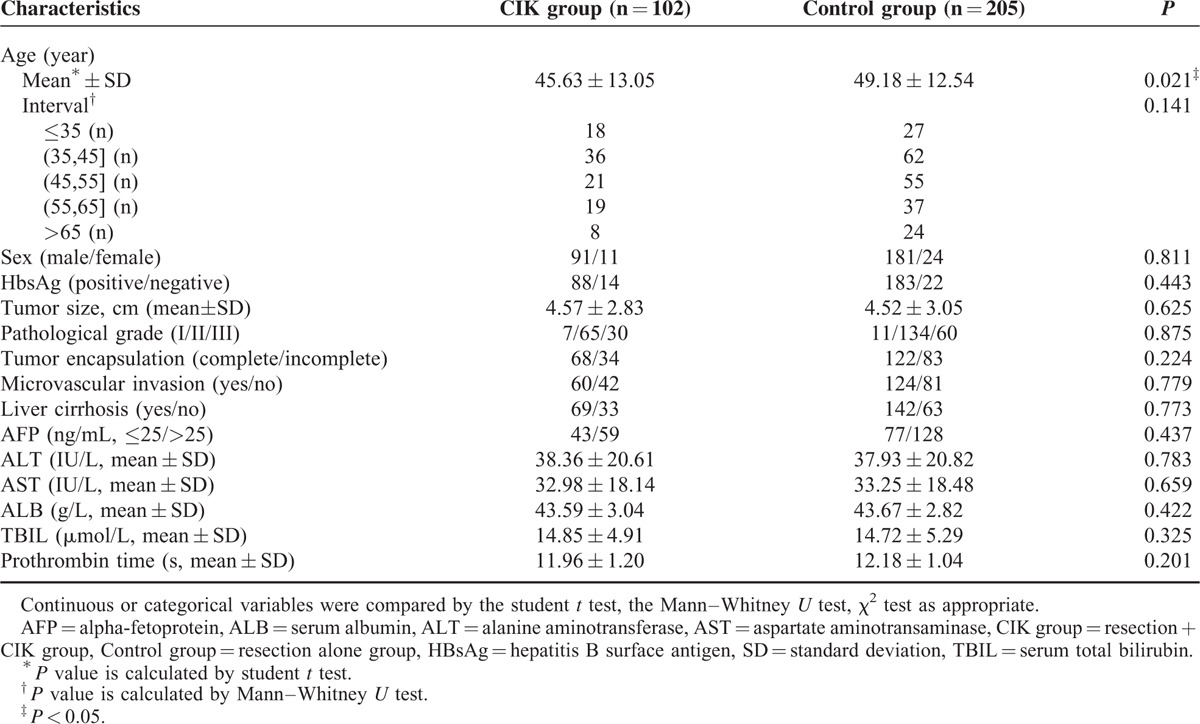
FIGURE 1.
Kaplan-Meier survival curves for DFS and OS in all cohort patients (n = 307). DFS = disease-free survival, OS = overall survival.
Adverse Effects of CIK Cell Therapy
All of the toxicity evaluation were prospectively collected from and defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). During and after each course of CIK cell transfusion, no serious adverse effects (infection, allergy, pulmonary, renal symptoms, hepatic function failure, or autoimmune disorder) were noted. Only 7 patients were reported to represent self-limiting shivering (grade 1 or 2) or light fever (no more than 38.5°C). In the subsequent 13.2 to 95.9 months of follow-up, no long-term side effects were reported.
Analysis of Disease-Free Survival and Overall Survival
Entire Patient Cohort
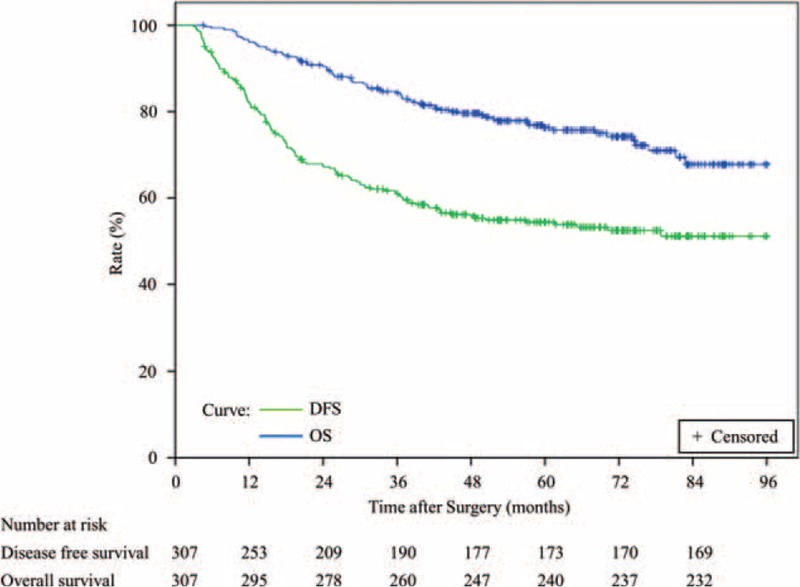
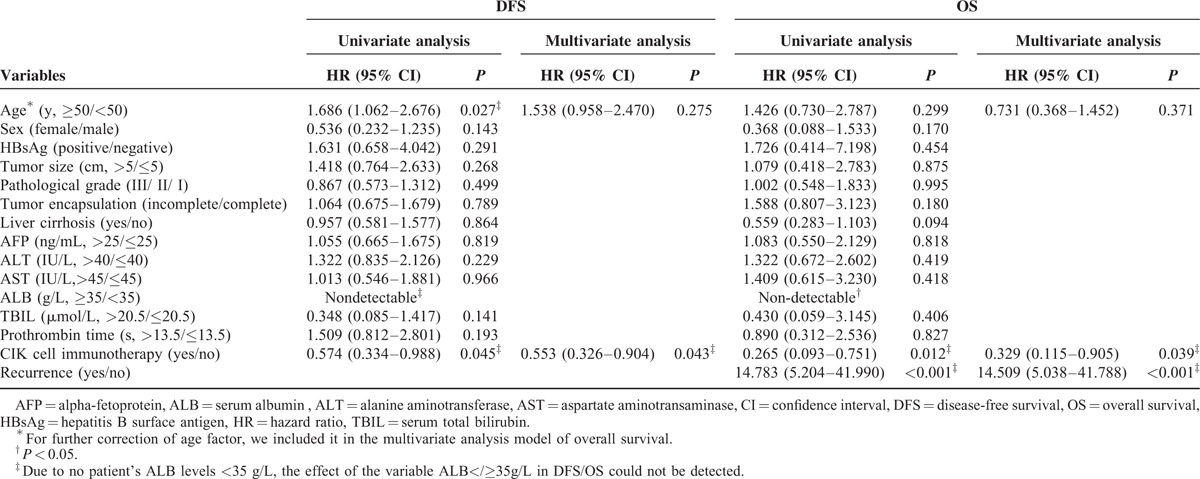
The 1-, 3-, and 5-year DFS rates were 85.3%, 68.2%, and 60.4%, respectively, in the CIK group (n = 102) and 79.8%, 57.3%, and 50.5%, respectively, in the control group (n = 205) (Figure 2a). A log-rank analysis showed marginal significant differences in DFS between the 2 groups (P = 0.055) and a univariate analysis revealed that 2 of the 15 variables were significant prognostic factors for DFS (Table 2). Multivariate analysis showed that tumor size was an independent risk factor for DFS (Table 2). In addition, the 1-, 3-, and 5-year OS rates were 99.0%, 93.0%, and 84.3%, respectively, in the CIK group and 94.1%, 79.4%, and 71.2%, respectively, in the control group (Figure 2b). A log-rank analysis showed that the CIK group had a significantly higher OS than the control group (P = 0.020), and a univariate analysis demonstrated that 5 of 16 variables were significant prognostic factors for OS (Table 2). Multivariate analysis showed that MVI status, the delivery of CIK cell immunotherapy, and recurrence situation were independent prognostic factors for OS (Table 2).
FIGURE 2.
DFS and OS Kaplan-Meier analysis of solitary HCC patients treated with curative resection and CIK cell therapy (CIK group, n = 102) or curative resection alone (control group, n = 205). (A) DFS curves for CIK versus control groups (P = 0.055). (B) OS curves for CIK versus control groups (P = 0.020). CIK = cytokine-induced killer, DFS = disease-free survival, HCC = hepatocellular carcinoma, OS = overall survival.
TABLE 2.
Clinicopathological variables associated with DFS and OS in all cohort patients identified by univariate analysis and multivariate analysis

Further Analysis Based on MVI Stratification
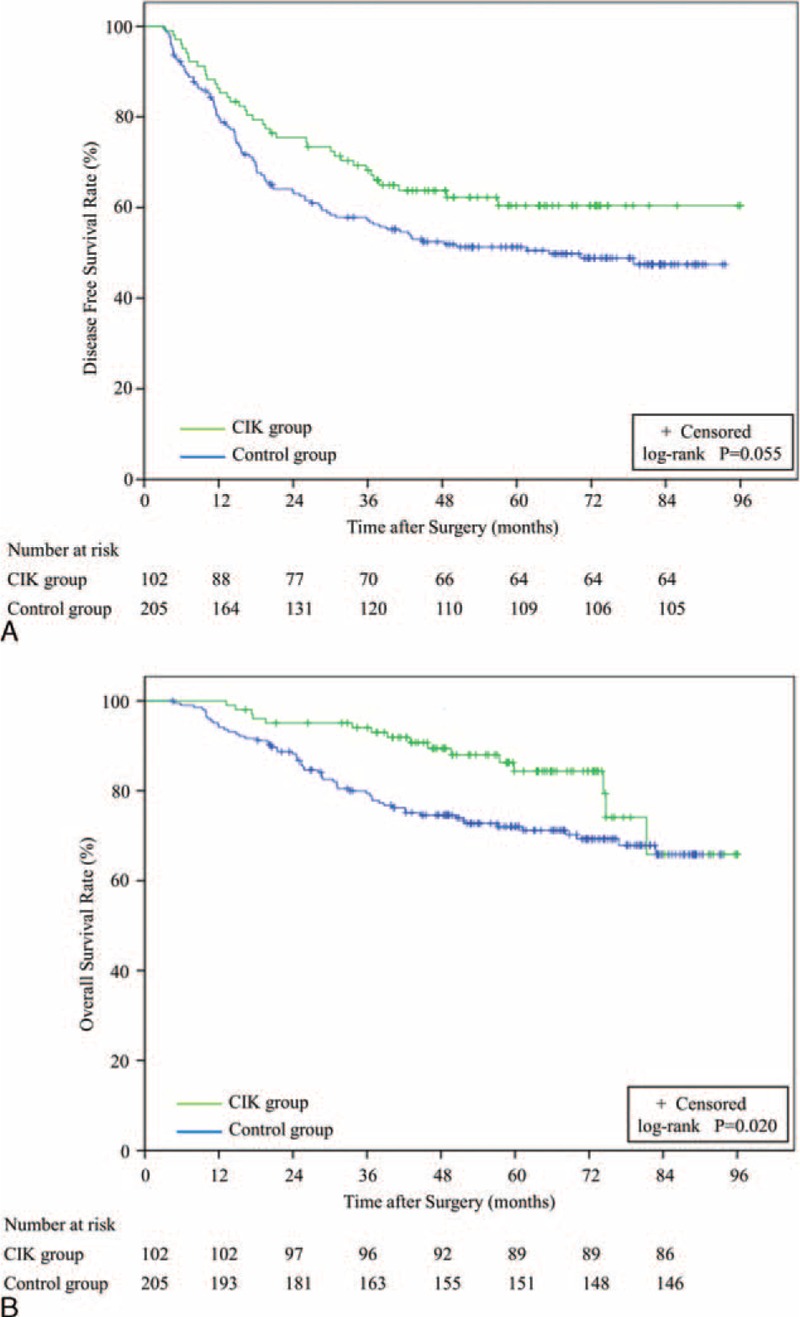
The 1-, 3-, and 5-year DFS rates for MVI-positive patients were 81.0%, 54.5%, and 45.8%, respectively, in the CIK group (n = 42), and 74.8%, 47.9%, and 42.5%, respectively, in the control group (n = 81) (Figure 3a). The 1-, 3-, and 5-year OS rates for MVI-positive patients were 97.6%, 85.3%, and 72.8%, respectively, in the CIK group, and 90.0%, 68.6%, and 64.1%, respectively, in the control group (Figure 3b). A log-rank analysis showed that for MVI-positive patients, the CIK group did not have significantly higher DFS and OS rates than the control group (PDFS = 0.439, POS = 0.374). All variables between the 2 groups were statistically comparable (see Table 1 supplemental content, which illustrates the baseline characteristics of MVI-positive cohort patients).
FIGURE 3.
DFS and OS Kaplan-Meier analysis of solitary HCC patients treated with curative resection and CIK therapy (CIK group) or curative resection alone (control group) and further grouped by MVI status. The survival of MVI-positive patients in the CIK group (n = 42) and the control group (n = 81) was analyzed according to (A) DFS curves (P = 0.439), or (B) OS curves (P = 0.374). The survival of MVI-negative patients in the CIK group (n = 60) and the control group (n = 124) was analyzed according to (C) DFS curves (P = 0.042), or (D) OS curves (P = 0.007). CIK = cytokine-induced killer, DFS = disease-free survival, HCC = hepatocellular carcinoma, MVI = microvascular invasion, OS = overall survival.
Conversely, the 1-, 3-, and 5-year DFS rates for MVI-negative patients were 88.3%, 76.0%, and 70.2%, respectively, in the CIK group (n = 60), and 83.0%, 62.4%, and 54.1%, respectively, in the control group (n = 124) (Figure 3c). The 1-, 3-, and 5-year OS rates were 98.3%, 96.4%, and 91.6%, respectively, in the CIK group and 96.8%, 85.3%, and 75.7%, respectively, in the control group (Figure 3d). A log-rank analysis showed there was a significant difference, both in DFS and OS, between MVI-negative patients in either group (PDFS = 0.042, POS = 0.007). Univariate analysis showed that 2 of 14 variables and 2 of 15 variables were calculated as significant prognostic factors for DFS and OS, respectively (Table 3). Multivariate analysis showed that CIK cell immunotherapy was identified as an independent prognostic factor for both DFS and OS. In addition, recurrence situation was also identified as an independent prognostic factor for OS (Table 3). All variables between the 2 groups were statistically comparable (for age, it was statistically comparable between the 2 groups when divided into 5 consecutive intervals; see Table 2 supplemental content, which illustrates the baseline characteristics of MVI-negative cohort patients).
TABLE 3.
Clinicopathological variables associated with DFS and OS in microvascular-invasive-negative cohort patients identified by univariate analysis and multivariate analysis
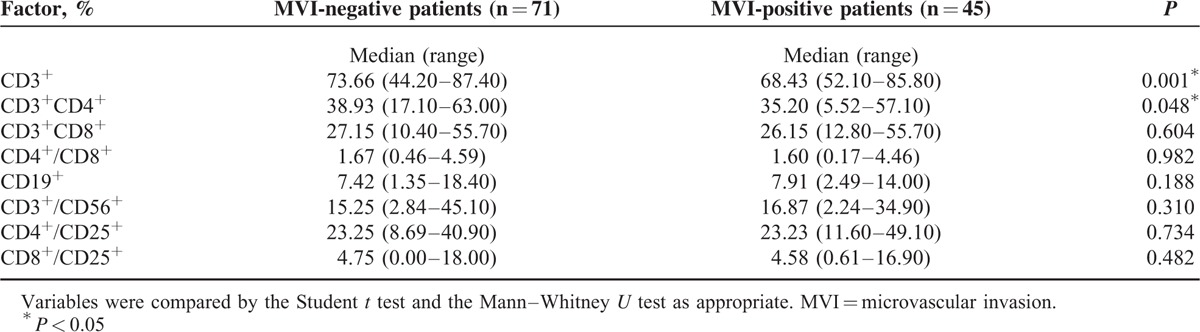
Lymphocyte Subset Analysis
In this study, 116 patients underwent a preoperative flow cytometry test to characterize the subsets of their lymphocyte populations. Of these patients, 71 were MVI-negative and the other 45 were MVI-positive. The immunophenotyping report generated for each patient characterized the lymphocyte subsets according to the percentage of CD3+, CD3+/CD4+, CD3+/CD8+, CD4+/CD8+, CD19+, CD3+/CD56+, CD4+/CD25+, and CD8+/CD25+. The comparison of lymphocyte subsets between MVI-negative and -positive patients is shown in Table 4. The results demonstrated that the median percentages of CD3+ (Mann-Whitney U test) and of CD3+/CD4+ cells (Student t test) from MVI-negative patients were significantly higher than those of MVI-positive patients (CD3+: 73.66% vs 68.43%, respectively; CD3+/CD4+: 38.93% vs 35.20%, respectively).
TABLE 4.
Preoperative lymphocyte subset analysis by flow cytometry for solitary hepatocellular carcinoma patients grouped according to microvascular invasion status
DISCUSSION
In this study, we investigated the efficacy of CIK cell therapy for solitary HCC patients after curative resection. To our best knowledge, this is the first study to perform this analysis with attention to MVI stratification. The results demonstrated that, for patients with solitary HCC, postsurgical CIK therapy was only beneficial for MVI-negative patients and had no statistically significant benefit for MVI-positive patients.
Indeed, as seen in Table 2, MVI was identified as an independent risk factor for OS in our study. Other studies have also found that MVI was closely associated with poor outcome of patients with HCC who received curative resection.23,25 In addition, Sumie et al 25 demonstrated that HCC patients with MVI had a higher frequency of micrometastasis, which Poon et al26 reported was an important contributor to early recurrence. Another study also showed that MVI was a significant predictor for early recurrence and early death within 2 years after hepatectomy in patients with solitary HCC.27 Likewise, in our study, there were a total of 64 patients with MVI (21 in the CIK group and 43 in the control group) who suffered recurrence, 78% of which recurred within 2 years (15/21 in the CIK group and 35/43 in the control group). This suggests that intrahepatic micrometastasis may be present in MVI-positive patients before resection. Previous studies have also reported that MVI was highly correlated with extrahepatic metastasis in HCC following curative resection.28,29 Similarly, in our study, 19 patients suffered extrahepatic metastasis as their initial recurrence. Among them, MVI-positive patients accounted for 74% (14/19 patients), which was overwhelmingly higher than MVI-negative patients (5/19 patients). The high risk of intrahepatic and extrahepatic metastasis suggests that MVI-positive patients possess a higher tumor burden than MVI-negative patients. As an immunotherapeutic modality, Linn et al12 thought CIK transfusion may be more effective in patients with a lower tumor burden. In line with this suggestion, CIK therapy did not seem to be beneficial to the MVI-positive patients in our cohort.
As is known, HCC has been associated with the development of numerous immunosuppression mechanisms, including but not confined to the yield of immunosuppressive cytokines of TGF-β and prostaglandins, the impairment of antigen-presenting cells, the generation of inhibitory macrophages, the promotion of regulatory T cells, and the induction of myeloid-derived suppressor cells,30–33 all of which combine to create a microenvironment favorable to tumor angiogenesis and metastasis. In general, it is known that T–cell-mediated immune responses play a significant role in antitumor activity.34 Specifically in HCC, studies have found a lack of CD4+ helper T cells,35 decreased cytotoxic effector function of CD8+ T cells,36 and inhibited function and reduced frequency of NK cells,37 whereas in our study, patients in the CIK group received transfusions of optimized and viable CIK cell populations, which contained ≥70% CD3+ cells, ≥30% CD3+/CD56+, cells and ≥40% CD8+ cells. What is more, the CD3+/CD56+ subset of NK-like T cells is unique, as it is a more terminally differentiated, late-effector T-cell population that possesses stronger cytotoxicity and a higher proportion of CD8+ cells than early effector T cells (ie, the CD3+/CD56− subset).12,38 For immunotherapy of HCC, the most optimal time for administration has been found to be in the adjuvant setting following liver resection.30 Similarly, our findings support this recommendation and suggest that CIK transfusion can improve DFS and OS in solitary HCC patients with negative MVI status when administered 1 month postoperatively.
In our study, approximate 90% of the patients had HBV-related HCC. It was widely accepted that high viral load was associated with poor prognosis of HBV-related HCC after curative resection.39,40 And Shi et al41 also reported that autologous CIK transfusion could inhibit HBV replication. Interestingly, in our cohort, patients receiving antiviral therapy with nucleotide/nucleoside analogs in CIK group (53.9%, 55/102) accounted for similar proportion as those in control group (51.2%, 105/205), and the patients with high HBV-DNA load (log copies/mL ≥4) between the 2 groups were also statistically comparable (CIK group 38/102, control group 70/205, χ2 test: P = 0.591). Thus, we hypothesized that CIK transfusion may be a possible contributor helping reduce recurrence risk or prolong survival through suppressing HBV activity in our cohort, particularly among MVI-negative patients, which a future RCT is needed to further testify. In addition, 73.5% of the patients in our CIK group had tumors that were ≤5 cm in size (vs 73.2% in the control group), indicating that our cohort had a generally smaller tumor burden than the cohort of a previous CIK-related study, in which 40% of the patients had tumors ≤5 cm.42 This suggests that tumor-induced immunosuppression may have been weaker in our patients, and that adjuvant CIK therapy could have more of an impact on immunity and tumor killing in such patients, compared with those in previous studies. Furthermore, for HCC patients with solitary tumor and negative MVI, the tumor would most likely be eradicated completely after curative resection and with minimal residual lesions, if present,20 which would make them ideal candidates for CIK immunotherapy. Therefore, these factors and the combination of our results suggest that solitary HCC patients who have relative small tumor burden and no MVI appear to be the ideal cohort for postsurgical CIK therapy.
In addition, according to Table 4, the median percentage of CD3+ and CD3+/CD4+ populations of MVI-positive patients were significantly lower than in MVI-negative patients, which suggests that the T–cell-mediated immune response is more poignantly suppressed in patients that also have MVI. Besides bolstering cytotoxic T lymphocyte activity, CD4+ T cells also assist in tumor rejection,43 but gradually become deficient over the course of cancer progression44 (Table 4). Thus, for the MVI-positive patients in our cohort, it is our belief that transfusing only 1 kind of immune cell (CIK cells) was not sufficient to improve DFS and OS significantly. While not statistically significant in this study, we did notice that the 2 Kaplan-Meier OS curves for MVI-positive patients in the CIK and control groups crossed at just about 72 months post-treatment (Figure 3b), and that the 1-, 3-, and 5-year OS rates of MVI-positive patients in the CIK group were not significantly different, but higher than those in the control group. According to Pan et al,22 long-term treatment (ie, >10–20 cycles of CIK therapy) positively affected the survival of postoperative HCC patients. In this study, 87% of the CIK group patients received only 4 cycles of CIK therapy. Therefore, it is possible that an HCC patient with MVI could benefit from longer term CIK therapy; however, this observation would need to be investigated by a larger prospective study. Furthermore, if the lymphocyte subset analysis of each MVI-positive/-negative patient (before and after ≥4 cycles of CIK therapy) could be performed, each patient's immune status could be identified and the therapy better tailored. Indeed, CIK therapy is not completely without toxicity. But studies from our institution and other institutions have confirmed its safety.18,22,45 Fever, chills, fatigue, and vomiting were reported as the common adverse events among a part of CIK-receiving patients, but these symptoms were mild and self-limiting, which were observed in the current study. Patients who received CIK therapy rarely developed serious side effects, and the safety would contribute to its clinical application in selected HCC patients. Because of the retrospective nature, this study has some limitations: first, the cohort scale is not large enough, especially the patient number in CIK group; second, the patients are not randomly assigned to each group, which may lead to potential selection bias; third, this study was conducted at a single center. Therefore, a multicenter RCT with large scale is needed to verify these findings.
In summary, this study showed that adjuvant CIK cell transfusion can improve survival in solitary HCC patients with no MVI after curative resection and demonstrated the importance of evaluating MVI in future studies of CIK therapeutic efficacy.
Supplementary Material
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALB = serum albumin, ALT = alanine aminotransferase, AST = aspartate aminotransaminase, CD = cluster of Differentiation, CI = confidence interval, CIK = Cytokine-induced killer cells, CT = computed tomography, DFS = disease-free survival, H&E = hematoxylin and eosin, HBsAg = hepatitis B surface antigen, HCC = hepatocellular carcinoma, HR = hazard ratio, IFN-γ = interferon-γ, IL = interleukin, LAK = lymphokine-activated killer cells, MRI = magnetic resonance imaging, MVI = microvascular invasion, NA = nucleotide/nucleoside analogs, NK = natural killer, OS = overall survival, PBMC = peripheral blood mononuclear cells, PET-CT = positron emission tomography-computed tomography, RCT = randomized controlled trial, RFA = radiofrequency ablation therapy, SD = standard deviation, SYSUCC = Sun Yat-sen University Cancer Center, TACE = transarterial chemoembolization, TBIL = serum total bilirubin, TGF-β = transforming growth factor-β, UICC = Union for International Cancer Control.
J-LC and X-ML contributed equally to this work.
The work was supported by the International Program for PhD Candidates of Sun Yat-Sen University (J-LC); the Sun Yat-sen University Clinical Research 5010 Program (Grant No. 2007043) (S-PL); the National Natural Science Foundation of China (No. 81171890) (S-PL) and the National Basic Research Program of China (973 Program, No.2013CB910304) (S-PL).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008; 48 suppl 1:S20–S37. [DOI] [PubMed] [Google Scholar]
- 3.Lai EC, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995; 221:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila MA, Berasain C, Sangro B, et al. New therapies for hepatocellular carcinoma. Oncogene 2006; 25:3866–3884. [DOI] [PubMed] [Google Scholar]
- 5.Brittenden J, Heys SD, Ross J, et al. Natural killer cells and cancer. Cancer 1996; 77:1226–1243. [DOI] [PubMed] [Google Scholar]
- 6.Taketomi A, Shimada M, Shirabe K, et al. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer 1998; 83:58–63. [DOI] [PubMed] [Google Scholar]
- 7.Ladhams A, Schmidt C, Sing G, et al. Treatment of non-resectable hepatocellular carcinoma with autologous tumor-pulsed dendritic cells. J Gastroenterol Hepatol 2002; 17:889–896. [DOI] [PubMed] [Google Scholar]
- 8.Maksan SM, Araib PM, Ryschich E, et al. Immune escape mechanism: defective resting and stimulated leukocyte-endothelium interaction in hepatocellular carcinoma of the rat. Dig Dis Sci 2004; 49:859–865. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Wolf IG, Negrin RS, Kiem HP, et al. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 1991; 174:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefterova P, Marten A, Buttgereit P, et al. Targeting of natural killer-like T immunologic effector cells against leukemia and lymphoma cells by reverse antibody-dependent cellular cytotoxicity. J Immunother 2000; 23:304–310. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 1993; 21:1673–1679. [PubMed] [Google Scholar]
- 12.Linn YC, Hui KM. Cytokine-induced NK-like T cells: from bench to bedside. J Biomed Biotechnol 2010; 2010: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994; 153:1687–1696. [PubMed] [Google Scholar]
- 14.Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 2008; 112:2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang FS, Liu MX, Zhang B, et al. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J Gastroenterol 2002; 8:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui J, Wang N, Zhao H, et al. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer 2014; 134:342–351. [DOI] [PubMed] [Google Scholar]
- 17.Hao MZ, Lin HL, Chen Q, et al. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer 2010; 29:172–177. [DOI] [PubMed] [Google Scholar]
- 18.Huang ZM, Li W, Li S, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother 2013; 36:287–293. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellularcarcinoma. Oncoimmunology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802–807. [DOI] [PubMed] [Google Scholar]
- 21.Hui D, Qiang L, Jian W, et al. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis 2009; 41:36–41. [DOI] [PubMed] [Google Scholar]
- 22.Pan K, Li YQ, Wang W, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol 2013; 20:4305–4311. [DOI] [PubMed] [Google Scholar]
- 23.Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008; 15:1375–1382. [DOI] [PubMed] [Google Scholar]
- 24.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954; 7:462–503. [DOI] [PubMed] [Google Scholar]
- 25.Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014; 21:1002–1009. [DOI] [PubMed] [Google Scholar]
- 26.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000; 89:500–507. [PubMed] [Google Scholar]
- 27.Kondo K, Chijiiwa K, Makino I, et al. Risk factors for early death after liver resection in patients with solitary hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2005; 12:399–404. [DOI] [PubMed] [Google Scholar]
- 28.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 2011; 117:4475–4483. [DOI] [PubMed] [Google Scholar]
- 29.Jun L, Zhenlin Y, Renyan G, et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist 2012; 17:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korangy F, Hochst B, Manns MP, et al. Immune responses in hepatocellular carcinoma. Dig Dis 2010; 28:150–154. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Ding T, Pan W, et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 2009; 125:1640–1648. [DOI] [PubMed] [Google Scholar]
- 32.Yang XH, Yamagiwa S, Ichida T, et al. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol 2006; 45:254–262. [DOI] [PubMed] [Google Scholar]
- 33.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt N, Neumann-Haefelin C, Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig Dis 2012; 30:483–491. [DOI] [PubMed] [Google Scholar]
- 35.Witkowski M, Spangenberg HC, Neumann-Haefelin C, et al. Lack of ex vivo peripheral and intrahepatic alpha-fetoprotein-specific CD4+ responses in hepatocellular carcinoma. Int J Cancer 2011; 129:2171–2182. [DOI] [PubMed] [Google Scholar]
- 36.Nagao M, Nakajima Y, Hisanaga M, et al. The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo? Hepatology 1999; 30:413–421. [DOI] [PubMed] [Google Scholar]
- 37.Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428–437. [DOI] [PubMed] [Google Scholar]
- 38.Linn YC, Lau SK, Liu BH, et al. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology 2009; 126:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Lu JH, Zhai J, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol 2012; 38:683–691. [DOI] [PubMed] [Google Scholar]
- 41.Shi M, Fu J, Shi F, et al. Transfusion of autologous cytokine-induced killer cells inhibits viral replication in patients with chronic hepatitis B virus infection. Clin Immunol 2009; 132:43–54. [DOI] [PubMed] [Google Scholar]
- 42.Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother 2008; 31:63–71. [DOI] [PubMed] [Google Scholar]
- 43.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamphorst AO, Ahmed R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy 2013; 5:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148:1383–1391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


