Abstract
This prospective cohort study was conducted to assess the duration of daytime napping and its effect combined with night sleep deprivation on the risk of developing high HOMA-IR (homeostasis model assessment of insulin resistance) index and disadvantageous changes in glycosylated hemoglobin (HbA1c) levels.
A total of 5845 diabetes-free subjects (2736 women and 3109 men), 30 to 65 years of age, were targeted for this cohort study since 2008. Multiple adjusted Cox regression models were performed to evaluate the single and joint effects of daytime napping on the risk of an elevated HbA1c level and high HOMA-IR index.
After an average of 4.5 years of follow-up, >30 minutes of daytime napping was significantly associated with an increased risk of an elevated HbA1c level (>6.5%) in men and women (all P trend < 0.05). Hazard ratios (HRs) for an HbA1c level between 5.7% and 6.4% were also significant in the entire cohort and women, but nonsignificant in men. HRs (95% confidence interval, CIs) for the high HOMA-IR index in the entire cohort, men, and women were 1.33 (1.10–1.62), 1.46 (1.08–1.98), and 1.47 (1.12–1.91), respectively. The combination of sleep deprivation with no naps or >30 minutes napping and the combination of no sleep deprivation with >30 minutes daytime napping were all associated with an HbA1c level >6.5% (HR = 2.08, 95% CI = 1.24–3.51; HR = 4.00, 95% CI = 2.03–7.90; and HR = 2.05, 95% CI = 1.29–3.27, respectively). No sleep deprivation combined with >30 minutes daytime napping correlated with a high risk of an HbA1c level between 5.7% and 6.4% and high HOMA-IR index (HR = 2.12, 95% CI = 1.48–3.02; and HR = 1.35, 95% CI = 1.10–1.65, respectively).
Daytime napping >30 minutes was associated with a high risk of an elevated HbA1c level and high HOMA-IR index. No sleep deprivation combined with napping >30 minutes carries a risk of abnormal glucose metabolism. Sleep deprivation combined with brief daytime napping <30 minutes was not associated with a risk for an elevated HbA1c level and high HOMA-IR index.
INTRODUCTION
Napping appears to be a highly prevalent phenomenon worldwide, especially in China. Of older Chinese adults, 61.7% of men and 46.8% of women practice habitual daytime napping.1 Napping is reported to be related to poor sleep and poor health/functional status, such as a high risk for all-cause, cardiovascular disease-specific, cancer-specific, diabetes-specific, and Alzheimer disease-specific mortality rates2–4; however, the cause or symptoms associated with the high risks for development of these diseases is unknown.5
Most of the research used to assess the risk of sleep on the development of type 2 diabetes (T2DM) is based on fasting blood glucose (FBG) levels, but short-term changes in diet, stress, and many other confounding factors are known to affect glucose testing.6 Glycosylated hemoglobin (HbA1c) is superior to daily blood glucose monitoring and more reflective of microvascular complications than glucose and has been recognized as the gold standard in the assessment of average glycemia over time.6,7 There is only 1 cross-sectional study focusing on the relationship between daytime napping hours and HbA1c levels.8 Thus, long-term follow-up studies are warranted using the exact duration of daytime napping as an exposure factor to uncover the longitudinal effect on disease-related biochemical parameters, such as changes in HbA1c, and the temporal relationship.
Insulin, which reflects intra- and extracerebral endocrine function, increases after eating in healthy individuals. Insulin has been reported to affect rapid eye movement (REM) and non-REM sleep time directly via the brain chemistry of neurotransmitters, such as serotonin, catecholamines, and acetylcholine.9 Insulin resistance is the common feature among many chronic diseases, including atherosclerosis, diabetes, dyslipidemia, and metabolic syndrome and is usually related to obstructive sleep apnea and night sleep deprivation through circadian disruptions of hormones, increased obesity, and/or the autonomic nervous system.10–14 This evidence supports a role for insulin as an influencing factor of siestas; however, data regarding daytime napping hours as an influence on insulin are scant; there are few prospective cohort studies on the relationship between daytime napping hours and homeostasis model assessment of insulin resistance (HOMA-IR) index, which is the most commonly used surrogate measure of insulin resistance in vivo.15 Although epidemiologic research has investigated napping and no napping and the correlations with pathologic states, such as stroke and myocardial infarction, different lengths of daytime napping and the effect on the pathogenesis of chronic diseases has seldom been assessed. However, short and long naps may have distinct effects on fatigue levels, immune function, and sleep-wake cycles.16,17 Lovato and Lack18 concluded that brief naps contain no slow wave electroencephalography (EEG) activity and have benefits such as a substantial increase in alertness through rapid dissipation of inhibition in the wake-active cells related to the sleep-switch mechanism. In addition, the length of daytime naps and the timing of daytime melatonin production are strongly associated with brief naps.19 A 20-minute nap before the postlunch dip has been reported to benefit sleepiness, subjective ratings of performance level, and the EEG arousal level.20 Indeed, the effects of long nap hours are still unknown, especially with respect to insulin resistance and HbA1c levels.
The role of night sleep deprivation in patients with diabetes, obesity, and metabolic syndrome has received more attention.21 Daytime napping has been considered a beneficial lifestyle to counteract the effects of sleep deprivation;22 however, long and frequent naps during the day are questionable sleep habits in terms of sleep rhythm disturbance, which calls into question the benefit of different lengths of daytime naps on neuroendocrine regulation.23 For short night sleepers, it is still unknown how long the daytime siesta can benefit health. Disadvantageous changes in HbA1c levels and the incidence of insulin resistance are commonly considered to be related to pivotal metabolic pathways in many chronic diseases.24,25 Thus, the joint effects of changes in HbA1c levels and elevated HOMA-IR index may contribute to the compensatory effects of daytime napping and the shortage of night sleep.
Therefore, we conducted this prospective cohort study to evaluate the single effects of the length of daytime napping and its joint effects on the risks for high HOMA-IR index and disadvantageous changes in HbA1c levels.
MATERIALS AND METHODS
Subjects
The study population was based on the cohort study for the risk of chronic noncommunicable disease and was conducted between March 2008 and June 2013 in Harbin, China (ChiCTR-ECH-12002938; www.chictr.org). Of a total of 7696 participants, 1851 (24.1%) were excluded at the time of enrollment for the following reasons: 1154 (15.0%) had T2DM and 697 (9.1%) were lost to follow-up or had missing sleep habit and related data. A total of 5845 subjects (2736 women and 3109 men; 30–65 years of age) had complete values for all analytic variables. Person-years of follow-up were calculated from the date of enrollment to the date of the initial development of elevated HbA1c levels and high HOMA-IR index or the date of follow-up, whichever occurred first. This study protocol was approved by the Harbin Medical University Ethics Committee.
Measurements
Information on sleep habits was derived from self-reported questionnaires, which included daytime napping minutes, night sleeping hours, sleep quality, insomnia status, use of hypnotics, and snoring. Individuals were categorized by daytime napping hours as follows: 0, 0 to 30 minutes, and >30 minutes. During the health examinations, data on health-related behaviors, demographics, anthropometric indices, and medical history were also collected. Participants were also asked the frequencies of bad mood and psychological pressure, and those who often or always had a bad mood or felt stressed were categorized into “bad mood” and “psychological pressure” groups. The International Physical Activity Questionnaire (short version) was adopted to evaluate physical activity. According to the metabolic equivalent scores (metabolic equivalents per minute per week), individuals were classified into 1 of 3 levels representing low, moderate, and vigorous physical activity levels. Current smoking status was surveyed by daily consumption of cigarettes. Daily alcohol consumption was calculated by the varieties of alcoholic beverages, the frequency, and the amount of alcohol consumed during a meal. We organized the data on tea drinking habits as weekly consumption. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured 3 times after a 10-minute rest in a sitting position, and the mean of the 3 measured results was calculated. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
Blood was collected after an overnight fast (without any form of medication), centrifuged at 2500 g for 15 minutes to obtain serum, and then cooled and stored at −80°C. The HbA1c and serum insulin levels were determined using an autoimmunoassay analyzer (AIA-2000 ST; Tosoh Corporation, Tokyo, Japan). FBG, triglyceride (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C) were determined using a Roche Modular P800 Automatic Biochemical Analyzer (Roche Diagnostics, Mannheim, Germany). According to the HbA1c criteria recommended by the American Diabetes Association, abnormal glucose metabolic states, such as prediabetes and diabetes, were categorized as an HbA1c level <5.7%, 5.7% to 6.4%, and ≥6.5%.26 The HOMA-IR index was calculated by the following formula: fasting insulin (mU/L) × FBG (mmol/L)/22.5.27 The high HOMA-IR index was defined as HOMA-IR levels >2.50.28
Statistical Analysis
One-way analysis of variance was performed to compare the baseline continuous variables. Pearson χ2 tests were used to assess the differences between proportions according to the categories of daytime napping. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) in the general population and separately in men and women, which were estimated to evaluate the association between daytime napping and the risk of an elevated HbA1c level and high HOMA-IR index. After stratification by sex, in model 3, age, SBP, smoking status, alcohol use, physical activity level, psychological pressure, bad mood, use of hypnotics, sleep quality, night sleep duration, tea consumption, and the prevalence of stroke, cardiovascular disease, mental disease, insomnia, and snoring, as well as serum FBG, TG, TC, and HDL-C levels, were adapted to Cox models. The analysis of women was also adjusted for menopausal status as a potential confounding factor.
Combining daytime napping with categories of night sleep deprivation, joint analyses were performed. Less than 6 hours of sleep per night has been defined as sleep deprivation according to previous research.29 No naps combined with no sleep deprivation was used as the referent. The statistical analysis of the data was carried out by IBM SPSS Statistics version 20.0 (IBM, Armonk, NY). All P values were 2-tailed. P values for trend <0.05 were considered statistically significant.
RESULTS
The baseline characteristics according to daytime napping minutes are shown in Table 1. Compared with participants with no naps, participants with >30 minutes of daytime napping were more likely to be current smokers, to be long night sleepers, to have less insomnia, and to have higher rates of poor sleep quality, mental disease, stroke, and cardiovascular disease and lower rates of bad mood. Serum FBG, HbA1c, and TG levels were also significantly higher in participants with >30 minutes of daytime napping. In addition, significant associations existed between daytime napping and age, BMI, SBP, snoring, tea consumption, and 2-hour postprandial blood glucose (PBG), HOMA-IR, and serum TC levels. No significant differences in sex, DBP, alcohol consumption, physical activity level, use of hypnotics, psychological pressure, and HDL-C levels were demonstrated between daytime napping groups.
TABLE 1.
Baseline Characteristics of Participants According to Daytime Napping Durations
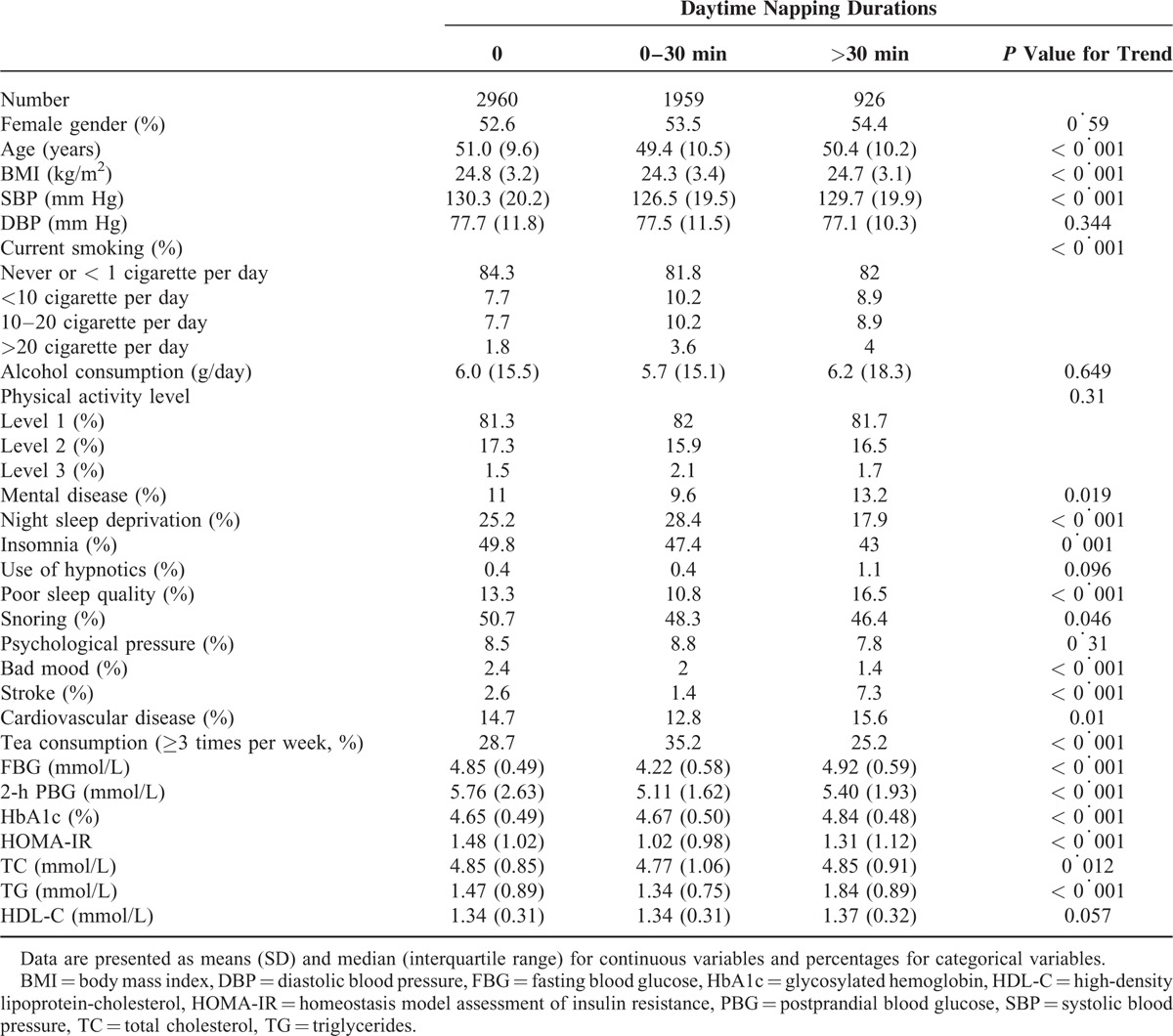
During a mean of 4.5 years of follow-up, 317 participants (5.4%) had HbA1c levels between 5.7% and 6.4%, and 231 participants (4.0%) had HbA1c levels >6.5%. The associations between long daytime napping and the risk of elevated HbA1c levels were confirmed among diabetes-free participants at baseline (Table 2). According to napping categories (0, 0–30, and >30 minutes), the total person-years were 13,401, 8808, and 4252, respectively. The incidence rates for HbA1c levels between 5.7% and 6.4% were 3.5%, 3.1%, and 11.0%, respectively, and the incidence rates for HbA1c levels >6.5% were 3.9%, 3.0%, and 8.3%, respectively. These incidences were significantly higher with increased daytime napping hours (all P trend < 0.001). After adjusting for all the potential confounding factors, including age, sex, SBP, smoking, alcohol use, physical activity, psychological pressure, bad mood, stroke, cardiovascular disease, mental illness, insomnia, use of hypnotics, sleep quality, night sleep duration, snoring, tea consumption, BMI, and FBG, TG, and HDL-C levels, the HRs for HbA1c levels between 5.7% and 6.4% and >6.5% according to baseline daytime napping durations still demonstrated that >30 minutes of daytime napping was a risk factor for an elevated HbA1c level.
TABLE 2.
Relative Risks for the Incidence of High HbA1c According to the Daytime Napping Durations
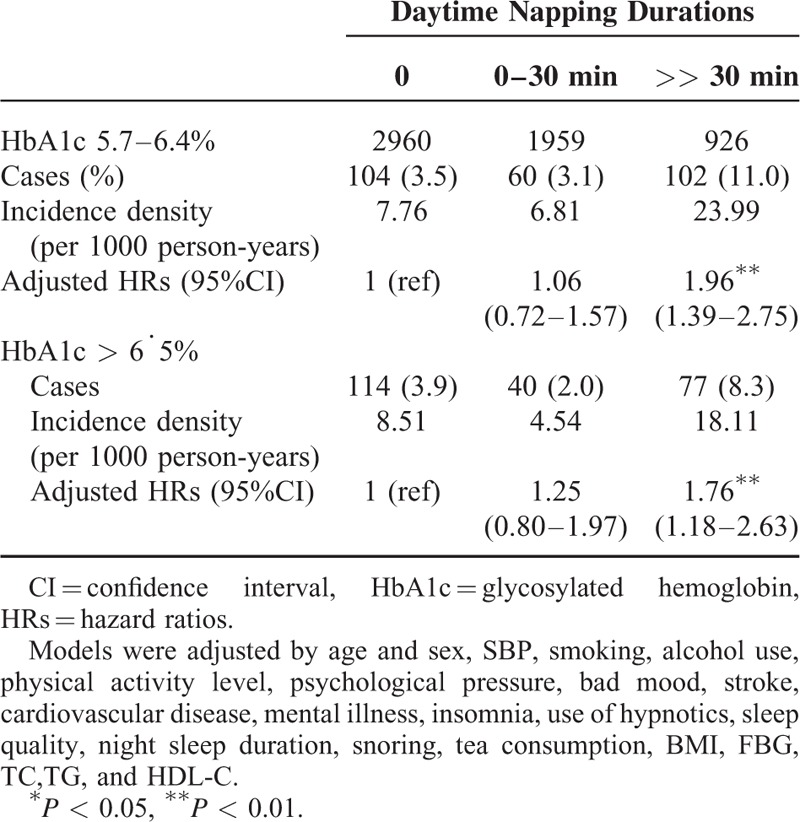
Table 3 presents the multivariate HRs for the high HOMA-IR index according to baseline daytime napping hours. The percentages of high HOMA-IR index significantly differed between daytime napping groups by 21.0%, 18.5%, and 29.3%, respectively (P trend < 0.001). After adjusting for all the potential confounding factors, the HRs for >30 minutes of daytime napping were still significantly higher than no napping.
TABLE 3.
Relative Risks for the High HOMA-IR Index According to the Daytime Napping Durations
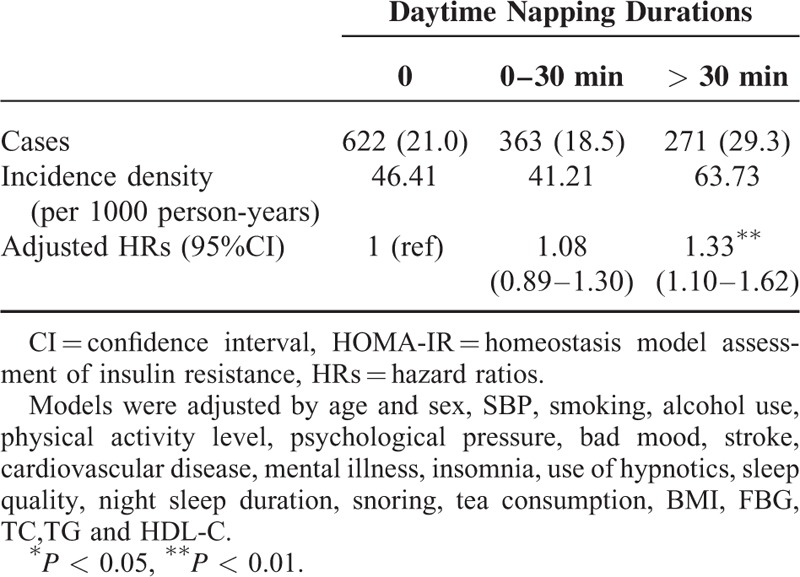
Figure 1 shows the HRs and 95% CIs for the incidence of elevated HbA1c levels and high HOMA-IR index in men and women. For women, menopause status as a potential confounding factor was also adjusted in the Cox regression models. In men (Figure 1A), the HRs (95% CI) of HbA1c levels between 5.7% and 6.4% were 1.00 (reference), 1.02 (0.55–1.87), and 1.39 (0.75–2.58). The HRs (95% CI) for HbA1c >6.5% were 1.00 (reference), 0.61 (0.26–1.42), and 2.01 (1.07–3.80). The HRs (95% CI) for the high HOMA-IR index were 1.00 (reference), 0.91 (0.70–1.19), and 1.46 (1.08–1.98). In women (Figure 1B), the HRs (95% CI) for HbA1c between 5.7% and 6.4% were 1.00 (reference), 1.07 (0.63–1.82), and 2.20 (1.43–3.38). The HRs (95% CI) for HbA1c >6.5% were 1.00 (reference), 1.36 (0.77–2.42), and 1.87 (1.04–3.35). The HRs (95% CI) for the high HOMA-IR index were 1.00 (reference), 1.06 (0.80–1.39), and 1.47 (1.12–1.91).
FIGURE 1.
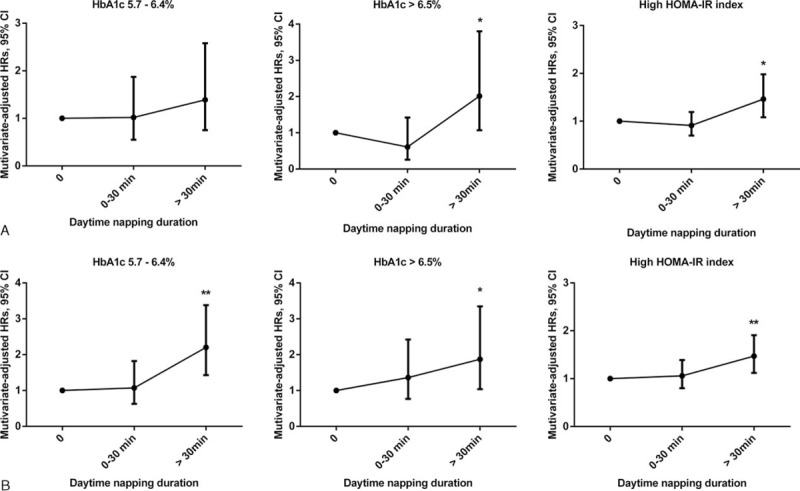
Hazard ratios (HRs) and 95% confidence intervals (CIs) for elevated glycosylated hemoglobin (HbA1c) levels and high HOMA-IR (homeostasis model assessment of insulin resistance) index according to daytime napping duration among men and women. Multivariate hazard ratios (HRs) were adjusted for age, sex, systolic blood pressure (SBP), smoking, alcohol use, physical activity, psychological pressure, bad mood, stroke, cardiovascular disease, mental illness, insomnia, use of hypnotics, sleep quality, night sleep duration, snoring, tea consumption, body mass index (BMI), and fasting blood glucose (FBG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels. In men (A), the HRs (95% CI) for HbA1c levels between 5.7% and 6.4% were 1.00 (reference), 1.02 (0.55–1.87), and 1.39 (0.75–2.58). The HRs (95% CI) for HbA1c >6.5% were 1.00 (reference), 0.61 (0.26–1.42), and 2.01 (1.07–3.80). The HRs (95% CI) for the high HOMA-IR index were 1.00 (reference), 0.91 (0.70–1.19), and 1.46 (1.08–1.98). In women (B), the HRs (95% CI) for HbA1c levels between 5.7% and 6.4% were 1.00 (reference), 1.07 (0.63–1.82), and 2.20 (1.43–3.38). The HRs (95% CI) for HbA1c >6.5% were 1.00 (reference), 1.36 (0.77–2.42), and 1.87 (1.04–3.35). The HRs (95% CI) for the high HOMA-IR index were 1.00 (reference), 1.06 (0.80–1.39), and 1.47 (1.12–1.91). ∗P trend < 0.05, ∗∗P trend < 0.01. BMI = body mass index, CI = confidence interval, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, HRs = hazard ratios, SBP = systolic blood pressure, TG = triglyceride.
No multicollinearity existed between daytime napping and night sleeping duration in their relationships in the total population or in men or women analyzed separately. All the variance inflation factors (VIFs) were <10. In the total population, the VIF of naps and night sleeping were 1.15 and 1.08, respectively. Similar results were observed in men (VIFs of naps and night sleeping were 1.16 and 1.11) and women (VIFs were 1.18 and 1.09).
Figures 2 to 4 show the joint effects of daytime napping and night sleep deprivation on the incidence of high HbA1c levels (5.7%–6.4% and >6.5%) and high HOMA-IR index. For subjects with no naps and no night sleep deprivation as references, the multiple adjusted HRs (95% CI) for HbA1c levels between 5.7% and 6.4% were nonsignificant for those reporting no naps, 0- to 30-minute naps, and >30-minute naps among the participants with sleep deprivation (all P trend > 0.05). Nonsignificant HRs (95% CI) were observed (P trend > 0.05) for those reporting 0- to 30-minute naps. However, the HR was significant (P trend < 0.01) for those reporting >30-minute naps among the participants without sleep deprivation (Figure 2).
FIGURE 2.
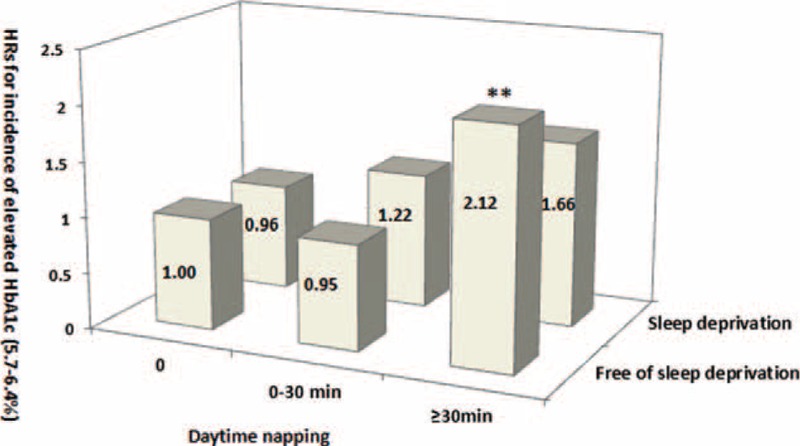
Joint analysis on sleep deprivation and day napping in relation to the risk of increased glycosylated hemoglobin (HbA1c) in diabetes-free Chinese adults at baseline. Multivariate hazard ratios (HRs) for the development of HbA1c levels of 5.7% to 6.4% were adjusted for age, sex, systolic blood pressure (SBP), smoking, alcohol use, physical activity, psychological pressure, bad mood, stroke, cardiovascular disease, mental illness, insomnia, use of hypnotics, sleep quality, night sleep duration, snoring, tea consumption, body mass index (BMI), and fasting blood glucose (FBG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels. ∗∗P trend < 0.01.BMI = body mass index, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, HRs = hazard ratios, SBP = systolic blood pressure, TG = triglyceride.
Among the participants with sleep deprivation, no daytime naps and napping >30 minutes were associated with an HbA1c level >6.5% (all P trend < 0.01). Among participants with no sleep deprivation, only >30 minutes of daytime napping correlated with a high risk for an HbA1c level >6.5% (P trend < 0.01; Figure 3). Among the subjects with sleep deprivation, long or short daytime nap duration was not significantly associated with the risk of high HOMA-IR index (all P trends > 0.05). Among those without sleep deprivation, only daytime napping >30 minutes increased the risk of the development of high HOMA-IR index (P trend < 0.01; Figure 4).
FIGURE 3.
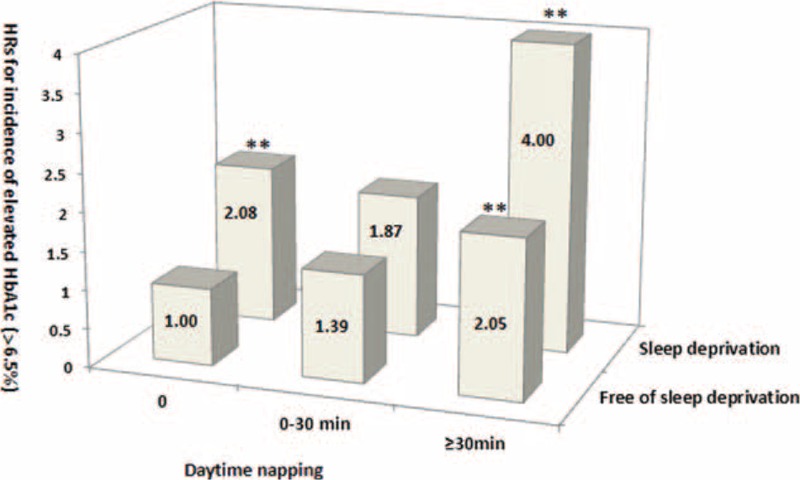
Joint analysis on sleep deprivation and day napping in relation to the risk of increased glycosylated hemoglobin (HbA1c) in diabetes-free Chinese adults at baseline. Multivariate hazard ratios (HRs) for the development of HbA1c >6.5% were adjusted for age, sex, systolic blood pressure (SBP), smoking, alcohol use, physical activity, psychological pressure, bad mood, stroke and cardiovascular disease, mental illness, insomnia, use of hypnotics, sleep quality, night sleep duration, snoring, tea consumption, body mass index (BMI), and fasting blood glucose (FBG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels. ∗∗P trend < 0.01. BMI = body mass index, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, HRs = hazard ratios, SBP = systolic blood pressure, TG = triglyceride.
FIGURE 4.
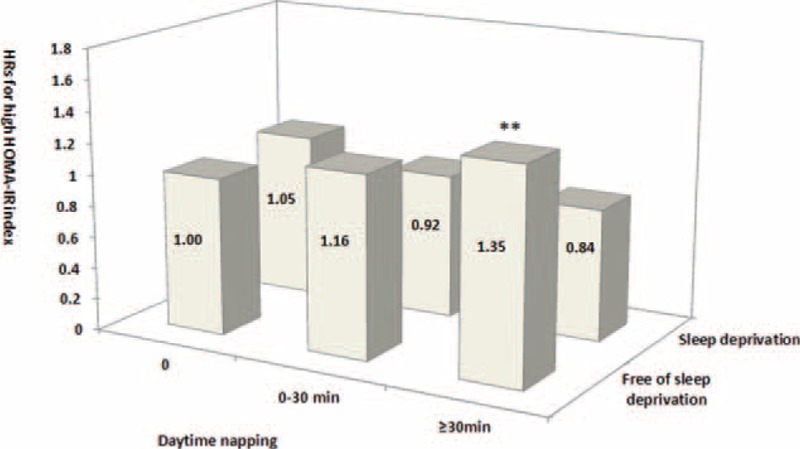
Joint analysis on sleep deprivation and daytime napping in relation to the risk for increased high HOMA-IR index in diabetes-free Chinese adults at baseline. Multivariate hazard ratios (HRs) for the development of high HOMA-IR index were adjusted for age, sex, systolic blood pressure (SBP), smoking, alcohol use, physical activity, psychological pressure, bad mood, stroke, cardiovascular disease, mental illness, insomnia, use of hypnotics, sleep quality, night sleep duration, snoring, tea consumption, body mass index (BMI), and fasting blood glucose (FBG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels. ∗∗P trend < 0.01. BMI = body mass index, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, HRs = hazard ratios, SBP = systolic blood pressure, TG = triglyceride.
DISCUSSION
Here we performed the first longitudinal cohort study showing the single effect of excessive daytime napping on the incidence of high HOMA-IR index and elevated HbA1c in the entire cohort and in men and women based on a large sample-sized population. We demonstrated that >30 minutes of daytime napping is associated with the development of elevated HbA1c levels and high HOMA-IR index over an average of 4.5 years of follow-up. Multiple adjusted HRs were significant in the entire cohort and in women and men analyzed separately (only for HbA1c >6.5%). In women, not only did long day nappers have an increased risk of an HbA1c level >6.5%, but the risk of an HbA1c level between 5.7% and 6.4% was significantly greater. Moreover, this is also the first longitudinal observational study on the combined effect of naps and night sleep deprivation in the prediction of developing abnormal glucose metabolism. For participants with sleep deprivation, both no naps and >30-minute daytime naps had elevated risks for developing high HbA1c levels (>6.5%) years later. No sleep deprivation combined with >30-minute daytime naps was associated with a high incidence of elevated HOMA-IR index and a high HbA1c level (5.7%–6.4%, and >6.5%).
Previous investigations on the effect of daytime napping have focused on FBG but not glucose metabolism indices such as HbA1c and insulin. Only 1 cross-sectional study indicated that middle-aged, diabetes-free adults with daytime napping had a significant elevation of HbA1c levels and high HOMA-IR index; it was speculated that the high risks for elevated HbA1c levels and high HOMA-IR index are the results of daytime napping.8 The results of our prospective study support the possibility that the causal correlation may not be reversed. This is also the first survey on siestas and the relationship with glucose metabolic parameters stratified by sex. Women were more likely to have increased HbA1c levels (>5.7%) if daytime napping was >30 min/d. In analyzing women, menopause status as a confounding factor was also considered in the multiple regression analysis because menopause related to sleep disturbances is usually considered important.30 Metabolic syndrome, atherosclerosis, and cardiovascular diseases are more prevalent after menopause than in premenopause; however, after adjusting for menopause, long daytime naps still had negative effects on the development of the elevated HOMA-IR index, especially on the change in HbA1c level longitudinally in women.31
As hypothesized, naps of long duration were observed to have adverse effects on the incidences of high HOMA-IR index and elevated HbA1c levels in the entire cohort and in men and women analyzed separately. This may be interpreted as follows. Both 60- and 90-minute naps have been reported to consist of both slow wave sleep (SWS) and REM, similar to that in nocturnal sleep.32 Subjects who are awakened from SWS and/or REM tend to have abnormal circadian rhythms, which were found to have an unfavorable influence on metabolic disorders.33 Visceral obesity and proinflammatory cytokines, such as interleukin-1 and tumor necrosis factor-α, exert metabolic effects associated with long sleep duration.34 Long sleep-related circadian and hormonal alterations may promote insulin resistance.35 Previously, people who napped more were thought to have less exercises. In this study, Pearson χ2 tests showed no significant differences existed between the daytime napping groups. With the physical activity level as a potential confounding factor, the adjusted HRs for high HbA1c and HOMA-IR index were still significant. However, we used the short form of international physical activity questionnaire to evaluate the physical activity levels, which discussed as a potential limitation in the following pages. Thus, the effect of physical activity on the association between daytime napping and abnormal glucose metabolism is still needed to be evaluated in the future. From our results, the association of elevated HOMA-IR index with napping >30 min only in the group free of sleep deprivation, but not in the group with sleep deprivation. Although the mechanisms on this remain unclear, Tietzel et al demonstrated that daytime siestas, especially for short naps, have short-term benefits following nocturnal sleep restriction.36 On the other hand, subjects free of sleep deprivation combined long naps have longer total sleep duration than those with sleep deprivation. Long daytime napping combined long nocturnal sleeping trend to increase the risk of coronary heart disease enhancing blood viscosity and fibrinolysis, which are verified related with insulin resistance.37,38
Previous evidence has indicated that short night sleep correlates with diabetes, insulin secretion dysfunction, and metabolic syndrome.39 Sleep deprivation leads to sleepiness during the day. Based on our results, participants with no sleep deprivation combined with no naps or napping <30 minutes had a nonsignificant risk for elevated HbA1c levels and high HOMA-IR index. Similarly, restricting inadequate daytime sleep opportunities results in improved sleep efficiency at night, which may benefit health.2,40 Additionally, arousal and sleeplessness are associated with not only the hypothalamus-pituitary-adrenal axis and central nervous system hyper-arousal but also the administration of glucocorticoids as well as the suppression of proinflammatory cytokine secretion.41 All of these proposals serve as the basis for a hypothesis explaining the resulting disorder of glucose metabolism. Additionally, in the present study, several important potential confounding factors commonly overlooked were also taken into account. Specifically, bad mood and psychological pressure were adjusted for depression, and the resulting insomnia contributed to the diminished deep SWS and disordered changes in REM sleep.42 The use of hypnotics can reduce the percentage of time spent in the deep sleep stages. In turn, altering 5- hydroxytryptamine type 2C antagonist levels involve the potency of appetite, weight gain, and insulin secretion.43–45
The major strength of this study was the large sample size, prospective study design, joint analysis, and multiple adjustments for potential confounding factors. This was also the first study to assess sex-specific napping hours and the relationship with glucose metabolic markers including the high HOMA-IR index and HbA1c. There were some limitations in the present research. First, the participants recruited for this study were limited to Chinese adults, and the conclusions may not be extended to other races and ethnicities. Second, the timing and the frequency of daytime napping, as well as the data on work shifts, were not collected; previous studies suggested these factors may disturb sleep and the circadian rhythm.46 The information on sleep habits was self-reported and not obtained using the Pittsburgh Sleep Quality Index or polysomnography; however, we have adjusted multiple factors affecting sleep quality and sleep duration from self-reported questionnaires to minimize the bias. In addition, obstructive sleep apnea was not screened, which could be a potential confounding factor of insulin resistance and abnormal glucose metabolism.47 Instead, we adjusted self-reported snoring in the regression models. Moreover, dietary factors such as coffee intake were not included in the multiple adjusted models, although the traditional use of coffee compensates for sleepiness and is positively related to sleep disorders.48 However, the coffee consumption rate is still low in Chinese residents. Instead, tea consumption was included in the multiple adjusted models. Additionally, although the short form of “The International Physical Activity Questionnaire” proven reliability shows it can be used with care in repeated measures studies, in recent years, it is also controversial for tending to overestimate the amount of physical activity reported compared to an objective device in some studies. Thus, it is necessary to perform further experiments to ensure whether physical activity is a pivotal cause for the correlation between napping and abnormal glucose metabolism.49 Finally, further analysis of the mechanisms underlying these associations is still warranted.
In summary, this cohort study indicated that adults with daytime napping >30 minutes could be at higher risk factor for elevated HbA1c levels and high HOMA-IR index years later. These conclusions were observed consistently in the Chinese general population, in men, and especially in women. Sleep deprivation combined with brief daytime napping <30 minutes was not associated with a risk for an elevated HbA1c level and high HOMA-IR index. The most appropriate duration of naps must be assessed in a controlled study under the hypothesis that prolonged naps might be a risk factor for diabetes.
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, EEG = electroencephalography, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein-cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, HR = hazard ratio, REM = rapid eye movement, SBP = systolic blood pressure, SWS = slow wave sleep, T2DM = type 2 diabetes, TC = total cholesterol, TG = triglyceride, VIF = variance inflation factor.
Clinical Trial Registry: http://www.chictr.org
Trial number: ChiCTR-ECH-12002938
Financial support: this study is supported by National Natural Science Foundation of China (grant No. 81472980)
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wu J, Xu G, Shen L, et al. Daily sleep duration and risk of metabolic syndrome among middle-aged and older Chinese adults: cross-sectional evidence from the Dongfeng–Tongji cohort study. BMC Public Health 2015; 15:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens JF, Buysse DJ, Hall M, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. J Clin Sleep Med 2010; 6:330–335. [PMC free article] [PubMed] [Google Scholar]
- 3.Park M, Comella CL, Leurgans SE, et al. Association of daytime napping and Parkinsonian signs in Alzheimer's disease. Sleep Med 2006; 7:614–618. [DOI] [PubMed] [Google Scholar]
- 4.Asplund R. Daytime sleepiness and napping amongst the elderly in relation to somatic health and medical treatment. J Intern Med 1996; 239:261–267. [DOI] [PubMed] [Google Scholar]
- 5.Leng Y, Ahmadi-Abhari S, Wainwright NW, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open 2014; 4:e006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins T. HbA1c for screening and diagnosis of diabetes mellitus. Endocrine 2013; 43:266–273. [DOI] [PubMed] [Google Scholar]
- 7.Koenig RJ, Peterson CM, Jones RL, et al. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med 1976; 295:417–420. [DOI] [PubMed] [Google Scholar]
- 8.Baoying H, Hongjie C, Changsheng Q, et al. Association of napping and night-time sleep with impaired glucose regulation, insulin resistance and glycated haemoglobin in Chinese middle-aged adults with no diabetes: a cross-sectional study. BMJ Open 2014; 4:e004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangiah S, Caldwell DF. Reduction of rapid eye movement (REM) sleep by glucose alone or glucose and insulin in rats. Life Sci 1988; 42:1425–1429. [DOI] [PubMed] [Google Scholar]
- 10.Azman M, Sani A, Kamaruddin NA. Insulin resistance using HOMA model in obstructive sleep apnea: a cross sectional study. Ann Saudi Med 2014; 34:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambadiari V, Triantafyllou K, Dimitriadis GD. Insulin action in muscle and adipose tissue in type 2 diabetes: the significance of blood flow. World J Diabetes 2015; 6:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Androutsos O, Moschonis G, Mavrogianni C, et al. Identification of lifestyle patterns, including sleep deprivation, associated with insulin resistance in children: the Healthy Growth Study. Eur J Clin Nutr 2014; 68:344–349. [DOI] [PubMed] [Google Scholar]
- 13.Morselli L, Leproult R, Balbo M, et al. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab 2010; 24:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butte NF, Puyau MR, Adolph AL, et al. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc 2007; 39:1257–1266. [DOI] [PubMed] [Google Scholar]
- 15.Esteghamati A, Ashraf H, Khalilzadeh O, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab 2010; 7:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun 2011; 25:16–24. [DOI] [PubMed] [Google Scholar]
- 17.Endo S, Kobayashi T, Yamamoto T, et al. Persistence of the circadian rhythm of REM sleep: a variety of experimental manipulations of the sleep-wake cycle. Sleep 1981; 4:319–328. [DOI] [PubMed] [Google Scholar]
- 18.Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res 2010; 185:155–166. [DOI] [PubMed] [Google Scholar]
- 19.Lockley S, Tabandeh H, Skene D, et al. Day-time naps and melatonin in blind people. Lancet 1995; 346:1491. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Hori T. The effects of a 20-min nap before post-lunch dip. Psychiatry Clin Neurosci 1998; 52:203–204. [DOI] [PubMed] [Google Scholar]
- 21.Di Milia L, Vandelanotte C, Duncan MJ. The association between short sleep and obesity after controlling for demographic, lifestyle, work and health related factors. Sleep Med 2013; 14:319–323. [DOI] [PubMed] [Google Scholar]
- 22.Gillberg M, Kecklund G, Axelsson J, et al. The effects of a short daytime nap after restricted night sleep. Sleep 1996; 19:570–575. [DOI] [PubMed] [Google Scholar]
- 23.Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr Opin Pulm Med 2006; 12:379–382. [DOI] [PubMed] [Google Scholar]
- 24.Reaven GM. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 25.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 2005; 42:987–1000. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 1989; 12:365–368. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012; 97:E223–E227. [DOI] [PubMed] [Google Scholar]
- 28.Son JW, Park C-Y, Kim S, et al. Changing clinical characteristics according to insulin resistance and insulin secretion in newly diagnosed type 2 diabetic patients in Korea. Diabetes Metab J 2015; 39:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson CL, Larkin EK, Patel S, et al. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011; 117:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruyneel M. Sleep disturbances in menopausal women: aetiology and practical aspects. Maturitas 2015; 81:406–409. [DOI] [PubMed] [Google Scholar]
- 31.Meirelles RM. Menopause and metabolic syndrome [in Portuguese]. Arq Bras Endocrinol Metab 2014; 58:91–96. [DOI] [PubMed] [Google Scholar]
- 32.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci 2003; 6:697–698. [DOI] [PubMed] [Google Scholar]
- 33.Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res 1999; 8:291–295. [DOI] [PubMed] [Google Scholar]
- 34.Choi K, Lee J, Park H, et al. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obesity 2008; 32:1091–1097. [DOI] [PubMed] [Google Scholar]
- 35.Arora T, Jiang CQ, Thomas GN, et al. Self-reported long total sleep duration is associated with metabolic syndrome the Guangzhou Biobank Cohort Study. Diabetes Care 2011; 34:2317–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep 2001; 24:293–300. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Yang H, He M, et al. Longer sleep duration and midday napping are associated with a higher risk of CHD incidence in middle-aged and older Chinese: the Dongfeng–Tongji cohort study. Sleep 2015; sp-00311-15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Hy, Li J, Xu M, et al. Elevated whole blood viscosity is associated with insulin resistance and non-alcoholic fatty liver. Clin Endocrinol 2015; 83:806–811. [DOI] [PubMed] [Google Scholar]
- 39.Darukhanavala A, Booth JN, Bromley L, et al. Changes in insulin secretion and action in adults with familial risk for type 2 diabetes who curtail their sleep. Diabetes Care 2011; 34:2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagmann-von Arx P, Perkinson-Gloor N, Brand S, et al. In school-age children who were born very preterm sleep efficiency is associated with cognitive function. Neuropsychobiology 2014; 70:244–252. [DOI] [PubMed] [Google Scholar]
- 41.Chrousos G, Vgontzas A, Kritikou I. HPA axis and sleep. Endotext [Internet], MDText.com. Inc South Dartmouth (MA) 2000. [Google Scholar]
- 42.Doerr J, Hirscher V, Riemann D, et al. Disturbances of slow-wave sleep and psychiatric disorders [in German]. Nervenarzt 2010; 81:347–354. [DOI] [PubMed] [Google Scholar]
- 43.Nonogaki K, Nozue K, Oka Y. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese A y mice. Biochem Biophys Res Commun 2006; 351:1078–1082. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Zhu Y, Zhou W, et al. Serotonin receptor 2C and insulin secretion. PloS One 2013; 8:e54250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi A, Sonoda R, Kimura Y, et al. Antiobesity effect of YM348, a novel 5-HT 2C receptor agonist, in Zucker rats. Brain Res 2004; 1011:221–227. [DOI] [PubMed] [Google Scholar]
- 46.Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) 2014; 62:292–301. [DOI] [PubMed] [Google Scholar]
- 47.Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Card AJ. Importance of sleep disorders in assessing the association between coffee consumption and all-cause mortality. Mayo Clinic Proc 2013; 88:1492. [DOI] [PubMed] [Google Scholar]
- 49.Lee PH, Macfarlane DJ, Lam T, et al. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic. Int J Behav Nutr Phys Activity 2011; 8:115.doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]


