Abstract
This study examines whether pelvic inflammatory disease (PID) facilitates the development of intracerebral hemorrhage (ICH).
By using outpatient claims data from the National Health Insurance Research Database (NHIRD) of Taiwan, we included the data of 25,508 patients who were newly diagnosed with PID between 1999 and 2004, and also from the Taiwan NHIRD, we randomly selected 102,032 women without PID, who were frequency-matched by age and entry-year and with 4 times the number of the PID patients, as the control cohort. We measured ICH risks associated with PID and comorbidities, including hyperlipidemia, diabetes, hypertension, ischemic heart disease, and atrial fibrillation, by the end of 2011.
In comparison with the controls, the ICH hazard was less in the PID group with an adjusted hazard ratio (aHR) of 0.67 (95% confidence interval [CI]:0.50–0.90), which was noted by calculation with the Cox proportional regression model. The ICH risk in the PID patients reduced progressively with the advance of age, with aHRs of 0.75 (95% CI:0.41–1.39) and 0.50 (95% CI:0.29–0.88), respectively, in the age <35-year and age ≥50-year groups. ICH risk lowered gradually with the progress of PID severity, from mild PID with an aHR of 0.72 (95% CI:0.53–0.98) to severe PID with that of 0.30 (95% CI:0.10–0.92). PID patients without any comorbidites had lower ICH risk (aHR = 0.63, 95% CI:0.42–0.94) than the controls without any comorbidites did.
Our findings revealed that PID is associated with reduced ICH development, especially for older patients.
INTRODUCTION
Intracerebral hemorrhage (ICH) represents only ∼10% to 15% of all stroke types.1–3 However, the outcome for patients with ICH is more severe, with a higher mortality and morbidity, and expends a greater amount of socioeconomic and health resources compared with ischemic stroke.1–3 Certain arteriolar pathological alterations have been noted in the cerebral arterioles of ICH patients (eg, death and the proliferation of arteriolar smooth muscle cells, ectasia and the subsequent formation of microaneurysms in arterioles,1–3 and microaneurysm rupture in arterioles).1,4,5 These arteriolar pathological variations in ICH could be triggered by certain conventional cardiovascular risk factors, including hyperlipidemia,3,6 diabetes,1,3,4,7,8 hypertension,1–8 ischemic heart disease (IHD),2,7,8 and atrial fibrillation (AF).4,7,8 However, in ∼10% to 25% of patients with ICH, the risk factors for ICH remain unclear,9,10 especially in younger patients.1,9,10
Numerous studies have presented powerful evidence that coronary microaneurysms could develop after the emergence of arterial ectasia in the coronary arteries, which could be triggered by chronic inflammatory processes.11–13 Moreover, previous investigations have linked ICH with various chronic cerebral arteriolar inflammations induced by autoimmune diseases (eg, rheumatoid arthritis [RA]14,15 and systemic lupus erythematosus [SLE]14,16) as well as viral infections (eg, varicella-zoster virus [VZV]17,18 and human immunodeficiency virus [HIV]).17,19 However, the precise mechanisms linking chronic inflammatory diseases with ectasia in cerebral arterioles and the subsequent generation and breaking of cerebral microaneurysms remain unclear.
Pelvic inflammatory disease (PID), a chronic inflammatory condition caused by an infection emerging from the cervix to the upper genital tract, is commonly diagnosed in young women.20–22 PID is characterized by frequent complications from the infections of Chlamydia trachomatis (C trachomatis) and Neisseria gonorrhoeae (N gonorrhoeae).20–22 As reported in previous investigations, type 1 T helper cells (Th1), which are primarily responsible for cellular immunity and enhance the secretion of proinflammatory cytokines, and type 2 T helper cells (Th2), which are responsible for humoral immunity and modulate the functions of Th1, both play a critical role in the immune responses of PID induced by C trachomatis and N gonorrhoeae.23–25 Tseng et al26 found that a milder form of allergic rhinitis (AR), a Th2-modulated inflammatory disorder, could reduce the risk of ICH, and in an investigation with a short follow-up period (2004–2005),27 Chen et al found an increased risk of ischemic stroke among patients with PID. However, whether PID, a disease involving the immune response of both Th1 and Th2 cells, advances or attenuates the development of ICH remains unclear. With a large number of enrollees in the National Health Insurance (NHI) program of Taiwan, for our study, we used this claims database to measure the correlation between PID and ICH over a follow-up period of 13 years (1999–2011).
MATERIALS AND METHODS
Data Source
We used the Longitudinal Health Insurance Database (LHID), which consists of 1 million patient data from 1996 to 2000 that were extracted from the catalogue of beneficiaries in the National Health Insurance Research Database (NHIRD).26,28 The NHIRD, which comprises >98% of the population of Taiwan, was established on the basis of the NHI program implemented in 1995. The contents of the LHID include information on patients’ medical registry facilities, their demographic data, and the types of medical care they underwent. The healthcare records of outpatients and inpatients in the LHID were required under the allowances of the insurance authority. To ensure patient privacy, the patient identification numbers used for linking data files were encrypted before the public release of the data. The disease diagnoses were determined in accordance with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Study Patients
We included participants of the NHI program who were newly diagnosed with PID (ICD-9-CM code 614) at the start of 1999 until the end of 2004, and with no history of stroke (ICD-9-CM codes 430-438), in the PID group according to the LHID, the data of which were drawn from the NHIRD. The first date of PID diagnosis was circumscribed as the entry date. The experimental group consisted of 25,508 women diagnosed with PID. By contrast, the control cohort comprised 4 times the number of patients in the PID group from the NHIRD, and they included women with neither PID nor a history of stroke (n = 102,032) who were frequency matched by age and entry year.
Outcome and Relevant Variables
The PID and control cases were followed until December 31, 2011, to evaluate those diagnosed with ICH (ICD-9-CM code 431). The variables relevant to ICH were age, and comorbidities, including hyperlipidemia (ICD-9-CM code 272), diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401-405), IHD (ICD-9-CM codes 410-414), and AF (ICD-9-CM code 427.32). We also evaluated the ICH cases in association with these variables.
Statistical Analysis
The spreads of the discrete and continuous variables between the PID and control cohorts were assessed by conducting the chi-square test and t test, respectively. The incidence rate of ICH per 1000 person-years was evaluated and computed from the date of entry until the first date of ICH occurrence, withdrawal from the insurance program, death, or December 31, 2011. The Cox regression model was used to determine the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) in order to measure the risk of ICH development between the 2 groups. Further assessments of ICH risk were conducted by stratifying by age (<35 years, 35–49 years, and ≥50years) and the common comorbidities of ICH.
For data assessment, the ICH risk was also evaluated in relation to PID severity, which was defined as patients receiving inpatient or outpatient medical services because of PID. We further categorized PID severity as patients with PID without hospitalization (mild group) and patients with hospitalization because of PID (severe group). The cumulative ICH risk for both PID and control groups was plotted through the analyses by using the Kaplan–Meier model, and the log-rank test was conducted to determine the differences between the 2 cohorts. Significance was circumscribed with a 2-tailed P value < 0.05. All statistical calculations were executed using SAS (version 9.1, SAS Institute Inc., NC).
Ethics Statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2–115). The IRB also specifically waived the consent requirement.
RESULTS
On the basis of the age distributions, no significant differences were found between the PID and control groups (Table 1). More than 90% of the PID patients were <50 years. Compared to control cohort, PID cohorts were with more comorbidities including hypertension (5.64% vs 5.08%), hyperlipidemia (4.76% vs 3.45%), diabetes (2.45% vs 1.89%), and IHD (2.84% vs 1.91%).
TABLE 1.
Demographic Data and Comorbidities of the Pelvic Inflammatory Disease and Control Groups
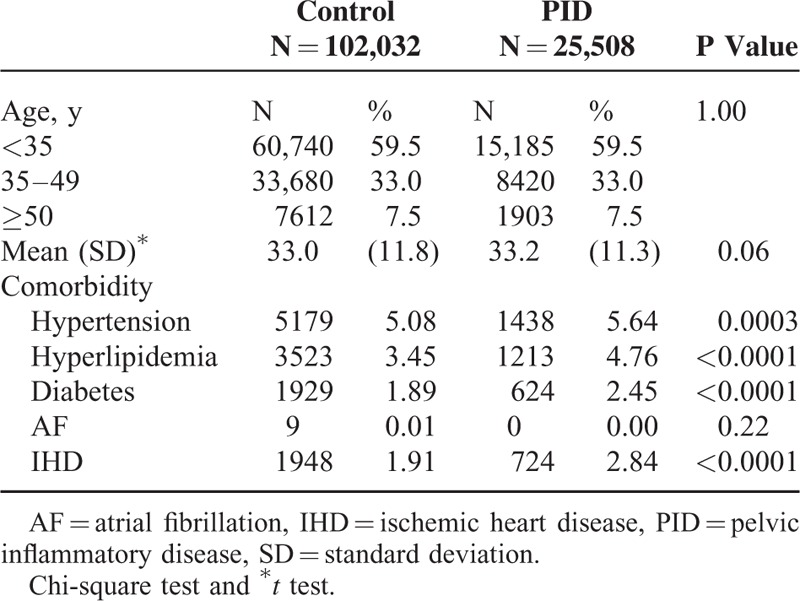
The overall ICH incidence in the PID and comparison cohorts was 2.02 and 3.10 per 1000 person-years, respectively (Table 2). After adjusting for age and the comorbidities of hyperlipidemia, diabetes, hypertension, IHD, and AF, we found that the aHR of ICH development was 0.67-fold (95% CI:0.50–0.90) for the PID group compared with those without PID. The ICH incidence rate in both groups increased with older age, and the age-specific risk for ICH in the PID group was lower than in the control cohort for all age groups, especially for older patients (Table 3). The incidence rate of ICH increased in conjunction with comorbidities in both cohorts, even though the comorbidity-specific risk for ICH in the PID group was still lower than in the control cohort, either with or without comorbidities (Table 3). All patients, with or without PID, but with the comorbidities of hypertension, diabetes, or IHD, were at a greater risk of ICH, especially those with hypertension (Table 2).
TABLE 2.
Incidence Rate and Hazard Ratio for Intracerebral Hemorrhage, Stratified by Demographic Factors and Comorbidities
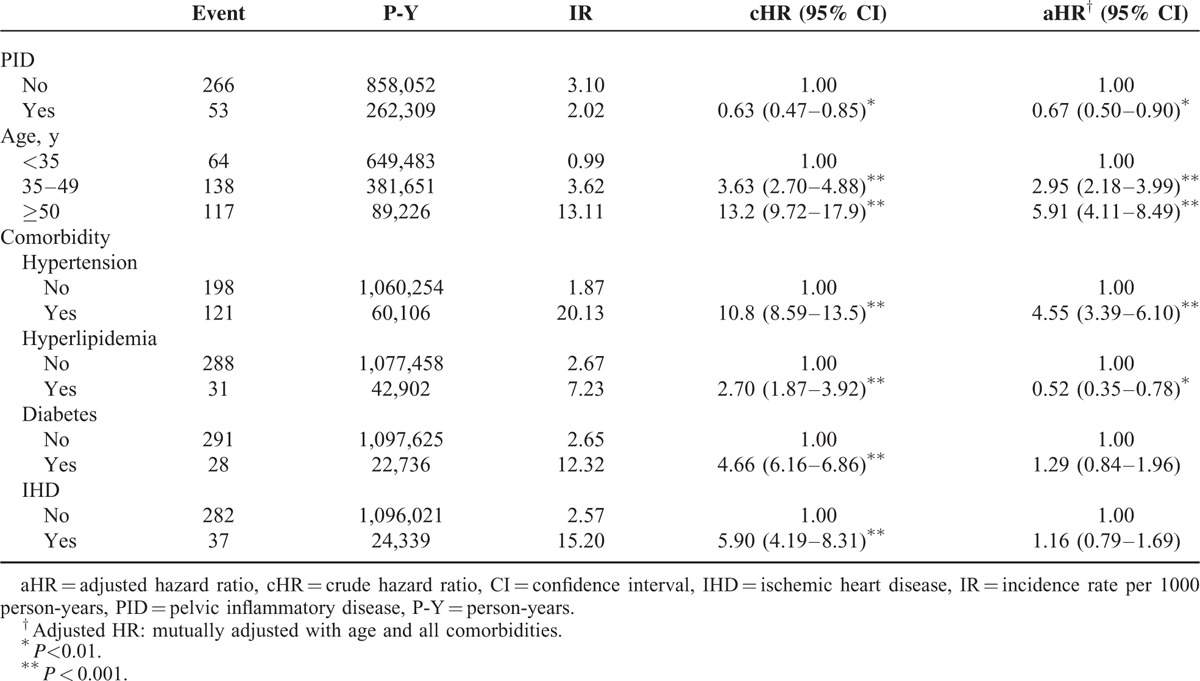
TABLE 3.
Incidence and Hazard Ratio for Intracerebral Hemorrhage Between the Pelvic Inflammatory Disease and Control Groups, Stratified by Demographic Factors and Comorbidities
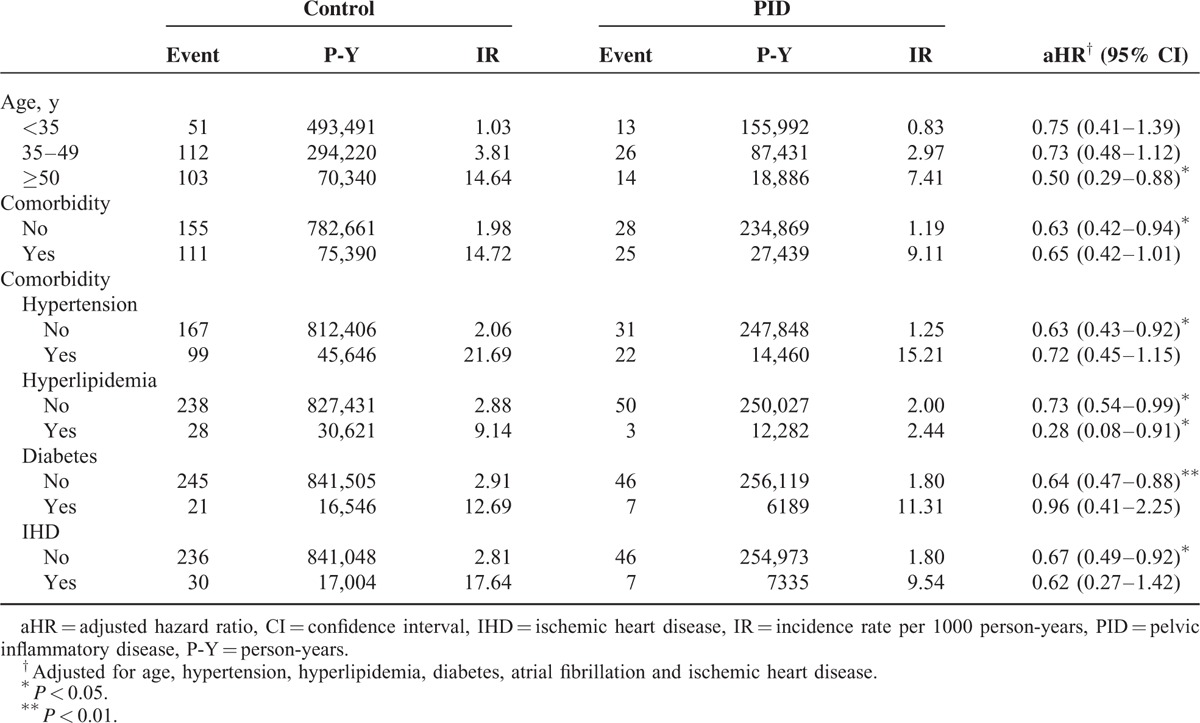
Our estimation results on the correlation between PID severity and ICH risk revealed a dose-response effect regarding the attenuation in ICH development; in other words, the risk of ICH decreased with a worsening PID severity (Table 4). Compared with the control group, the aHR for patients with PID stratified by severity was 0.72 (95% CI:0.53–0.98) for the mild group and 0.30 (95% CI:0.10–0.92) for the severe group. The Kaplan–Meier graph displayed that the cumulative incidence of ICH was lower for the PID group than for the control cohort (log-rank P = 0.003) (Figure 1).
TABLE 4.
Incidence and Hazard Ratio for Intracerebral Hemorrhage, Stratified by Whether Patients Diagnosed With Pelvic Inflammatory Disease Were Hospitalized During the Study Period
FIGURE 1.
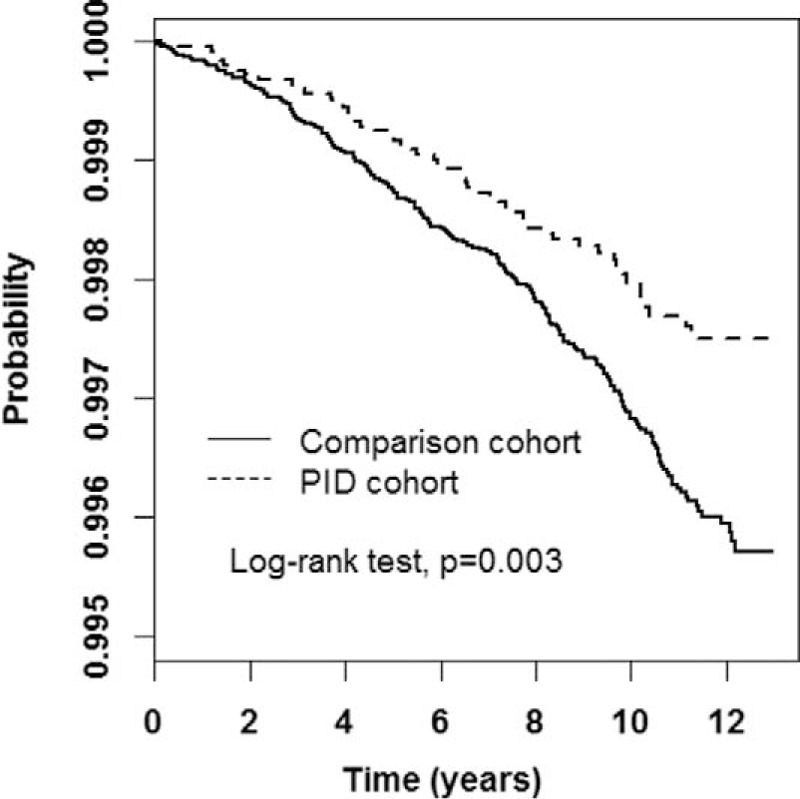
Cumulative intracerebral-hemorrhage-free rates between pelvic inflammatory disease (PID) and comparison cohorts, analyzed through the Kaplan–Meier model. PID = pelvic inflammatory disease.
DISCUSSION
Associations of extended ICH risk because of increased arterial ectasia in cerebral arterioles with inflammatory processes (eg, viral infections withVZV17,18 and HIV17,19) as well as dysimmunity disorders (eg, RA14,15 and SLE14,16) have been reported in previous investigations. In the present retrospective cohort research, we compared PID patients against those without this disease over a 13-year follow-up period (1999–2011). A 33% decrease in the ICH risk was found in the PID cohort (Table 2). The ICH risk diminished for patients with either mild or severe PID, and we discerned a dose-response effect regarding the reduction of ICH risk on PID severity (Table 4). As demonstrated in previous studies,29–31 Th2-associated immune responses have been shown to be correlated with anti-inflammatory properties, which might play a critical role in reducing ICH risk in patients with PID.
Age is the most crucial risk factor for ICH.1–9 However, among all the patients examined, regardless of whether they were diagnosed with PID, those at an advanced age had a higher age-specific ICH risk (Table 2), and the incidence rate of ICH rose gradually with age in both the PID and control groups under study (Table 3). Moreover, the aHR of ICH was lower in patients with PID compared with the control group, regardless of age stratification (Table 3), and ICH risk decreased steadily in conjunction with the severity of PID (Table 4). These findings further support the association between PID and the attenuation of ICH development.
Comorbidities for Intracerebral Hemorrhage
In several investigations, the authors have combined the risk of ICH with the male gender,1–8 older age,1–9 and the comorbidities of hyperlipidemia,3,6 diabetes,1,3,4,7,8 hypertension,1–8 IHD,2,7,8 and AF.4,7,8 In the present study, the ICH risk for patients with PID increased in combination with the comorbidities of diabetes and hypertension (Table 3).
STRENGTHS AND LIMITATIONS
With the advantages of using a large sample as well as the availability of a low loss-to-follow-up rate in a longitudinal arrangement, we could provide analyses with further stratification of variables, including age, prevalent comorbidities for ICH, and the severity of PID. The dose–response effect on reducing ICH risk associated with PID severity further strengthened the validity of our findings. Comorbidities could lead to variations in the link between PID and ICH development. In order to prevent healthcare fraud, the insurance claims for reimbursements for inpatient and outpatient services are surveyed strictly in the NHI program of Taiwan. The monitoring system for the NHI program lends further validity to the diagnoses reported in insurance claims.
However, our study was still subject to several limitations. First, other potential risk factors for cardiovascular diseases (eg, possible immunity variations resulting from PID and physical inactivity, which might affect patients by reducing the risk of ICH development) could not be obtained from the NHI database. Second, the immunity-correlated laboratory data, including the serum counts of Th1 and Th2, serum immunoglobulin levels, C-reactive protein serum levels, and the erythrocyte sedimentation rate were unavailable in the NHIRD. Hence, we could not assess the association between the different immune conditions in individual PID patients and ICH risk. Third, data on patients’ private behavior (eg, cigarette smoking and alcohol consumption habits) were unavailable. Nevertheless, the low smoking rate among women in Taiwan (<3.5%)32 implies that cigarette smoking did not appear to be a major confounder in the link between PID and ICH. Fourth, although we noted a higher prevalence of comorbidities for ICH in patients with PID compared with the control group, the results revealed that even with these risk factors, which could further advance the development of ICH, PID was still found to have a lower ICH risk. In numerous studies,29–31,33,34 Th2, which plays a crucial role in the generation of immunity in PID,20–22 has been found to be closely related to the control of anti-inflammatory responses. However, the precise mechanisms by which Th2-related anti-inflammatory effects protect PID patients from ICH development remain unclear. Future investigations on this issue are warranted to further confirm the correlation between PID and ICH development.
CONCLUSIONS
The findings of this study revealed that the overall risk of ICH was lower in patients with PID; however, ICH risk may increase relative to a younger age as well as with certain common comorbidities of ICH. Further investigations are required to verify the findings of our study. These findings can serve as a reference for ICH prevention in PID patients, especially for those of a younger age.
Footnotes
Abbreviations: CIs = confidence intervals, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases Ninth Revision Clinical Modification, ICH = intracerebral hemorrhage, NHIRD = National Health Insurance Research Database, PID = pelvic inflammatory disease.
M-CL and C-HK contributed equally to this study.
Author contributions: these authors’ individual contributions were as follows—C-HT and C-HK designed the study. C-HM carried out the statistical analysis. All authors drafted the manuscript. M-CL and C-HK revised the manuscript. All authors approved the final version submitted for publication.
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sutherland GR, Auer RN. Primary intracerebral hemorrhage. J Clin Neurosci 2006; 13:511–517. [DOI] [PubMed] [Google Scholar]
- 2.Labovitza DL, Sacco RL. Intracerebral hemorrhage: update. Curr Opin Neurol 2001; 14:103–108. [DOI] [PubMed] [Google Scholar]
- 3.Ariesen MJ, Claus SP, Rinkel GJE, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003; 34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 4.Ferro JM. Update on intracerebral hemorrhage. J Neurol 2006; 253:985–999. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 6.Bozluolcay M, Nalbantoglu M, Gozubatik-Celik RG, et al. Hypercholesterolemia as one of the risk factors of intracerebral hemorrhage. Acta Neurol Belg 2013; 113:459–462. [DOI] [PubMed] [Google Scholar]
- 7.Hamad A, Hamad A, Sokrab TEO, et al. Stroke in Qatar: a one-year, hospital-based study. J Stroke Cerebrovasc Dis 2001; 10:236–241. [DOI] [PubMed] [Google Scholar]
- 8.Mayo NE, Nadeau L, Daskalopoulou SS, et al. The evolution of stroke in Quebec: a 15-year perspective. Neurology 2007; 68:1122–1127. [DOI] [PubMed] [Google Scholar]
- 9.Karatay M, Mehmetoğlu R, Erdem Y, et al. The relation between surgically treated spontaneous intracerebral hematomas and mortality: retrospective evaluation of 72 cases. Türk Nöroloji Dergisi 2012; 18:83–87. [Google Scholar]
- 10.Lai SL, Chen ST, Lee TH, et al. Spontaneous intracerebral hemorrhage in young adults. Eur J Neurol 2005; 12:310–316. [DOI] [PubMed] [Google Scholar]
- 11.Li JJ, Nie SP, Qian XW, et al. Chronic inflammatory status in patients with coronary artery ectasia. Cytokine 2009; 46:61–64. [DOI] [PubMed] [Google Scholar]
- 12.Nichols L, Lagana S, Parwani A. Coronary artery aneurysm: a review and hypothesis regarding etiology. Arch Pathol Lab Med 2008; 132:823–828. [DOI] [PubMed] [Google Scholar]
- 13.Dogan A, Tuzun N, Turker Y, et al. Matrix metalloproteinases and inflammatory markers in coronary artery ectasia: their relationship to severity of coronary artery ectasia. Coron Artery Dis 2008; 19:559–563. [DOI] [PubMed] [Google Scholar]
- 14.Zöller B, Li X, Sundquist J, et al. Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immunemediated diseases: a nationwide follow-up study from Sweden. BMC Neurol 2012; 12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmqvist M, Gränsmark E, Mantel Ä, et al. Occurrence and relative risk of stroke in incident and prevalent contemporary rheumatoid arthritis. Ann Rheum Dis 2013; 72:541–546. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan E. Stroke subtypes among young patients with systemic lupus erythematosus. Am J Med 2005; 118:1415.e1-1415.e7. [DOI] [PubMed] [Google Scholar]
- 17.Nagel MA, Mahalingam R, Cohrs RJ, et al. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets 2010; 10:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2008; 8:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbs MR, Berger JR. Stroke in HIV infection and AIDS. Expert Rev Cardiovasc Ther 2009; 7:1263–1271. [DOI] [PubMed] [Google Scholar]
- 20.French CE, Hughes G, Nicholson A, et al. Estimation of the rate of pelvic inflammatory disease diagnoses: trends in England, 2000–2008. Sex Transm Dis 2011; 38:158–162. [DOI] [PubMed] [Google Scholar]
- 21.Ross J. Pelvic inflammatory disease. Medicine 2010; 38:255–259. [Google Scholar]
- 22.Herzog SA, Heijne JCM, Althaus CL, et al. Describing the progression from Chlamydia trachomatis and Neisseria gonorrhoeae to pelvic inflammatory disease: systematic review of mathematical modeling studies. Sex Transm Dis 2012; 39:628–637. [DOI] [PubMed] [Google Scholar]
- 23.Chen KS, Wang PH, Yang S-F, et al. Significant elevation of a Th2 cytokine, interleukin-10, in pelvic inflammatory disease. Clin Chem Lab Med 2008; 46:1609–1616. [DOI] [PubMed] [Google Scholar]
- 24.Vats V, Agrawal T, Salhan S, et al. Primary and secondary immune responses of mucosal and peripheral lymphocytes during Chlamydia trachomatis infection. FEMS Immunol Med Microbiol 2007; 49:280–287. [DOI] [PubMed] [Google Scholar]
- 25.Escobar A, Candia E, Reyes-Cerpa S, et al. Neisseria gonorrhoeae induces a tolerogenic phenotype in macrophages to modulate host immunity. Mediators Inflamm 2013; 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng C-H, Chen J-H, Lin C-L, et al. Decreased risk of intracerebral hemorrhage among patients with milder allergic rhinitis. QJM 2015; doi:10.1093/qjmed/hcv104 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Chen P-C, Tseng T-C, Hsieh J-Y, et al. Association between stroke and patients with pelvic inflammatory disease: a nationwide population-based study in Taiwan. Stroke 2011; 42:2074–2076. [DOI] [PubMed] [Google Scholar]
- 28.Tseng C-H, Chen J-H, Muo C-H, et al. Increased risk of ischaemic stroke amongst patients with chronic osteomyelitis: a population-based cohort study in Taiwan. Eur J Neurol 2015; 22:633–639. [DOI] [PubMed] [Google Scholar]
- 29.Graber JJ, Dhib-Jalbut S. Protective autoimmunity in the nervous system. Pharmacol Ther 2009; 121:147–159. [DOI] [PubMed] [Google Scholar]
- 30.Gimsa U, Wolf SA, Haas D, et al. Th2 cells support intrinsic anti-inflammatory properties of the brain. J Neuroimmunol 2001; 119:73–80. [DOI] [PubMed] [Google Scholar]
- 31.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000; 117:1162–1172. [DOI] [PubMed] [Google Scholar]
- 32.Health Promotion Administration, Taiwan. Taiwan tobacco control annual report 2014. Taipei: Ministry of Health and Welfare, Taiwan, 2014. [Google Scholar]
- 33.Toscano MA, Commodaro AG, Ilarregui JM, et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol 2006; 176:6323–6332. [DOI] [PubMed] [Google Scholar]
- 34.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res 2001; 88:877–887. [DOI] [PubMed] [Google Scholar]


