Abstract
Bacterial vaginosis (BV) is considered as a trigger for an inflammatory response that could promote adverse pregnancy outcome (APO). We hypothesized that BV-related inflammation could be counterbalanced by anti-inflammatory and mucosal homeostatic responses that could participate in pregnancy outcomes.
A total of 402 vaginal self-samples from pregnant women in their first trimester were screened by Nugent score. In this population, we enrolled 23 pregnant women with BV but without APO, 5 pregnant women with BV and developing APO, 21 pregnant women with intermediate flora, and 28 random control samples from pregnant women without BV or APO.
BV without APO in pregnant women was associated with 28-fold interleukin-8, 5-fold interleukin-10, and 40-fold interleukin-22 increases in expression compared to controls. BV associated with APO in pregnant women shared 4-fold increase in tumor necrosis factor, 100-fold decrease in interleukin-10, and no variation in interleukin-22 expressions compared to controls. Next-generation sequencing of vaginal microbiota revealed a shift from obligate anaerobic bacteria dominance in BV without APO pregnant women to Lactobacillus dominance microbiota in BV with APO.
Our results show that the anti-inflammatory and mucosal homeostatic responses to BV may determine outcome of pregnancy in the setting of BV possibly through effects on the vaginal microbiota.
INTRODUCTION
Bacterial vaginosis (BV) is a dysbiosis of the vaginal microbiota characterized by a shift from the normal Lactobacillus-dominant species to an increased abundance of anaerobic species and genital mycoplasmas.1 BV is a risk factor for Trichomonas vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, human papillomavirus, herpes simplex virus type-2, human immunodeficiency virus infection, pelvic inflammatory disease, and posthysterectomy wound infections in women of reproductive age.2 During pregnancy, while most BV remain clinically asymptomatic, the occurrence of BV before 13 completed weeks of amenorrhea increases the risk of adverse pregnancy outcomes (APOs) including preterm birth, spontaneous preterm labor, preterm prelabor rupture of membranes, recurrent abortion, or miscarriage.3 However, the pathophysiological mechanism through which BV affects the ongoing pregnancy remains unclear. To date, BV has only been considered as a trigger for an adverse inflammatory response during pregnancy. Indeed, an excess of proinflammatory cytokines during pregnancy is linked to APO.4,5 However, during pregnancy the immune balance is in favor of an anti-inflammatory/immunosuppressive state to tolerate foreign paternal antigens present in the fetus.6 Therefore, the interaction between the dysbiosis of BV and the specific anti-inflammatory balance of pregnancy might be involved. In other settings of mucosal microbiotal dysbiosis/immune interaction such as inflammatory bowel diseases, the critical immune responses involve proinflammatory cytokines, interleukin-17 (IL-17), interleukin-18 (IL-18), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-1β (IL-1β), tumor necrosis factor (TNF); mucosal wound repair cytokines such as interleukin-22 (IL-22); and anti-inflammatory cytokines such as interleukin-10 (IL-10).7,8 Therefore, in a pilot study of pregnant women with BV in their first trimester, we studied the relation between the vaginal mucosal immune balance and pregnancy outcome.
MATERIALS AND METHODS
Patient Selection
We screened BV in all pregnant women ages 18 years or older with a gestational age of <13 completed weeks of amenorrhea at the time of screening during routine pregnancy follow-up consults. During the consult, a short-standardized questionnaire collected the age, the educational level, whether they had smoked since the beginning of pregnancy, and whether they had any history of APO. APO including preterm birth, spontaneous preterm labor, preterm premature rupture of membranes, recurrent abortion or miscarriage, and antibiotic therapy before the delivery were collected 10 months later from the medical records. Each woman performed a vaginal self-sample with 2 swabs. One swab was used to identify BV, intermediate flora (IF), and normal flora using the Nugent score. Following the constitution of the groups of women with BV and IF, a control group of an equal number samples was randomly sampled from the much larger pool of swabs with normal Nugent score. The second vaginal swab was discharged in 1 mL of transport media (Eswab, Coban, Italy) and stored at −80°C for ribonucleic acid (RNA) extraction and flow cytometry.
Nugent Score
Vaginal secretions were spread on a clean microscopy slide and heat-fixed within 4 h. The vaginal smear was then Gram-stained. Examining several microscopic fields at a 10-fold magnification assessed bacterial and cellular abundance. Nugent score was established as described elsewhere.9 Scores of 0 to 3 were considered normal (lactobacillus dominant), 4 to 6 were labeled as intermediate (mixed morphotypes), and 7 to 10 were indicative of BV (absence of lactobacilli and predominance of the other 2 morphotypes).
Quantitative Real-Time Polymerase Chain Reaction
RNA extractions were performed using GeneJet RNA Purification Kit (Fermentas, Thermo fisher scientific Waltham, MA, USA, K0731). Isolated RNAs were reverse-transcribed to complementary deoxyribonucleic acid (cDNA) with the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). The resulting cDNA (equivalent to 5 ng of total RNA) was amplified using the SYBR Green Real-Time Polymerase Chain Reaction kit and detected on a Stratagene Mx3005P (Agilent Technologies, Santa Clara, CA). RNA quality was assessed using a NanoDrop ND-8000 spectrophotometer. Real-time polymerase chain reaction was performed with the forward and reverse primers that were designed using Primer express software, version 1.0 (Applied Biosystems).
Specific primers for: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5′-ACCCACTCCTCCACCTTTGA-3′ and 3′-CATACCAGGAAATGAGCTTGACAA-5′), TNF (5′-CCCAGGCAGTCAGATCATCTTC-3′ and 5′-AGCTGCCCCTCAGCTTGA-3′), IL-17 (5′-ACTACAACCGATCCACCTCAC-3′ and 5′-ACTTTGCCTCCCAGATCACAG-3′), IL-1β (5′-AAACCTCTTCGAGGCACAAG-3′ and 5′-GTTTAGGGCCATCAGCTTCA-3′), IL-18 (5′-ACTGTACAACCGCAGTAATACGC-3′ and 5′-AGTGAACATTACAGATTTATCCC-3′), IL-22 (5′-TGAATAACTAACCCCCTTTCCCTG-3′ and 5′-TGGCTTCCCATCTTCCTTTTG-3′), IL-8 (5′-TAGCAAAATTGAGGCCAAGG-3′ and 5′-AAACCAAGGCACAGTGGAAC-3′), and IL-6 (5′-GGTACATCCTCGACGGCATCT-3′ and 5′-GTGCCTCTTTGCTGCTTTCAC-3′) were used for amplification in triplicate assays. On completion of polymerase chain reaction amplification, a deoxyribonucleic acid (DNA) melting curve analysis was carried out in order to confirm the presence of a single amplicon. β-Actin was used as an internal reference gene in order to normalize the transcript levels. Relative messenger RNA levels (2−ΔΔCt) were determined by comparing the polymerase chain reaction amplification cycle thresholds (Ct) for the gene of interest and β-actin gene (ΔCt) and ΔCt values for treated and control groups (ΔΔCt).
Given that APO, regardless BV presence, has been shown to be associated with cytokine levels, quantitative real-time polymerase chain reaction results of pregnant women that presented APO were interpreted separately from pregnant women without APO.
Next-Generation Sequencing
Next-generation sequencing libraries were prepared by amplification of hypervariable regions of the 16S ribosomal DNA gene with the Ion 16S Metagenomics kit, followed by library generation using the Ion Plus Library kit (Life Technologies, Carlsbad, CA). Barcoded libraries were quantified and assessed for quality using the Agilent 2100 BioAnalyzer (Agilent Technologies). Libraries were pooled in equimolar amounts and sequenced on an Ion PGM Platform using a Ion 314 Chip Kit v2 and the Ion PGM Sequencing 400 kit (Life Technologies).
Statistical Analysis
Statistical analysis was carried out using Prism 6 software (GraphPad). One-way analysis of variance (ANOVA) followed by multiple comparison tests or t test was used for all comparisons. Significance was accepted at P < 0.05.
RESULTS
A total of 402 vaginal self-samples from pregnant women in their first trimester were screened by Nugent score. BV and IF were found in 28 and 21 pregnant women, respectively. Among the 28 pregnant women with BV, 5 miscarried between 18 and 22 completed weeks of amenorrhea. For the study, 4 groups were individualized post hoc: pregnant women with BV but without APO (BV-APO−; n = 23), pregnant women with BV and developing APO (BV-APO+; n = 5), pregnant women with IF (n = 21), and random samples from pregnant women with normal Nugent screening (controls; n = 28).
The general characteristics of the studied population are presented in Table 1. Antimicrobial treatment by clindamycin was noted for 5 pregnant women of our cohort in which 4 women belonged to BV-APO+ group and 1 to BV-APO− group. Neutrophil count was under 20 cells per field at a 10-fold magnification for 26/28 BV, 19/21 IF, and 25/28 controls (data not shown). Finally, no history of sexually transmitted disease was reported in our cohort.
TABLE 1.
Population Characteristics of Studied Pregnant Women
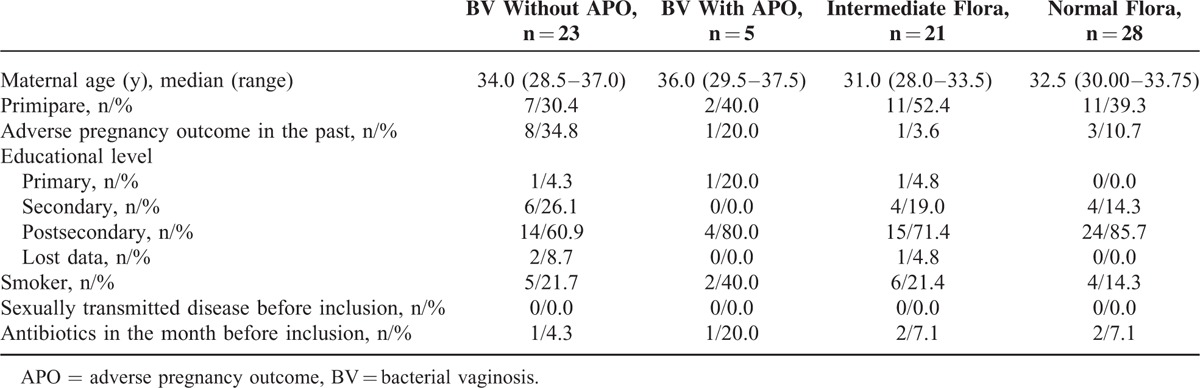
BV-APO− pregnant women showed significantly increased IL-22, IL-10, and IL-8 expression in vaginal samples compared to controls (Figure 1A and B). Additionally, pregnant women with BV showed significantly decreased IL-17 and IL-6 expression (Figure 1C). IL-1β, IL-10, and TNF expression did not significantly differ (Figure 1C). For pregnant women with IF compared to controls, IL-1β was the only observed increase in cytokine expression.
FIGURE 1.
Relative gene expression of proinflammatory, mucosal homeostatic, and anti-inflammatory cytokines in the vagina pregnant women. APO = adverse pregnancy outcome, BV = bacterial vaginosis, IF = intermediate flora.
Among the 5 BV-APO+ pregnant women, IL-10 expression decreased 100-fold in vaginal samples compared with controls and more than 1000-fold compared with BV-APO− (Figure 1B). IL-8 and IL-22 messenger RNA were not significantly increased in these 5 samples compared with controls and differed significantly compared with BV-APO− (Figure 1A). By contrast, TNF expression was increased compared with both controls and BV-APO− (Figure 1C).
Given these results, we wanted to find out if the vaginal microbiota composition might differ between BV-APO+ pregnant women and BV-APO− pregnant women. Next-generation sequencing of 2 patients with BV-APO+ revealed that the vaginal microbiota was largely dominated by Lactobacillus sp, which represent 84.2% and 67.5% of the reads in the 2 patients with BV-APO+. Lactobacillus iners was the only Lactobacillus sp found (84.2% of the reads) for 1 patient and L. iners and L. jensenii were the only 2 Lactobacillus sp found for the second patient. Additionally, obligate anaerobes were drastically decreased in both BV-APO+ parturients when compared with BV-APO− pregnant women (Figure 2). Indeed, the microbiota of these 2 BV-APO− pregnant women were characterized by a high proportion of sequences from obligate anaerobe (67.5% and 44.4% in BV-APO− compared with 8.3% and 1.4% in BV-APO+).
FIGURE 2.
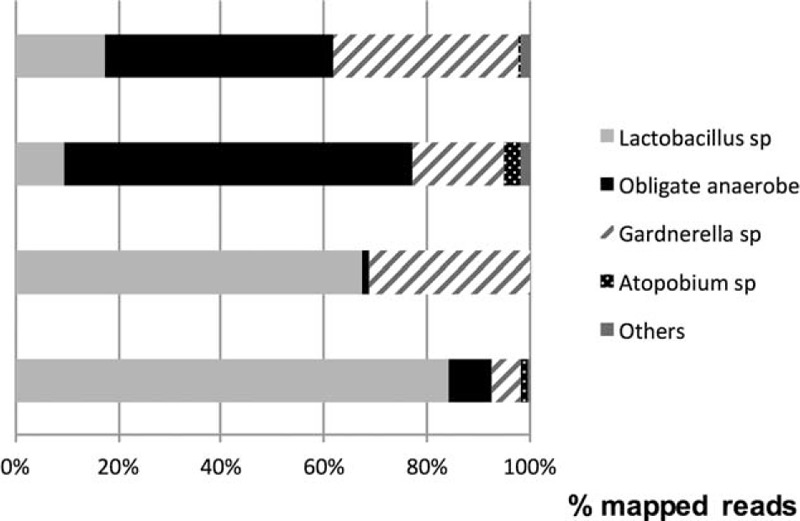
Vaginal microbiota composition of pregnant women with bacterial vaginosis but without adverse pregnancy outcome (BV-APO−) and pregnant women with bacterial vaginosis and developing adverse pregnancy outcome (BV-APO+).
Finally given that Lactobacillus sp dominate the BV-APO+ pregnant women, we verified the Gram stain performed on the vaginal smear to establish the Nugent score. Gram-variable polymorphous bacilli and gram-variable rod-shaped bacilli characterized the smears but not the typical gram-positive bacilli with regular parallel edges morphotype of Lactobacillus sp.
DISCUSSION
Our results show that in pregnant women, BV without APO is associated with a vaginal immune response consisting in increased IL-22, IL-8, and IL-10 expression. In contrast, BV with APO is associated with decreased IL-10, unmodified IL-22/IL-8, and increased TNF expression compared with healthy controls (Figure 3). Although a limited pilot study, with the inherent risk of selection bias, our study was conducted in samples from a very general population of pregnant women and not a specific population at very high risk of BV. Of note, our population is exempt of patients with known histories of sexually transmitted diseases that could modify the vaginal immune response.
FIGURE 3.
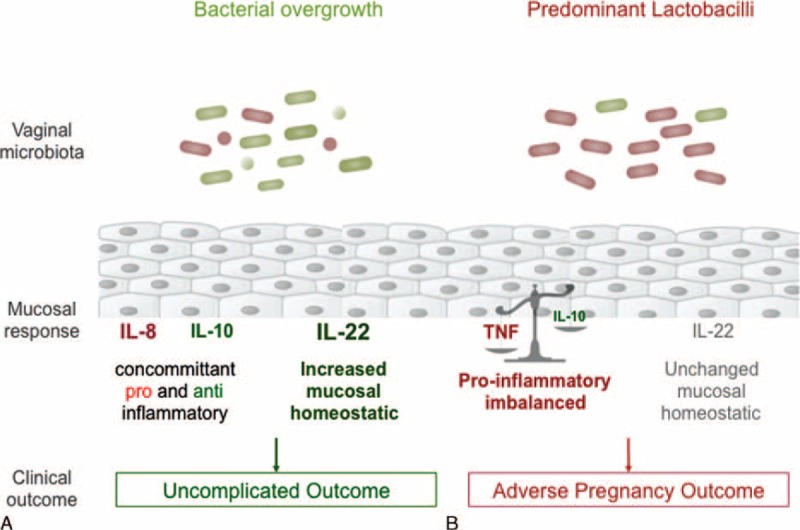
Proposed underlying mechanisms of outcomes of pregnancy in the setting of bacterial vaginosis. (A) The typical microbiota observed in bacterial vaginosis (varied obligate anaerobic bacteria and limited Lactobacilli and Gardnerella) elicit a mucosal response characterized by concomitant increases in both pro- and anti-inflammatory cytokines (IL-8 and IL-10, respectively) further balanced by an increased mucosal homeostatic response (increased IL-22) when compared to controls without bacterial vaginosis. This microbiota/response profile is associated with uncomplicated pregnancy outcomes despite bacterial vaginosis. (B) Another vaginal microbiota observed in certain cases of bacterial vaginosis (predominant Lactobacilli and lacking obligate anaerobes) elicit a different mucosal response characterized by a proinflammatory imbalance (increased TNF and 100-fold decrease in IL-10) and an unchanged homeostatic mucosal response (unchanged IL-22) when compared to controls without bacterial vaginosis. This microbiota/response profile is associated with adverse pregnancy outcomes. IL = interleukin, TNF = tumor necrosis factor.
While high neutrophil recruitment is a strong marker of vaginitis due to sexually transmitted bacteria,10 we observe limited neutrophil recruitment in BV as previously reported.11 This observation is in contradiction with the fact that IL-8 is known to chemo-attract immune cells such as neutrophils to the site of injury.12 However, it is also well-known that during pregnancy an immune “re-balancing” toward IL-10 synthesis occurs to tolerate the fetus.6,13 We observe such an IL-10 increase in BV without APO. This IL-10 increase can explain the significant decrease we observe in other proinflammatory cytokines such as IL-6 and IL-17. In turn by inhibiting proinflammatory mediators effects at the materno-fetal interface,14,15 this increase in IL-10 could explain why concomitant IL-8-dependent neutrophil recruitment is limited. Additionally, IL-10 increase is consistent with an immune cellular profile we observed in an attempt at flow cytometry of vaginal samples of BV without APO (n = 3) which showed expansion of CD4+ T-lymphocytes and natural killer cells compared with controls from parturients without BV nor APO (n = 3) (data not shown). Indeed, IL-10 produced by both natural killer cells and subsets of CD4+ T-lymphocytes (T-regulatory lymphocytes) at the materno-fetal site6,16,17 is known to inhibit CD8+ T-lymphocyte expansion.
The most marked cytokine profiles are an increased vaginal anti-inflammatory response in BV with successful pregnancy outcome while this response is deeply depressed in BV with APO. Outside of the setting of BV, an anti-inflammatory state of the mother's immune system has been shown to be associated with successful pregnancy outcome.6 Conversely, increased expression of proinflammatory cytokines such as TNF is usually associated with several APOs.18–21 Thus, our finding that the vaginal anti-inflammatory immune response to BV we observe may participate in pregnancy outcome in this setting.
Ours is the first study to report that the response to BV with successful pregnancy outcome includes a major (40-fold) increase in IL-22 while this specific response is lacking in BV with APO. Upon damage, IL-22 participates in mucosal homeostasis by inducing mucosal antimicrobial peptide and mucus synthesis, by promoting wound repair and reinforcement of epithelial tight junctions, and by avoiding invasion by commensal bacteria.8,22 Potential for damage requiring IL-22-dependent mechanisms of protection exists in BV. Indeed, vaginal microbiota synthesizes several virulence factors such as sialidase, which can lyse mucins, favor bacterial adherence, and counteract immunoglobulin A protection leading in fine to mucosal damage.23,24 This novel finding suggests several possibilities. First, the combined IL-10 anti-inflammatory response with an increase in the IL-22 mucosal homeostatic response may be the protective appropriate vaginal immune response to BV. Second, the major known mucosal homeostatic roles of IL-22, antimicrobial peptide expression, mucin expression, tight junction reinforcement, and epithelial wound repair, may be enough in themselves to determine BV outcome.
Taken as a whole, the profile we observe in vaginal swabs of pregnant women with BV but without APO may consist in an adapted anti-inflammatory (5-fold IL-10 increase), strong mucosal homeostatic (40-fold IL-22 increase) response to BV-induced inflammation (28-fold IL-8 increase, with neutrophil recruitment limited by the concomitant anti-inflammatory response).
Interestingly, this cytokine profile is associated with a shift of the vaginal microbiota characterized by a dramatic decrease of obligate anaerobic bacteria and an increase of L. iners or L. jensenii that dominate the vaginal microbiota. Both L. iners and L. jensenii belong to the normal vaginal microbiota. While a high proportion of L. iners in the vaginal microbiota have been reported as associated with prematurity25,26 implying a pathogenic causative role. However, an increase in the amount of L. iners in the vaginal bacteria can be considered an indirect marker of a transitional change of the microbiota under environmental conditions such as those observed following antimicrobial treatment of BV.27 Furthermore, the previous studies showing the association between dominant L. iners group in the vaginal microbiota and prematurity did not study concomitant anti-inflammatory IL-10 and IL-22 synthesis. Indeed, in our cohort, neither L. iners nor L. jensenii are associated with IL-22 increase implying that these bacteria probably did not induce mucosal injury.
Obligate anaerobes, however, are known to synthesize strong inducers of mucosal IL-10 expression such as butyrate in the digestive tract.28 Therefore, the major decrease of anaerobes in the vaginal microbiota of BV-APO+ women could participate in the concomitant observed decrease in vaginal IL-10. Furthermore, oral and vaginal anti-anaerobic metronidazole treatment of BV has been shown to be associated with reduced levels of both IL-8 and IL-10.29 Taken together, our results may provide a possible explanation to the observed inefficiency of antimicrobials to prevent BV-related APO in clinical trials.30
Taken as a whole, our results highlight that “debugging” the vaginal microbiotal metabolome by determining the quantity of organic compounds synthesized by the microbiota known to induce anti-inflammatory mucosal responses or mucosal damage in different groups of pregnant women would be a relevant goal for further study. Finally, increasing the study sample size may allow confirmation of our results and possible identification of different cytokine/microbiota profiles.
Footnotes
Abbreviations: APO = adverse pregnancy outcome, BV = bacterial vaginosis, Ct = cycle threshold, DNA = deoxyribonucleic acid, GAPDH = glyceraldehyde 3-phosphate dehydrogenase, IF = intermediate flora, IL = interleukin, RNA = ribonucleic acid, TNF = tumor necrosis factor.
RD, DS, BG, KF, and GB conceived the study; EF, RD, SG, and SD performed the experiments; EF, RD, EK, DS, and SD wrote the first draft of the manuscript. All authors discussed the results and commented on the manuscript.
The Ethical Committee of our institution approved the study was on December 20, 2011 (No. A0125437).
PHRC 11-2011 of French Ministry of Health
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Spiegel CA. Bacterial vaginosis. Clin Microbiol Rev 1991; 4:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin DH. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci 2012; 343:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1995; 173:1231–1235. [DOI] [PubMed] [Google Scholar]
- 4.Kheshtchin N, Gharagozloo M, Andalib A, et al. The expression of Th1- and Th2-related chemokine receptors in women with recurrent miscarriage: the impact of lymphocyte immunotherapy. Am J Reprod Immunol 2010; 64:104–112. [DOI] [PubMed] [Google Scholar]
- 5.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today 1997; 18:478–482. [DOI] [PubMed] [Google Scholar]
- 6.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 2009; 15:517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troncone E, Marafini I, Pallone F, et al. Th17 cytokines in inflammatory bowel diseases: discerning the good from the bad. Int Rev Immunol 2013; 32:526–533. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012; 336:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simhan HN, Krohn MA. First-trimester cervical inflammatory milieu and subsequent early preterm birth. Am J Obstet Gynecol 2009; 200:e1–e4.377. [DOI] [PubMed] [Google Scholar]
- 11.Holmes KK, Spiegel C, Amsel AR, et al. Nonspecific vaginosis. Scand J Infect Dis Suppl 1981; 26:110–114. [PubMed] [Google Scholar]
- 12.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 1992; 307:97–101. [DOI] [PubMed] [Google Scholar]
- 13.Piccinni M-P. T cells in normal pregnancy and recurrent pregnancy loss. Reprod Biomed Online 2006; 13:840–844. [DOI] [PubMed] [Google Scholar]
- 14.Fortunato SJ, Menon R, Lombardi SJ. Interleukin-10 and transforming growth factor-beta inhibit amniochorion tumor necrosis factor-alpha production by contrasting mechanisms of action: therapeutic implications in prematurity. Am J Obstet Gynecol 1997; 177:803–809. [DOI] [PubMed] [Google Scholar]
- 15.Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. Endocrine Soc 2013; 83:4332–4337. [DOI] [PubMed] [Google Scholar]
- 16.Kopcow HD, Allan DSJ, Chen X, et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA 2005; 102:15563–15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lash GE, Robson SC, Bulmer JN. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010; 31 (Suppl):S87–S92. [DOI] [PubMed] [Google Scholar]
- 18.Shimada S, Nishida R, Takeda M, et al. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from sporadic miscarriage. Am J Reprod Immunol 2006; 56:193–200. [DOI] [PubMed] [Google Scholar]
- 19.Vitoratos N, Papadias C, Economou E, et al. Elevated circulating IL-1beta and TNF-alpha, and unaltered IL-6 in first-trimester pregnancies complicated by threatened abortion with an adverse outcome. Mediators Inflamm 2006; 2006:30485–30486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Shazly S, Makhseed M, Azizieh F, et al. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol 2004; 52:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Gargano JW, Holzman C, Senagore P, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J Reprod Immunol 2008; 79:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev 2013; 252:116–132. [DOI] [PubMed] [Google Scholar]
- 23.Cauci S, Driussi S, Monte R, et al. Immunoglobulin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. Am J Obstet Gynecol 1998; 178:511–515. [DOI] [PubMed] [Google Scholar]
- 24.Cauci S, Hitti J, Noonan C, et al. Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol 2002; 187:877–881. [DOI] [PubMed] [Google Scholar]
- 25.Petricevic L, Domig KJ, Nierscher FJ, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 2014; 4:5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kacerovsky M, Vrbacky F, Kutova R, et al. Cervical microbiota in women with preterm prelabor rupture of membranes. PLoS ONE 2015; 10:e0126884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakobsson T, Forsum U. Lactobacillus iners: a marker of changes in the vaginal flora? J Clin Microbiol 2007; 45:3145–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yudin MH, Landers DV, Meyn L, et al. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol 2003; 102:527–534. [DOI] [PubMed] [Google Scholar]
- 30.Brocklehurst P, Gordon A, Heatley E, et al. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013; 1:CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]


