Abstract
The goal of rectal cancer treatment is to minimize the local recurrence rate and extend the disease-free survival period and survival. For this aim, obtainment of negative circumferential radial margin (CRM) plays an important role. This study evaluated predictive factors for positive CRM status and its effect on patient survival in mid- and distal rectal tumors.
Patients who underwent curative resection for rectal cancer were included. The main factors were demographic data, tumor location, surgical technique, neoadjuvant therapy, tumor diameter, tumor depth, lymph node metastasis, mesorectal integrity, CRM, the rate of local recurrence, distant metastasis, and overall and disease-free survival. Statistical analyses were performed by using the Chi-squared test, Fisher exact test, Student t test, Mann–Whitney U test and the Mantel–Cox log-rank sum test.
A total of 420 patients were included, 232 (55%) of whom were male. We observed no significant differences in patient characteristics or surgical treatment between the patients who had positive CRM and who had negative CRM, but a higher positive CRM rate was observed in patients undergone abdominoperineal resection (APR) (P < 0.001). Advanced T-stage (P < 0.001), lymph node invasion (P = 0.001) and incomplete mesorectum (P = 0.007) were encountered significantly more often in patients with positive CRM status. Logistic regression analysis revealed that APR (P < 0.001) and open resection (P = 0.046) were independent predictors of positive CRM status. Moreover, positive CRM was associated with decreased 5-year overall and disease-free survival (P = 0.002 and P = 0.004, respectively).
This large single-institution series demonstrated that APR and open resection were independent predictive factors for positive CRM status in rectal cancer. Positive CRM independently decreased the 5-year overall and disease-free survival rates.
INTRODUCTION
Surgery remains the main stay of curative treatment for rectal cancer. The purpose of surgical treatment is to minimize the local recurrence rate, extend the disease-free survival period, and preserve patient quality of life. The total mesorectal excision (TME) technique described by Heald, which consists of resection of the rectum within the mesorectal envelope, allows for the removal of the mesorectum en bloc along with the fascia recti propria.1,2 The local recurrence rate in rectal cancer patients exceeded 25% before implementation of the TME technique, whereas the local recurrence rate was reduced to 4% to 5% with the implementation of TME.2–4 Neoadjuvant radiotherapy, which was pioneered by the Swedish Study Group, is more efficient with respect to postoperative sequelae, as surgery results in trauma to the tissue, which results in poor perfusion and oxygenation. Neoadjuvant radiotherapy improves local recurrence and survival rates by reducing the tumor size and stage.4–8 Subsequent studies demonstrated that chemotherapy treatment with neoadjuvant radiotherapy resulted in better outcomes regarding local recurrence and survival rates when compared with radiotherapy alone.6–9 Contemporary neoadjuvant treatment of middle and distal rectal tumors in a neoadjuvant setting (stages II–III) is the gold standard to increase the effectiveness of radiotherapy, provide negative surgical margins, enhance the chance of sphincter-preserving surgery and improve local recurrence and survival rates.
Quirke et al10 defined the concept of the circumferential radial margin (CRM) as the distance from the tumor to the mesorectal fascia, which challenged the belief that local recurrences originate from the distal margin of the anastomosis site. This group demonstrated that CRM is a prognostic factor for local recurrence and the majority of local recurrences originate from the residual tumor remaining on the pelvic wall after the initial resection (ie, CRM involvement).10 Reduction of local recurrence rates can be achieved by increasing surgical experience and skills as well as by increasing our understanding of the CRM concept and the significance of the removal of the mesorectum en bloc through anatomical and pathological assessments. TME and the status of the CRM are the key parameters for a successful resection. The former parameter provides an evaluation of surgical quality, and the latter parameter predicts local recurrence, systemic spread, and survival rates.5,11,12
In this study, we investigated the effects of a positive CRM on local recurrence and survival rates based on mesorectal excision completeness as well as the causes of CRM positive or negative status.
MATERIALS AND METHODS
Approval was obtained from the ethics committee (No: 2012/741-1059), and patients with histologically confirmed tumors located in the middle and lower rectum, who had undergone curative surgery between January 2005 and December 2012, were included in the study. Stage IV cases at the initial diagnosis and patients with synchronous colorectal tumors or tumors on the proximal rectum were excluded (Figure 1). The data were recorded in Microsoft Excel® software and were evaluated retrospectively using SPSS® software (Statistical Package for Social Sciences, Inc., Chicago, IL, ABD) for Windows, version 21.0.
FIGURE 1.
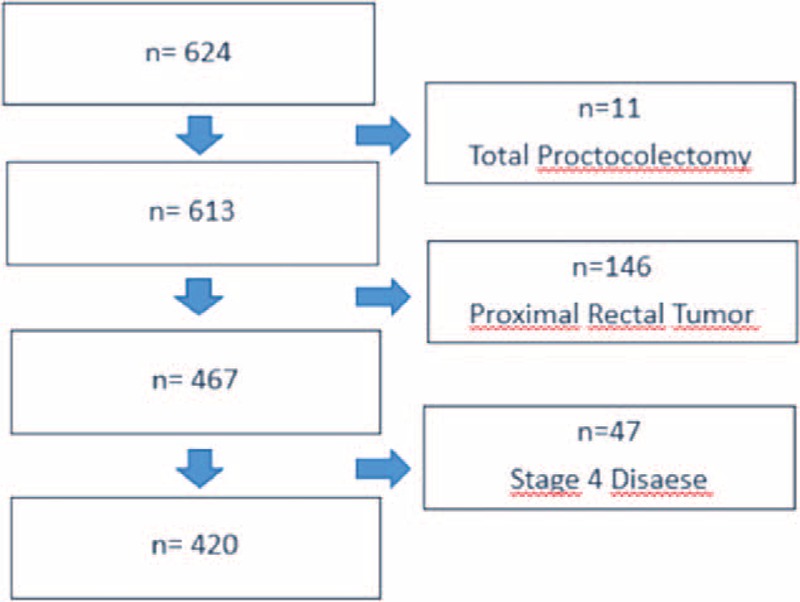
Exclusion criteria.
Gender, age, body mass index (BMI), tumor location (middle or distal), neoadjuvant treatment, surgical technique, surgery type, tumor diameter (mm), tumor invasion depth (T), circumferential margin, lymph node invasion (N), and completeness of the mesorectal resection were obtained from the database. Evaluations of the completeness of mesorectum excision were standardized in our Pathology Department after 2007. Therefore, the series included in the analysis was restricted to 371 patients who satisfied the criteria for completeness of mesorectum resection. CRM was recorded as positive in cases with a ≤1 mm distance between the tumor and the fascia propria recti.13
Informed consent was obtained from all patients. The preoperative staging evaluation included abdomen and chest computerized tomography scans, pelvic magnetic resonance imaging, with or without endorectal ultrasonography. Neoadjuvant chemoradiotherapy or radiotherapy was performed in patients with T3> tumors and/or N (+) on imaging. Surgery was performed 8 weeks after neoadjuvant chemoradiotherapy and 4 weeks after short-term radiotherapy.
All patients underwent TME surgery with curative intent by 6 experienced colorectal and laparoscopic surgeons who completed their learning curve in laparoscopic surgery between 2002 and 2005. Laparoscopic surgery was recommended to all patients. Some patients underwent open surgery because they did not consent to laparoscopic surgery or had previous abdominal surgery. During the surgery, inferior mesenteric artery and vein were ligated at their origins. Then, rectum was mobilized throughout the Holly Plan to the levator muscle level, adhering to the principles of TME. In patients, who underwent sphincter preserving surgery, anastomosis was performed by circular stapler or it was done by hand sewn. The techniques in the intra-abdominal section were similar for abdominoperineal resection (APR) procedure. After mobilizing the rectum up to the levator muscle, end colostomy was created and abdomen part of the operation was finished. The wide perineal incision covering sphincters was done. Perineal dissection was performed up to tip of coccyx posteriorly, and then it was completed anteriorly and laterally. Specimens were examined in 0.5-cm tissue sections after at least 72 hours of fixation to ensure proper circumferential margin examination. Adjuvant treatment, consisting of 4 doses of 5-fluorouracil/folic acid, was given to all patients who received neoadjuvant treatment as well as to patients with pT3 and patients with lymph node invasion. Clinical follow-ups were obtained upon clinical visits, rehospitalization and the other procedures such as endoscopy, radiological studies. The missing data of the patients about possible relapse, metastasis, and survival rates; whom were not found on the records, were collected and updated according to the conversation with the patients through the phone calls and clinical visits.
Statistical Analysis
The patient cohorts with negative and positive CRM associated with mid- and distal localized rectal tumors were compared for differences in demographic, clinical, and pathological characteristics using bivariate analysis. The Chi-squared test or Fisher exact test was used to compare categorical variables. Continuous variables were examined for normality of distribution using the Shapiro–Wilk test. Student t test was used to analyze normally distributed variables, and the nonparametric Mann–Whitney U test was used for the analysis of nonnormally distributed values.
All variables in the bivariate analyses were entered into a forward logistic regression model to correct for selection bias and to identify independent predictors of CRM. Overall and disease-free 5-year survival rates for patients who completed 60 months of follow-up after recovery from surgery were analyzed in our comparison of patients with negative- and positive-CRM using the Mantel–Cox log-rank sum test. Patients with local recurrence and distant metastases were compared for overall and at 5-year follow-ups using bivariate analysis.
RESULTS
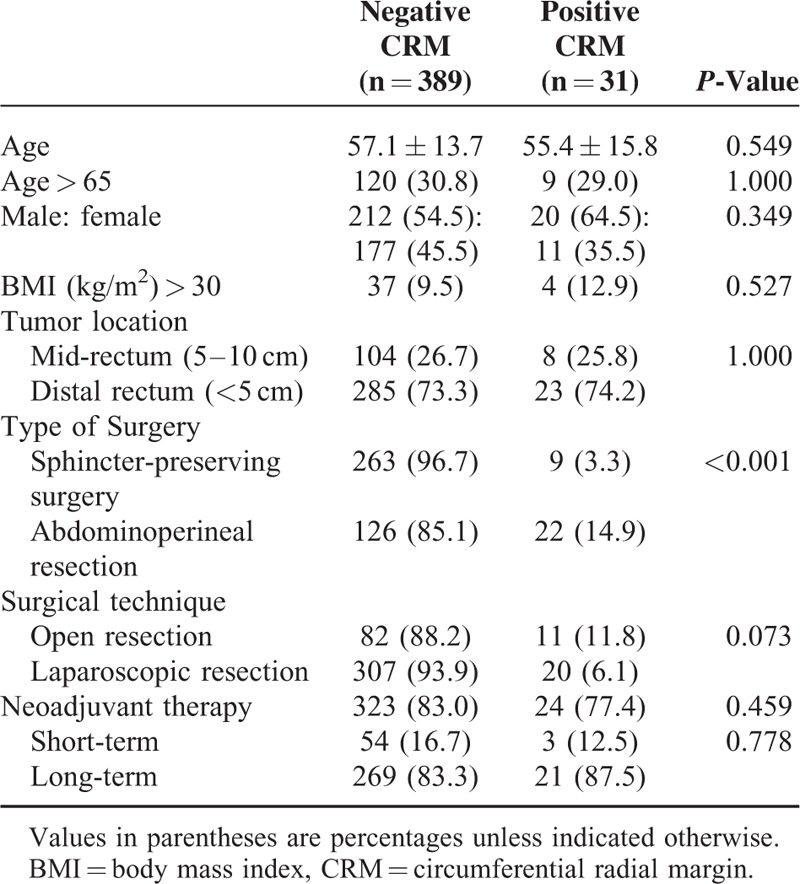
Analysis of the patient demographic characteristics revealed that 232 patients were male (55%), and 188 patients were female (45%). The mean age of the patients was 58 years (range 19–91 years). A total of 347 (83%) patients received neoadjuvant treatment. Laparoscopic surgery was performed on 327 (78%) patients. The rate of sphincter-preserving surgery was 65% (n = 272). A total of 31 cases (7.4%) exhibited positive CRMs as verified by pathological examination. Comparisons between patients with negative and positive CRMs revealed no significant differences in demographics, patient characteristics, tumor location (mid or distal), neoadjuvant therapy, or laparoscopic resection; however, a higher positive CRM rate was observed in patients who received APR (14.9 vs. 3.3, P < 0.001) (Table 1). APR rate was found similar between patients operated with laparoscopic surgery and patients operated with open surgery [laparoscopic: 35.2% (115/327) and open: 35.5% (33/93); P = 1.000].
TABLE 1.
Demographics and Patient Characteristics
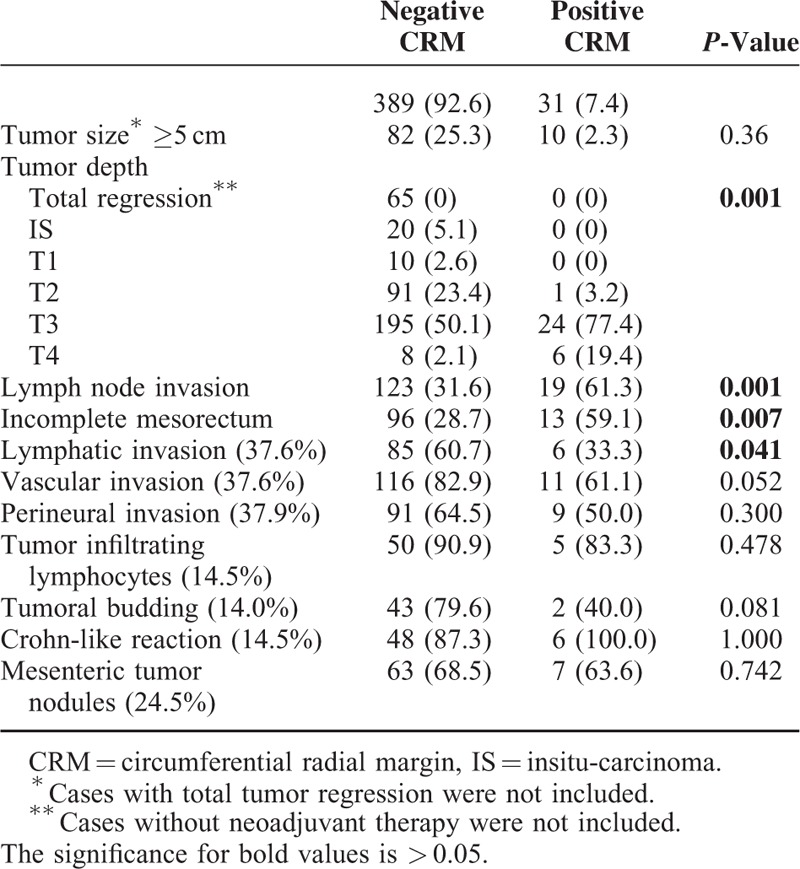
Comparisons of pathological risk factors revealed that advanced T-stages (P < 0.001), lymph node invasion (P = 0.001) and incomplete TME rates (P = 0.007) were encountered significantly more often in patients with positive CRMs (Table 2). Except lymphatic invasion, minor pathologic features of tumors (perineural invasion, vascular invasion, etc.) did not effect positive CRM status, but data of these factors was obtained only 37.5% of patients. Since patients were not randomized for surgical technique (open or laparoscopic) in the present study, we evaluated tumor's diameter relative to surgical technique (open or laparoscopic) to show that surgeons did not use tumor diameters as a criteria for deciding about the surgical technique. The tumor diameter (tumor size ≥5 cm) did not differ between laparoscopic and open surgery group (laparoscopic: 33.8% vs. open: 23.6; P = 0.082).
TABLE 2.
Pathologic Risk Factors
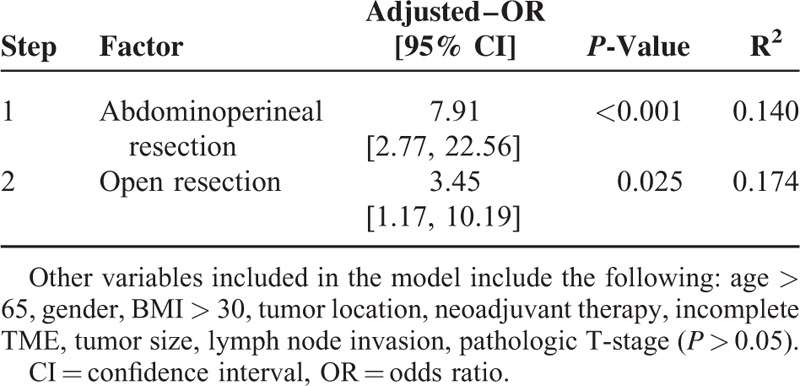
All variables in the bivariate analyses were entered into a forward logistic regression model, which revealed that APR and open resection were independent predictors of positive CRM (Table 3).
TABLE 3.
Independent Predictors of CRM Involvement
The follow-up rate for this study was greater than 95%. The mean follow-up period was 51.4 ± 24.4 months. Twenty-seven (6.4%) patients experienced local recurrence, and 72 (17.1%) patients experienced metastases to distant organs.
Overall, 5 (16.1%) patients with positive CRMs and 22 (5.7%) patients with negative CRMs experienced local recurrence (P = 0.040). Overall, 12 (38.7%) patients with positive CRMs and 60 (15.4%) patients with negative CRMs experienced metastases to distant organs (P = 0.002).
Overall and disease-free 5-year survival rates were analyzed based on negative and positive CRM status. Positive CRM was associated with significantly decreased 5-year overall and disease-free survival rates (Table 4) (Figures 2 and 3).
TABLE 4.
Survival Analysis (Log-Rank Sum Test)
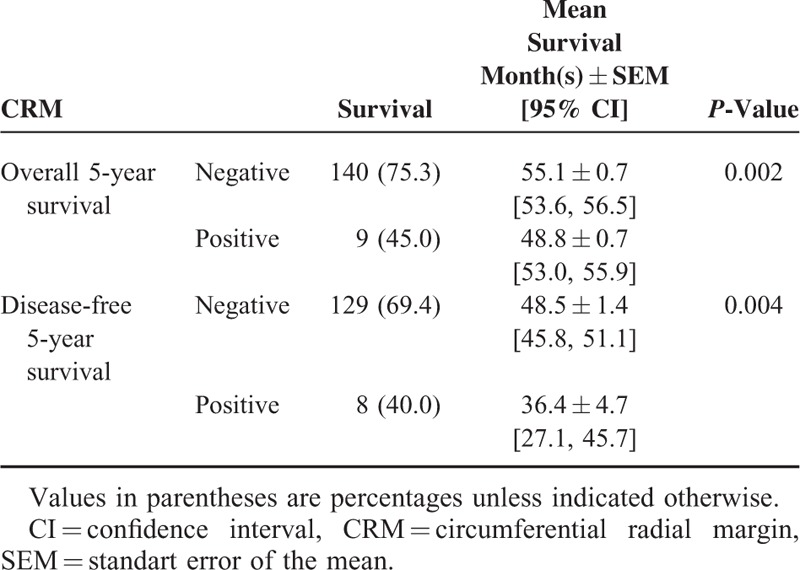
FIGURE 2.
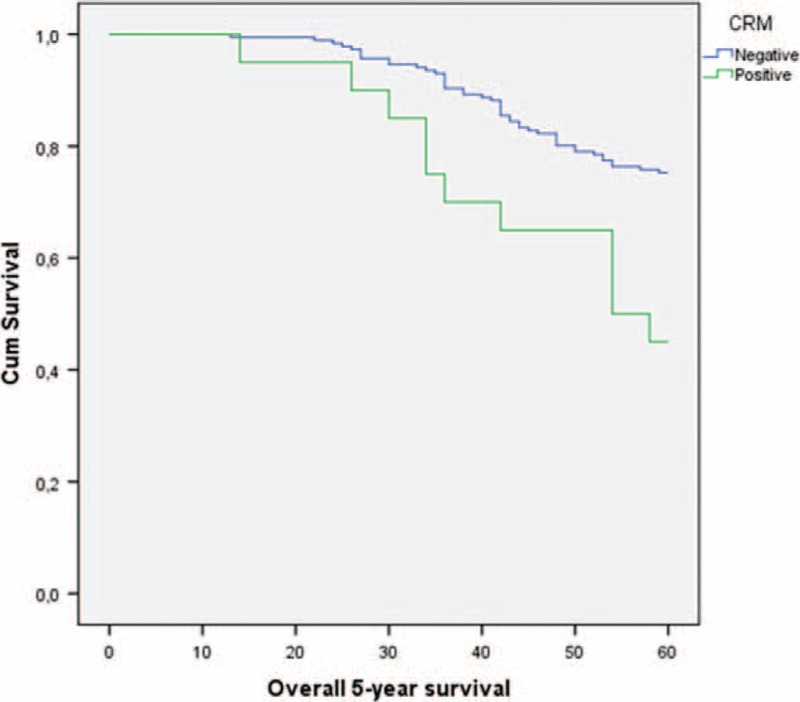
Five-year overall survival curves according to CRM using Kaplan–Meier analysis.
FIGURE 3.
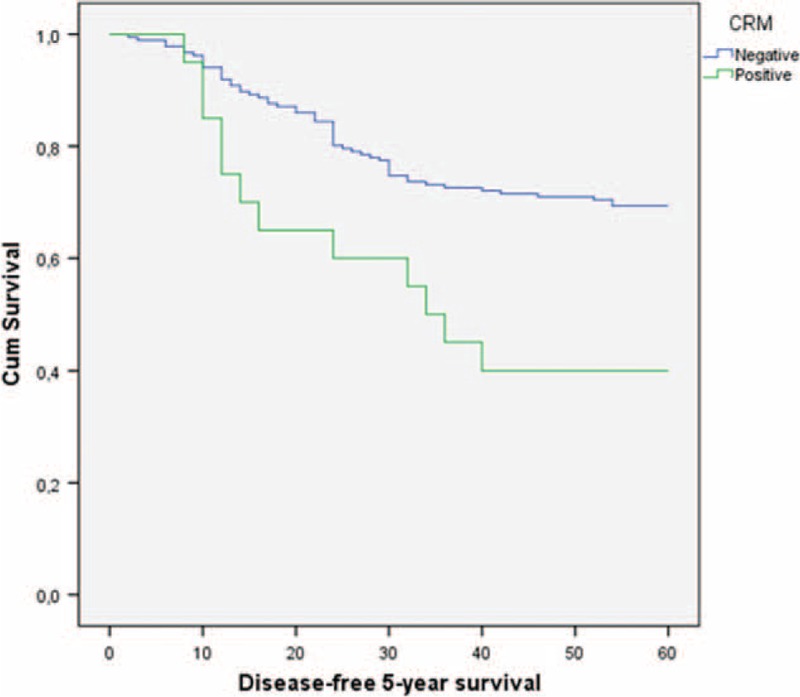
Five-year disease-free survival curves according to CRM using Kaplan–Meier analysis.
Cox-regression analysis was performed to identify independent predictors for survival based on surgical technique. When laparoscopic resection, incomplete mesorectum, APR and CRM status were entered into a stepwise logistic regression model, CRM involvement was the only independent predictor of survival (overall 5-year survival AOR: 3.6 [1.6, 8.2], P = 0.002; disease-free 5-year survival AOR: 3.1 [1.4, 7.0], P = 0.006).
DISCUSSION
The factors influencing rectal cancer outcomes are classified as tumor, patient, and surgeon related. Surgical skills and experience, comprehension of the significance of 2 essential concepts, and the selection of the appropriate surgical technique are accordingly important for the reduction in the rate of local recurrence. These concepts include the removal of the mesorectum en bloc (TME) and the achievement of negative circumferential margins. A significant correlation has been observed between TME performance and the subsequent development of local recurrence.2,14 The notion of TME has been a major concept associated with the reduction of local recurrence, but differences in the competence of the surgeon performing the technique should not be overlooked.15 CRM status initially proposed by Quirke et al10 is the most influential factor for local recurrence rates. Following TME, in last 15 years, we have observed two major variations in rectal cancer treatment, utilizing neoadjuvant treatment and performing minimal invasive surgery. Although many previous studies indicated CRM positivity as a prognostic factor on rectal cancer long-term outcomes, recent study from Sweden claimed that the relationship between CRM (+) and local recurrence is less important than previously stated and they contributed it to the innovation of pre- and postoperative oncological treatment.10,13,14,16
The present cohort study, in which multiple factors including these new treatment approaches with demographic, clinic, operative, and pathologic data were evaluated to determine the predictive factors on CRM positive in extraperitoneal rectal cancer, stated that tumor invasion depth, lymph node positivity, incompleteness of mesorectal integrity and APR are associated with positive CRM whereas demographic features, surgical technique, tumor location, and neoadjuvant therapy method have no effect on CRM positivity. The logistic regression analysis revealed that APR increases CRM positivity by 7.9 times as open surgery increases it by 3.45 times. Cox-regression analysis showed that CRM was the only independent predictor on survival.
The correlation between positive CRM status and age, gender, or BMI has been previously investigated, but, as in our study, a statistically significant correlation was not observed.17–19 Rullie et al20 found that the median length of CRM can be extended with neoadjuvant treatment. In contrast, many studies have demonstrated that neoadjuvant treatments did not significantly affect CRM positivity.17,21,22 We also did not identify a positive effect of neoadjuvant treatment on CRM positivity. The sort of short- or long-term neoadjuvant treatment also did not impact on CRM positivity. Although some studies identified that tumors with diameters ≥5 or ≥4 cm were associated with increased positive CRM status, controversial study showed no significant correlation between tumor diameter and CRM positivity.18,19,23 We did not found any correlation between tumor diameter and CRM positivity. We also added tumor's diameter into the multivariate analyses and we still did not identify a significant correlation between tumor diameter and CRM positivity.
It has been shown that increasing T stage and lymph node invasion (N+) were associated with CRM positivity.17,19,21,24 Our study also demonstrated that significantly higher rates of CRM positivity were seen in patients with T3 and T4 tumors or N+ tumors. However, multivariate analysis revealed no significant correlation between these pathological features and CRM positivity. We believe that the reason for these results can be explained with utilizing neoadjuvant treatments for patients who had local advanced rectal cancer. Many studies demonstrated that surgery associated with the complete removal of the mesorectum reduces positive CRM status and local recurrence rates.21,25–27 An MRC 07 trial reported a local recurrence rate of 1% in cases of stage III rectal tumors in which complete removal of mesorectum was achieved.28 A statistically significant correlation between incomplete TME and positive CRM status was also observed in the present study.
The primary concerns associated with the laparoscopic surgical treatment of rectal cancers are distal surgical margin and CRM. Proper laparoscopic colorectal surgery requires an adequate level of experience in colorectal and laparoscopic surgeries. The learning curve for these techniques is long and difficult, which slows down the widespread use of laparoscopic rectum cancer surgery, increases the uncertainty of the oncological outcomes and unearths the need for high-capacity experienced centers to competently apply the technique. In the COLOR II and MRC CLASICC trials, no significant differences were found in CRM positivity between conventional and laparoscopic-assisted surgery of rectal tumors but COLOR II study stated that CRM positivity was significantly higher in the open surgery group than the laparoscopic surgery group for distally located tumors.29,30 In our previous study, which included proximal, middle, and distal tumors, CRM positivity did not differ between laparoscopy and open surgery groups.31 However, the mesorectum becomes thinner in the distal rectum and there is no mesorectum to act as a barrier at the anorectal area and many studies suggested that positive CRM involvement decreased as the distance from the anal verge increased.21,32 Therefore, in the present study, patients with proximal located rectal tumors were excluded and only patients with extraperitoneal located rectal tumors were included. Whereas surgical technique (open or laparoscopic) did not effect on positive CRM status, no significant correlation was found between tumor location (middle or distal) and positive CRM status. However, the logistic regression model revealed that open surgery was the second independent risk factor for positive CRM status with APR. Multivariate analysis also indicated that open surgery increased CRM positivity 3.45-fold (P < 0.001). A randomized trial COLOR II showed the same result as the present study relative to CRM positivity. They found that laparoscopic surgery was associated with lower CRM positivity in patients with distal located rectal tumors and they also observed less local recurrence in laparoscopic group than open surgery group.33 We believe that this result was due to the favorable effect of laparoscopic surgery, which can provide the ability to conduct precise dissection under an enlarged endoscopic view like the same comment was stated in COLOR II trial.33
Although the outcomes of rectal cancer treatment have improved remarkably with new treatment strategies in last 20 years, the worse local recurrence rate has been still observed in patients treated with APR compared to patients treated with sphincter-preserving surgery secondary to high CRM positivity as reported previously.17,18,21,34 CRM positivity in the present study was significantly higher in patients who underwent APR. In the multivariate analysis, APR was found an independent risk factor for positive CRM involvement and increased CRM positivity 7.9-fold. Subgroup analyses in the present study revealed that incomplete mesorectum excision, which is an another negative factor for CRM positivity, resulted in a 2-fold increase in the APR group (P < 0.001). The achievement of negative surgical margins is more difficult as the tumor approaches the anal verge because of the lack of mesorectal tissue and the restriction of the pelvic outlet on the sidewalls by pelvic bones.32,34,35 To handle this issue some surgeons recommended extralevator APR, a wider resection including the levator muscles,36,37 but the benefit of extralevator APR is not clear in the literature. Recently, the retrospective study from Denmark and the systematic review from China revealed that extralevator APR did not improve CRM positivity compared to standard APR.35,38 In the present study, extralevator APR technique was not used on patients.
Positive CRM status is an influential prognostic factor for local recurrence, systemic spread, and survival rates.10,13,24 Wibe et al14 observed a local recurrence rate of 22% in patients with positive CRMs and 5% in patients with negative CRMs in their study. Quirke et al21 showed that CRM positivity negatively affected local recurrence and 3-year disease-free survival rates. However, the multivariate analysis, in which all parameters were included, revealed that N stage, T stage, completeness of mesorectal excision and tumor location were risk factors for local recurrence but positive CRM was not a risk factor.21 This result has been debated in the literature because neoadjuvant treatment can compensate for poor surgical skills and CRM positivity.26 Overall, local recurrence rates and distant metastasis in the present series were statistically higher in patients with positive CRM status.
Our previous study found that positive CRM was significantly associated with overall and disease-free survival rates.31 Kennelly et al24 demonstrated that CRM positivity decreased survival. CRM positivity was a significant factor on survival rates and was even more important than the TNM staging system for prognosis when combined with lymph node invasion status.26,39 In the present study, it was found that positive CRM status significantly reduced overall and disease free 5-year survival rates.
The main limitation of the present study is the retrospective nature of our analysis. The goal of curative rectal cancer surgery is to achieve negative CRMs and to protect sphincter function. This goal encumbers the design of prospective randomized trials that evaluate predisposing factors for positive CRM in rectal cancer surgery. Another limitation to our study is the evaluation of the completeness of mesorectum integrity, as this factor was only evaluated at our institute after 2007. We would like to emphasize that the present study, in which 78% of the patients underwent laparoscopic surgery, presents results from a single high-volume center in colorectal surgery. We think that this is important because it has been previously shown that the hospital volume influences CRM positivity rate and long-term outcomes in rectal cancer treatment.40
CONCLUSIONS
Our retrospective analyses, in mid- and distal rectal cancer patients, revealed that tumor invasion depth, lymph node positivity, incomplete mesorectal excision, and APR increased positive CRM status. In the logistic regression analysis of our large single-institution series, APR and open resection were independent predictors of CRM status for extraperitoneal rectal cancer. Significant increases in distant organ metastasis and local recurrence were observed during the follow-up period in the CRM-positive group of patients. Overall and disease-free 5-year survival rates were significantly decreased in patients with positive CRM status. Evaluation of the impact of the surgical techniques on survival rates revealed that positive CRM was the only independent predictor of survival.
Footnotes
Abbreviations: APR = abdominoperineal resections, BMI = body mass index, CRM = circumferential radial margin, N = lymph node invasion, T = tumor invasion depth, TME = total mesorectal excision.
Contribution of author/coauthor: Metin Keskin: Substantial contributions to conception and design, acquisition of data, drafting the article/drafting the article or revising it critically for important intellectual content.
Adem Bayraktar: Substantial contributions to conception and design, acquisition of data, drafting the article.
Emre Sivrikoz: Substantial contributions to conception and design, acquisition of data, drafting the article.
Gülçin Yegen: Substantial contributions to conception and design, acquisition of data, drafting the article.
Bora Karip: Substantial contributions to conception and design, acquisition of data, drafting the article acquisition of data.
Esra Saglam: Drafting the article or revising it critically for important intellectual content.
Mehmet Türker Bulut: Drafting the article or revising it critically for important intellectual content.
Emre Balık: Drafting the article or revising it critically for important intellectual content/final approval of the version to be published.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Maunsell HW. A new method of excising the two upper portions of the rectum and the lower segment of the sigmoid flexure of the colon. Lancet 1892; 2:473–476. [DOI] [PubMed] [Google Scholar]
- 2.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982; 69:613–616. [DOI] [PubMed] [Google Scholar]
- 3.Den Dulk M, Krijnenb P, Corrie AM, et al. Improved overall survival for patients with rectal cancer since 1990: the effects of TME surgery and pre-operative radiotherapy. Eur J Cancer 2008; 44:1710–1716. [DOI] [PubMed] [Google Scholar]
- 4.Kusunoki M, Inoue Y. Current surgical management of rectal cancer. Dig Surg 2007; 24:115–119. [DOI] [PubMed] [Google Scholar]
- 5.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004; 232:335–346. [DOI] [PubMed] [Google Scholar]
- 6.Dahlberg M, Glimelius B, Påhlman L. Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Ann Surg 1999; 229:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12:575–582. [DOI] [PubMed] [Google Scholar]
- 8.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345:638–646. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–23. [DOI] [PubMed] [Google Scholar]
- 10.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986; 2:996–999. [DOI] [PubMed] [Google Scholar]
- 11.Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg 1983; 198:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernava AM, III, Moran M, Rothenberger DA, et al. A prospective evaluation of distal margins in carcinoma of the rectum. Surg Gynecol Obstet 1992; 175:333–336. [PubMed] [Google Scholar]
- 13.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis 1988; 3:127–131. [DOI] [PubMed] [Google Scholar]
- 14.Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 2002; 89:327–334. [DOI] [PubMed] [Google Scholar]
- 15.Moran BJ, Holm T, Brannagan G, et al. The English National Low Rectal Cancer Development Programme: key messages and future perspectives. Colorectal Dis 2014; 16:173–178. [DOI] [PubMed] [Google Scholar]
- 16.Nikberg M, Kindler C, Chabok A, et al. Circumferential resection margin as a prognostic marker in the modern multidisciplinary management of rectal cancer. Dis Colon Rectum 2015; 58:275–282. [DOI] [PubMed] [Google Scholar]
- 17.Rullier A, Gourgou-Bourgade S, Jarlier M, et al. Predictive factors of positive circumferential resection margin after radiochemotherapy for rectal cancer: the French randomised trial ACCORD12/0405 PRODIGE 2. Eur J Cancer 2013; 49:82–89. [DOI] [PubMed] [Google Scholar]
- 18.Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol 2013; 20:1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J, Kim H, Hur H, et al. Circumferential resection margin involvement in stage III rectal cancer patients treated with curative resection followed by chemoradiotherapy: a surrogate marker for local recurrence? Yonsei Med J 2013; 54:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rullier E, Goffre B, Bonnel C, et al. Preoperative radiochemotherapy and sphincter saving resection for T3 carcinomas of the lower third of the rectum. Ann Surg 2001; 234:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009; 373:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol 2005; 23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 23.Oh SJ, Shin JY. Risk factors of circumferential resection margin involvement in the patients with extraperitoneal rectal cancer. J Korean Surg Soc 2012; 82:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennelly RP, Rogers AC, Winter DC, et al. Multicentre study of circumferential margin positivity and outcomes following abdominoperineal excision for rectal cancer. Br J Surg 2013; 100:160–166. [DOI] [PubMed] [Google Scholar]
- 25.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002; 20:1729–1734. [DOI] [PubMed] [Google Scholar]
- 26.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008; 26:303–312. [DOI] [PubMed] [Google Scholar]
- 27.Hiranyakas A, da Silva G, Wexner SD, et al. Factors influencing circumferential resection margin in rectal cancer. Colorectal Dis 2013; 15:298–303. [DOI] [PubMed] [Google Scholar]
- 28.Quirke P, Sebag-Montefiore D, Steele R, et al. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by pre-operative short course radiotherapy. Preliminary results of the Medical Research Council (MRC) CR07 trial and CR0. ASCO Annu Meet Proc 2006; 24 (18 suppl): [Google Scholar]
- 29.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013; 14:210–218. [DOI] [PubMed] [Google Scholar]
- 30.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; 365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 31.Dural AC, Keskin M, Balık E, et al. The role of the laparoscopy on circumferential resection margin positivity in patients with rectal cancer: long-term outcomes at a single high-volume institution. Surg Laparosc Endosc Percutan Tech 2015; 25:129–137. [DOI] [PubMed] [Google Scholar]
- 32.Marr R, Birbeck K, Garvican J, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg 2005; 242:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 34.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol 2015; 33:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perdawood SK, Lund T. Extralevator versus standard abdominoperineal excision for rectal cancer. Tech Coloproctol 2015; 19:145–152. [DOI] [PubMed] [Google Scholar]
- 36.Huang A, Zhao H, Ling T, et al. Oncological superiority of extralevator abdominoperineal resection over conventional abdominoperineal resection: a meta-analysis. Int J Colorectal Dis 2014; 29:321–327. [DOI] [PubMed] [Google Scholar]
- 37.West NP, Finan PJ, Anderin C, et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol 2008; 26:3517–3522. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X, Sun T, Xie H, et al. Extralevator abdominoperineal excision for low rectal cancer: a systematic review and meta-analysis of the short-term outcome. Colorectal Dis 2015; 17:474–481. [DOI] [PubMed] [Google Scholar]
- 39.Gosens MJ, Van Krieken JH, Marijnen CA, et al. Improvement of staging by combining tumor and treatment parameters: the value for prognostication in rectal cancer. Clin Gastroenterol Hepatol 2007; 5:997–1003. [DOI] [PubMed] [Google Scholar]
- 40.Gietelink L, Henneman D, van Leersum NJ, et al. The influence of hospital volume on circumferential resection margin involvement: Results of the Dutch Surgical Colorectal Audit. Ann Surg 2014; DOI: 10.1097/SLA 0000000000001009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


