Abstract
We evaluated the prognostic value of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) parameters for limited-stage small-cell lung cancer (LS-SCLC).
We retrospectively enrolled 59 LS-SCLC patients who underwent pretreatment FDG PET/CT. Various PET parameters were measured in all malignant lesions, and we recorded the highest maximum standardized uptake value (SUVmax), and sum of metabolic tumor volume (MTVsum) and total lesion glycolysis (TLGsum). The relationship between the highest SUVmax and volumetric PET parameters was evaluated. The prognostic significances of PET parameters and clinical variables were assessed using Cox's proportional hazard regression analysis. Overall survival (OS) and progression-free survival (PFS) were assessed by the Kaplan–Meier method.
The SUVmax of the highest metabolic lesion had a significant positive correlation with MTVsum and TLGsum (P < 0.001). Upon multivariate analysis, the highest SUVmax was an independent predictor of OS (1 unit increase, hazard ratio [HR]: 1.133, P = 0.003) and MTVsum was a significant prognostic factor of PFS (10-cm3 increase, HR: 1.027, P = 0.034) after adjusting for age, sex, performance status, tumor stage, and treatment modality. The highest SUVmax was a prognostic factor for PFS with marginal significance (1 unit increase, HR: 1.078, P = 0.053). Patients with higher SUVmax (≥11) were also characterized by a significantly shorter median OS (P < 0.001) and PFS (P = 0.002) compared with patients with lower SUVmax.
The highest SUVmax is an independent prognostic factor for survival in LS-SCLC patients. Therefore, the highest SUVmax might be a possible imaging biomarker for risk stratification in LS-SCLC. A further study in a large cohort is needed to validate the prognostic significance of the parameter.
INTRODUCTION
Lung cancer remains the leading cause of cancer-related mortality, and small-cell lung cancer (SCLC) accounts for 14% to 20% of all lung cancers.1,2 SCLC is an aggressive disease characterized by a rapid doubling time, early development of distant metastasis, and poor prognosis. SCLC is usually classified according to the Veterans Affairs Lung Study Group as limited-stage (LS) or extensive-stage.3 LS is defined as a disease confined to the ipsilateral hemithorax and can be encompassed within a tolerable radiation port. Extensive disease is defined as a disease extending outside the ipsilateral hemithorax, including malignant pleural effusion. A recent study has revealed that the proportion of SCLC patients categorized as LS is increasing thus approximately 40% in the United States.1 There is a 20% to 25% chance of cure for minority of patients with LS-SCLC, while the patients with metastasis have a median survival of less than 1 year,4 thus reports about long-term survivals have shown wide range up to 18 years.5–8 Several studies have suggested that tumor-node-metastasis (TNM) staging can be applied to SCLC because the current, 2-tiered staging system does not accurately reflect prognosis.9–11 Very limited SCLC, thus stage I or II according to the TNM staging system, is characterized by a better prognosis than is LS-SCLC with advanced mediastinal or supraclavicular lymphadenopathy.12–14 Therefore, the risk stratification and prediction of clinical outcome of LS-SLCL is important.
18F-fluorodeoxyglucose positron emission tomography (FDG PET) is a valuable imaging tool for staging of SCLC.15–18 PET can upstage or downstage the disease and detect additional sites of disease that are missed by conventional computed tomography (CT). Furthermore, PET may be useful for predicting prognosis. Several studies have validated the prognostic significance of the metabolic parameters measured by FDG PET in SCLC.19–24 These parameters maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) reflect disease activity and tumor burden.
The standard treatment for LS-SCLC is chemotherapy with thoracic irradiation.2,25 Despite an initially favorable response to treatment, the prognosis remains poor. Treatment has not changed significantly for more than 30 years although numerous clinical trials have been performed interim. In addition, few advances have been noted in translational research of SCLC.2 Therefore, several investigators have experimented with different treatment modalities, including surgery. The most recent Japanese study, of this type which assessed 243 early-stage SCLC surgical resection cases, reported favorable results.26 Recent several studies investigated the prognostic value of volume-based parameters in SLCL, but conflicting results have been reported.20,21,24,27 Furthermore, it has not been fully explored which PET parameters showed better prognostic performance in LS-SCLC. Therefore, we evaluated the relationship between SUVmax and volume-based parameters, and investigated the prognostic roles of SUVmax and volume parameters in LS-SCLC patients.
METHODS
Patients
We reviewed the medical records of 150 consecutive patients with pathologically proven SCLC, who underwent pretreatment FDG PET/CT in Ajou Medical Center between September 2007 and May 2013. We excluded 86 patients with extensive-stage, and 5 others who refused treatment, yielding a final sample size of 59. The Ethics Committee of our institution (Ajou Institutional Review Board) approved this retrospective study, and requirement for informed consent was waived.
Demographic and clinical characteristics, and survival data, were obtained from patient medical records. All patients underwent bronchoscopy, contrast-enhanced chest CT, brain MRI, and FDG PET/CT scans during initial disease staging. Following staging work-up, surgery with adjuvant chemotherapy was conducted on 4 patients (6.8%); 41 patients (69.4%) underwent concurrent chemoradiation therapy, whereas 14 others received chemotherapy (n = 9) or radiation therapy (n = 5) alone, due to their poor conditions. First-line chemotherapy involved a platinum-based drug (cisplatin or carboplatin) with etoposide regimens at 3-week intervals between 4 and 6 cycles. As concurrent chemoradiation therapy, chest irradiation was initiated on day 1 of the second chemotherapy cycle, with 1.5 Gy given twice per day in 30 fractions.
PET/CT Imaging
FDG-PET/CT was performed using 2 types of dedicated PET/CT scanners: a Discovery ST (n = 45; GE Healthcare, Milwaukee, WI) and a Discovery STE (n = 14; GE Healthcare). All patients fasted for at least 6 hours prior to PET/CT; serum glucose levels at the time of FDG injection were <150 mg/dL. Unenhanced CT was performed 60 minutes after a 5 MBq/kg FDG injection using 8- or 16-slice helical CT (120 keV, 30–100 mA in AutomA mode; section width = 3.75 mm). Emission PET data were acquired from the thigh to the head for 2.5 minutes per frame in the 3-dimensional mode. Attenuation-corrected PET images using CT data were reconstructed by an ordered-subsets expectation maximization algorithm (20 subsets, 2 iterations).
Measurement of Metabolic PET Parameters
Two experienced nuclear medicine physicians reviewed all of PET/CT images on a dedicated workstation (GE Advantage Workstation 4.4; GE Healthcare). We measured the various PET parameters of all malignant hypermetabolic lesions, including lung and lymph nodes. Automatic volume of interest (VOI) using an isocontour threshold method based on SUV was used to calculate various metabolic PET parameters including SUVmax, average SUV (SUVavg), MTV, and TLG for each hypermetabolic lesion. The SUVmax was defined as the voxel with the highest count within the region of interest. Fixed SUV value of 2.5 was used to define VOI boundaries. MTVs were automatically calculated by summing the total volumes of voxels in the VOI. TLG was calculated by multiplying the SUVavg by the MTV of each hypermetabolic lesion.
We recorded the SUVmax of the highest metabolic lesion when multiple lesions were evident. MTVsum and TLGsum represent the sums of the MTV and TLG values, respectively, of all malignant lesions.
Statistical Analysis
We expressed data as means ± SDs for continuous variables and as percentages for categorical variables. All analyses were conducted using SPSS for Windows software (ver 18.0; IBM Inc, New York, NY). Pearson's correlation analysis was used to assess relationships between the highest SUVmax and volumetric PET parameters.
Variables included in survival analysis included age, sex, performance status, albumin and lactate dehydrogenase levels, tumor stage, treatment modality, and PET parameters. For the purposes of statistical analysis, clinical variables were grouped into 2 categories, with the exception of age and PET parameters.
Overall survival (OS) and progression-free survival (PFS) were used as measures of patient outcomes to evaluate the prognostic value of PET/CT. OS was defined as the time between the date of PET/CT to the date of death or last follow-up. PFS was defined as the time from the date of PET/CT to the date of initial progression or death. Survival curves were drawn using the Kaplan–Meier method and survival differences between groups compared using the log-rank test.
A univariate Cox's proportional hazards model was used to calculate the hazard ratios for selected probable predictors of OS and PFS. Three different multivariate Cox's proportional hazards models (the SUVmax, MTVsum, and TLGsum models) were used to evaluate the potential independent effects of PET parameters after adjusting for the effects of clinical variables.
RESULTS
Patient baseline demographic data and clinical characteristics are shown in Table 1. Of the 59 patients, 46 were male (78%) and 13 female (22%). Their mean age was 67 ± 9 years. According to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system, 3 patients were of stage I (5.1%), 14 of stage II (23.7%), and 42 of stage III (71.2%).
TABLE 1.
Patient Demographics and Clinical Characteristics
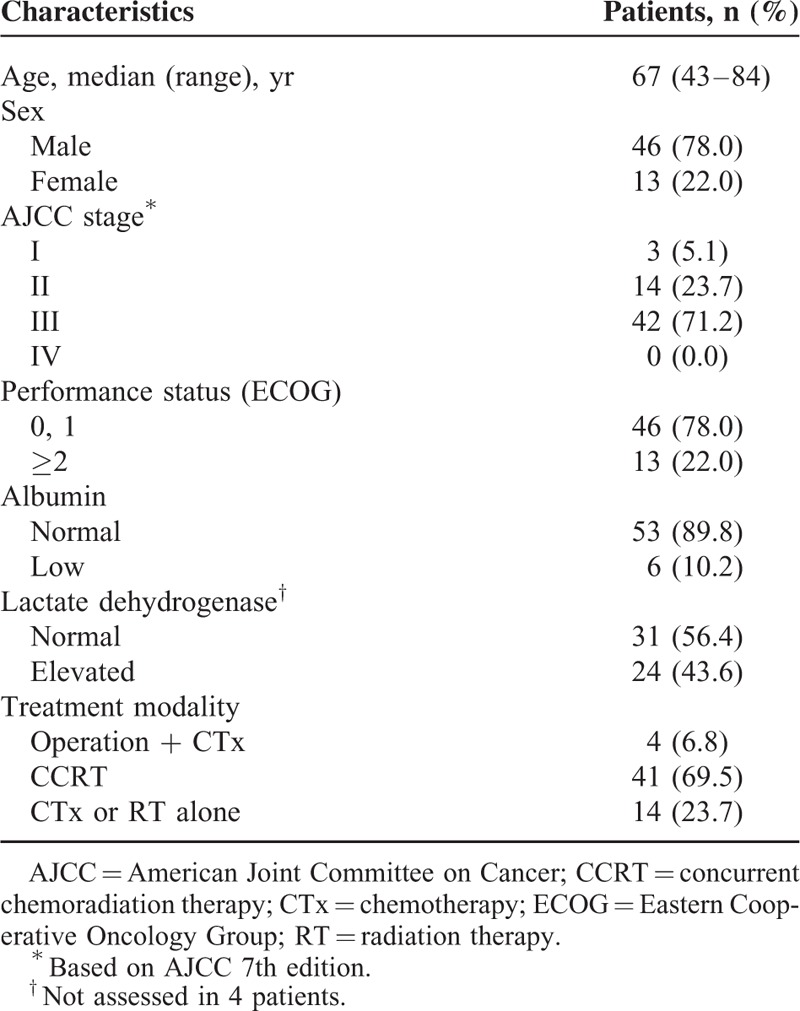
The median values of the highest SUVmax, MTVsum, and TLGsum were 11 (range, 6–21.1), 110 (range, 2–596), and 532 (range, 8–4,028), respectively. The highest SUVmax exhibited a significant positive correlation with MTVsum (r = 0.533, P < 0.0001; Figure 1A), and TLGsum (r = 0.599, P < 0.0001; Figure 1B).
FIGURE 1.
Correlation between the highest maximum standardized uptake value (SUVmax) and (A) the sum of metabolic tumor volume (MTVsum), and (B) the sum of total lesion glycolysis (TLGsum).
At the time of analysis, 15 (25%) patients were still alive and 44 (75%) had died. The estimated median OS and PFS were 16.9 months (range, 0.8–82.1 months) and 10.3 months (range, 0.8–82.1 months), respectively.
Univariate analysis revealed that age (P = 0.004), performance status (P = 0.004), chemotherapy (P = 0.007), the highest SUVmax (1 unit increase, P < 0.001), MTVsum (10-cm3 increase, P = 0.008), and TLGsum (100 units increase, P = 0.002) were significant predictors of OS. Age (P = 0.008), performance status (P = 0.001), lactate dehydrogenase level (P = 0.026), chemotherapy (P = 0.001), the highest SUVmax (P = 0.005), MTVsum (P = 0.002), and TLGsum (P = 0.001) were significant predictors of PFS (Table 2).
TABLE 2.
Results of Univariate Survival Analysis
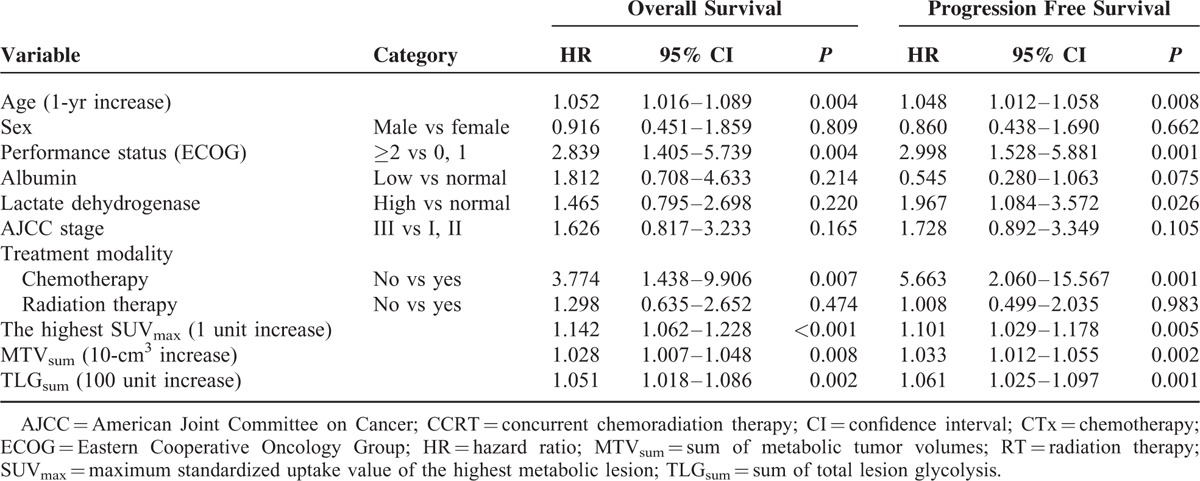
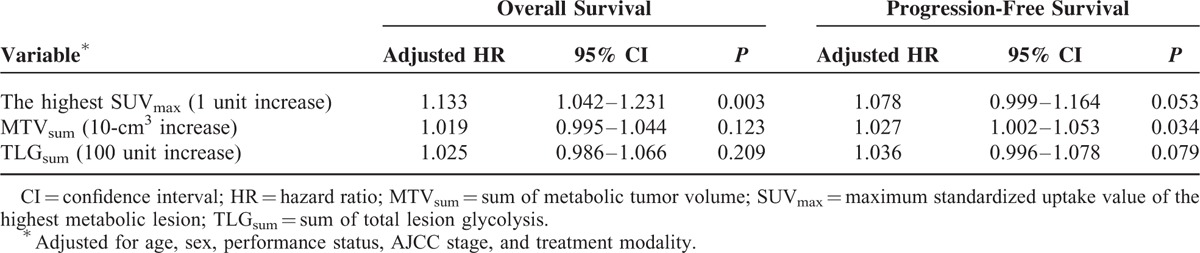
Upon multivariate analysis, only the highest SUVmax (hazard ratio [HR]: 1.133, 95% confidence interval [CI]: 1.042–1.231; P = 0.003) was an independent predictor of OS. MTVsum (HR: 1.027, 95% CI: 1.002–1.053; P = 0.034) was an independent predictor of PFS. In addition, the highest SUVmax (HR: 1.078, 95% CI: 0.999–1.164; P = 0.053) was found to be a prognostic factor with marginal significance (Table 3).
TABLE 3.
Results of Multivariate Survival Analysis
Kaplan–Meier curves exhibited significant differences in terms of both OS and PFS when patients were stratified by the median value of the highest SUVmax (Figure 2). In addition, we performed the Kaplan–Meier curve analysis in the 41 patients who underwent concurrent chemoradiation therapy. Patients with higher SUVmax (≥11) had significantly shorter OS (P < 0.032) but not PFS (P = 0.088) compared with patients with lower SUVmax (figure not shown).
FIGURE 2.
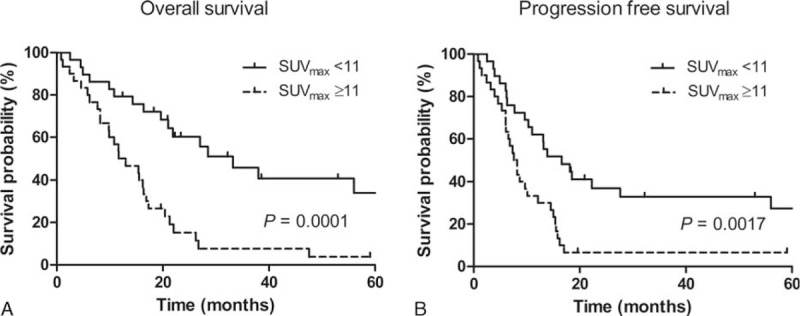
Kaplan–Meier survival curves for overall survival (A) and progression-free survival (B) of the 2 groups. The cut-off value was the median of the highest SUVmax.
DISCUSSION
The present study showed that the SUVmax of the highest metabolic lesion is an independent prognostic factor for survival in LS-SCLC patients. Our results suggest that the highest SUVmax is a promising imaging biomarker, and may facilitate risk stratification to identify surgical candidates or high risk patients managed as extensive stage.
We focused on LS-SCLC in this study. LS-SCLC encompasses a wide spectrum of diseases, from a single pulmonary nodule to advanced contralateral mediastinal and/or supraclavicular lymphadenopathy, and patients with LS-SCLC thus represent a heterogeneous group of patients; this was evidenced by our results. When we restaged our 59 patients according to the 7th edition of the TNM staging system, 17 patients (28.8%) were of stage I or II, 18 (30.5%) of stage IIIA, and the remaining 24 (40.7%) of stage IIIB. Therefore, different treatment modality may be applied in LS-SCLC; surgery may be possible for very early LS-SCLC and more vigorous treatment may be needed in advanced LS-SCLC. In addition, their overall survival durations ranged very widely between 0.8 and 82.1 months. Therefore, more discriminative prognostic markers are required to select the most appropriate treatment modality and to predict survival in LS-SCLC patients.
FDG PET has been widely used in lung cancer and yields useful data on metabolic parameters, allowing tumor classification. SUVmax is the value of the single voxel with the highest radiotracer concentration within the VOI, and is widely used because the parameter is easily measured and very reproducible. Whereas volume-based parameters are appropriate indicators of tumor burden, SUVmax, indicating the presence of malignant cells with high-level glucose metabolism, reflects tumor activity and aggressiveness.19,28 Previous reports evaluated several different kinds of SUVs in SCLC, including SUVmax of the primary tumor, the sum and mean of SUVmax, and the highest SUVmax among several malignant lesions.19–22,24 Although no standard means of SUV assessment is available, we selected the highest SUVmax method because this may represent the most aggressive part of multiple malignant lesions.
The clinical significance of volume-based PET parameters has been actively investigated in several kinds of malignancies including lung cancer.29–33 The prognostic significance of volumetric PET parameters in SCLC has been also analyzed by several investigations.20,21,24 In their subgroup analysis of LS-SCLC, Zhu et al20 and Oh et al21 demonstrated that patients with larger MTVs had significantly shorter median OS and PFS compared with those with smaller MTVs, as revealed by Kaplan–Meier survival curves. In the study by Park et al, sum of MTV and sum of TLG of intrathoracic hypermetabolic lesions were significant prognostic factors.24 Our univariate analysis also showed similar results, thus volumetric parameters may be useful to predict LS-SCLC prognosis. The latest paper investigating 120 LS-SCLC patients concluded that PET parameters including volumetrics do not have independent prognostic value.27 Although the study is the largest series yet reported of PET in LS-SCLC, they measured the PET parameters for primary tumor only. In the present study, we measured all malignant hypermetabolic lesions including both intrathoracic and extrathoracic space and evaluated the prognostic significance of the highest SUVmax, the sum of MTV, and sum of TLG. We guess the PET parameters for primary tumor only may be not adequate to reflect tumor burden or whole tumor characteristics when lesions are multiple, thus different results may be obtained. However, our analysis was performed in a small number of patients, a further study in a large cohort is mandatory.
In our multivariate analysis, the highest SUVmax was an independent prognostic factor for OS, whereas volume-based PET parameters were not. For PFS, the highest SUVmax was found to be a prognostic factor with marginal significance. Based on these results, we suggest that the presence of a malignant lesion with high-level glucose metabolism may be more important than is the total tumor burden of LS-SCLC. In addition, measurement of the highest SUVmax is more feasible in routine clinical practice, whereas measurement of MTVs or TLGs for multiple hypermetabolic lesions is both time-consuming and impractical.
The limitations of our study include the retrospective single-center design, the relatively small patient population, and the use of heterogeneous treatment modalities although patients predominantly received concurrent chemoradiation therapy. We used 2 different PET/CT scanners although we used a consistent imaging protocol for this study. Finally, we did not perform histopathologic studies for all hypermetabolic lesions on pretreatment PET, but follow-up studies were used for confirming malignancy.
CONCLUSION
The highest SUVmax and volume-based parameters were valuable prognostic factors in survival of LS-SCLC patients. The highest SUVmax is an independent prognostic factor; therefore, the parameter may be a possible indicator to select surgical candidates or high-risk patients managed as extensive stage. A further study using a larger population of patients is needed to validate the prognostic role of these biomarkers derived from FDG PET.
Acknowledgment
The authors thank Jung-Ae Lee (Department of Nuclear Medicine, Ajou University Hospital) for her assistance with the data analysis.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CT = computed tomography, FDG PET = 18F-fluorodeoxyglucose positron emission tomography, HR = hazard ratio, LS = limited-stage, MTV = metabolic tumor volume, OS = overall survival, PFS = progression-free survival, SCLC = small-cell lung cancer, SUV = standardized uptake value, TLG = total lesion glycolysis, VOI = volume of interest.
This work was supported by the new faculty research fund of Ajou University School of Medicine.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 2.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015; 121:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 2002; 37:271–276. [DOI] [PubMed] [Google Scholar]
- 4.Lally BE, Urbanic JJ, Blackstock AW, et al. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist 2007; 12:1096–1104. [DOI] [PubMed] [Google Scholar]
- 5.Lassen U, Osterlind K, Hansen M, et al. Long-term survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years—an analysis of 1,714 consecutive patients. J Clin Oncol 1995; 13:1215–1220. [DOI] [PubMed] [Google Scholar]
- 6.Jacoulet P, Depierre A, Moro D, et al. Long-term survivors of small-cell lung cancer (SCLC): a French multicenter study. Groupe d’Oncologie de Langue Francaise. Ann Oncol 1997; 8:1009–1014. [DOI] [PubMed] [Google Scholar]
- 7.Lewinski T, Zulawski M. Small cell lung cancer survival: 3 years as a minimum for predicting a favorable outcome. Lung Cancer 2003; 40:203–213. [DOI] [PubMed] [Google Scholar]
- 8.Tartarone A, Lerose R, Ardito R, et al. Long-term survival in small cell lung cancer: a case report and review of the literature. Future Oncol 2014; 10:523–528. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007; 2:1067–1077. [DOI] [PubMed] [Google Scholar]
- 10.Vallieres E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4:1049–1059. [DOI] [PubMed] [Google Scholar]
- 11.Jhun BW, Lee KJ, Jeon K, et al. Clinical applicability of staging small cell lung cancer according to the seventh edition of the TNM staging system. Lung Cancer 2013; 81:65–70. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd FA, Ginsberg RJ, Haddad R, et al. Importance of clinical staging in limited small-cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol 1993; 11:1592–1597. [DOI] [PubMed] [Google Scholar]
- 13.Urban T, Chastang C, Vaylet F, et al. Prognostic significance of supraclavicular lymph nodes in small cell lung cancer: a study from four consecutive clinical trials, including 1,370 patients. “Petites Cellules” Group. Chest 1998; 114:1538–1541. [DOI] [PubMed] [Google Scholar]
- 14.Argiris A, Murren JR. Staging and clinical prognostic factors for small-cell lung cancer. Cancer J 2001; 7:437–447. [PubMed] [Google Scholar]
- 15.Kamel EM, Zwahlen D, Wyss MT, et al. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med 2003; 44:1911–1917. [PubMed] [Google Scholar]
- 16.Bradley JD, Dehdashti F, Mintun MA, et al. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004; 22:3248–3254. [DOI] [PubMed] [Google Scholar]
- 17.Chong S, Lee KS. Spectrum of findings and usefulness of integrated PET/CT in patients with known or suspected neuroendocrine tumors of the lung. Cancer Imaging 2007; 7:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinjamuri M, Craig M, Campbell-Fontaine A, et al. Can positron emission tomography be used as a staging tool for small-cell lung cancer? Clin Lung Cancer 2008; 9:30–34. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Cho A, Cho BC, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clinl Cancer Res 2009; 15:2426–2432. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Ma T, Niu Z, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 2011; 73:332–337. [DOI] [PubMed] [Google Scholar]
- 21.Oh JR, Seo JH, Chong A, et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39:925–935. [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Lee JS, Mok JH, et al. Metabolic burden measured by (18)f-fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in patients with small cell lung cancer. Cancer Res Treat 2014; 46:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Kim JO, Jung CK, et al. Metabolic activity on [18f]-fluorodeoxyglucose-positron emission tomography/computed tomography and glucose transporter-1 expression might predict clinical outcomes in patients with limited disease small-cell lung cancer who receive concurrent chemoradiation. Clin Lung Cancer 2014; 15:e13–e21. [DOI] [PubMed] [Google Scholar]
- 24.Park SB, Choi JY, Moon SH, et al. Prognostic value of volumetric metabolic parameters measured by [18F]Fluorodeoxyglucose-positron emission tomography/computed tomography in patients with small cell lung cancer. Cancer Imaging 2014; 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Socinski MA, Bogart JA. Limited-stage small-cell lung cancer: the current status of combined-modality therapy. J Clin Oncol 2007; 25:4137–4145. [DOI] [PubMed] [Google Scholar]
- 26.Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese lung cancer registry in 2004. J Thorac Oncol 2014; 9:1140–1145. [DOI] [PubMed] [Google Scholar]
- 27.Ong LT, Dunphy M, Foster A, et al. Prognostic Value of Preradiotherapy 18F-FDG PET/CT Volumetrics in Limited-Stage Small-Cell Lung Cancer. Clin Lung Cancer. doi: 10.1016/j.cllc.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010; 5:612–619. [DOI] [PubMed] [Google Scholar]
- 29.Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg 2013; 257:364–370. [DOI] [PubMed] [Google Scholar]
- 30.Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol 2013; 14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyun SH, Ahn HK, Kim H, et al. Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014; 41:50–58. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Choi JY, Choi DW, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging 2014; 48:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang KJ, Lim I, Park JY, et al. The role of (18)F-FDG PET/CT as a prognostic factor in patients with synovial sarcoma. Nucl Med Mol Imaging 2015; 49:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


