Abstract
Background
Dexmedetomidine, a sedative agent, provides neuroprotection when administered during or before brain ischaemia. This study was designed to determine whether dexmedetomidine post-treatment induces neuroprotection against subarachnoid haemorrhage (SAH) and the mechanisms for this effect.
Methods
Subarachnoid haemorrhage was induced by endovascular perforation to the junction of the right middle and anterior cerebral arteries in adult rats. Dexmedetomidine was applied immediately or 2 h after onset of SAH. Neurological outcome was evaluated 2 days after SAH. Right frontal cortex area 1 was harvested 24 h after SAH for western blotting.
Results
Subarachnoid haemorrhage reduced neurological scores and increased brain oedema and blood–brain barrier permeability. These effects were attenuated by dexmedetomidine post-treatment. Neuroprotection by dexmedetomidine was abolished by PD98095, an inhibitor of extracellular signal-regulated kinase (ERK) activation. Phospho-ERK, the activated form of ERK, was increased by dexmedetomidine; this activation was inhibited by PD98095.
Conclusions
Dexmedetomidine post-treatment provides neuroprotection against SAH. This effect appears to be mediated by ERK.
Keywords: dexmedetomidine, extracellular signal-regulated kinase, post-treatment, subarachnoid haemorrhage
Editor's key points.
Subarachnoid haemorrhage (SAH) causes profound neurological deficits and has few effective pharmacological treatments.
In a rat model, dexmedetomidine given 2 h after onset of SAH attenuated brain oedema, blood–brain barrier permeability, and neurological deficits.
Inhibition of extracellular signal-regulated kinase prevented neuroprotection by dexmedetomidine, consistent with a role of ERK in its effect.
Subarachnoid haemorrhage (SAH) is a devastating disease that accounts for 5% of all strokes.1 About 85% of SAHs are caused by intracranial aneurysm rupture that often occurs in 40- to 60-yr-old patients,2 a very productive period of human life. Although the incidence of aneurismal SAH is low (∼1 incident per 10 000 persons yr−1),3 >50% of patients will die with aneurysm rupture.2 Most measures for treating SAH are supportive. Interventions are urgently needed to improve neurological outcome after SAH.
Dexmedetomidine is a highly selective agonist for α2-adrenergic receptors (α2ARs) and is used as a sedative agent with analgesic properties.4,5 Dexmedetomidine applied before or during brain ischaemia can provide neuroprotection against ischaemic brain injury in adult animals.6–11 However, this approach is limited clinically because most brain ischaemic events are not predicted. A neuroprotective strategy might be applicable if treatment after brain ischaemia can still provide neuroprotection. Application of dexmedetomidine after a traumatic insult provided protection to hippocampal slices in vitro.12 However, dexmedetomidine post-treatment-induced neuroprotection in vivo has not been shown. Also, it is not known whether dexmedetomidine is neuroprotective against SAH.
We hypothesized that dexmedetomidine post-treatment provides neuroprotection against SAH. Given that extracellular signal-regulated kinase (ERK) is involved in neuroprotection against ischaemic brain injury,11,12 we further hypothesized that dexmedetomidine post-treatment-induced neuroprotection in SAH is mediated by activation of ERK. These hypotheses were tested in an endovascular perforation model of SAH in rats.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 80-23, revised in 2011). Efforts were made to minimize the number of animals used and suffering of animals. This study is reported in accordance with Animal Research: Reporting In Vivo Experiments.13
Experimental protocols
In Experiment 1, 2-month-old male Sprague–Dawley rats weighing 300–370 g (Charles River Laboratories Inc., Wilmington, MA, USA) were randomly assigned to four groups, as follows: sham+saline, sham+dexmedetomidine, SAH+saline, and SAH+dexmedetomidine. Rats received dexmedetomidine or saline immediately and 24 h after the onset of SAH. Neurological scores were evaluated at 24 and 48 h after SAH (n=18). Subarachnoid haemorrhage grades, brain water content (n=8), and Evans blue content in brain tissue (n=10) were evaluated at 48 h after SAH. Evans blue extravasation was performed to reflect blood–brain barrier (BBB) permeability. To determine whether delayed application of dexmedetomidine would provide neuroprotection, two groups of rats were studied, as follows: SAH+saline and SAH+dexmedetomidine with application of dexmedetomidine at 2 and 24 h after onset of SAH. We delayed the first dose of dexmedetomidine for 2 h to increase the translational potential of our findings because the majority of patients with SAH experience a delay in treatment. Dexmedetomidine (1 µg ml−1; catalogue no. 0409-1638-02; Hospira, Lake Forest, IL, USA) was prepared freshly each day in normal saline. Dexmedetomidine 25 µg kg−1 or the same volume of saline was injected i.p.14 This dose is larger than that used in humans (usually 1 µg kg−1 i.v. as the loading dose for sedation) but is commonly used in rats.11,15 This dose often induces light sedation in rats.15,16
In Experiment 2, the groups were the same as for Experiment 1. Dexmedetomidine or saline was injected immediately after SAH was established. Right frontal cortex area 1 (Fr1) was harvested 24 h after SAH to measure the content of phospho-ERK by western blotting (n=8).
In Experiment 3, rats were randomly assigned to five groups, as follows: sham, SAH, SAH+PD98059, SAH+dexmedetomidine, and SAH+PD98059+dexmedetomidine. Dexmedetomidine was given immediately and 24 h after the onset of SAH. The ERK inhibitor PD98059 (PD) at 1 mg kg−1 was injected 30 min before dexmedetomidine application.17 PD98059 solution (0.25 mg ml−1) was prepared (Sigma-Aldrich Co., Louis, MO, USA) in normal saline containing 2% dimethyl sulfoxide (DMSO; Fisher Scientific, Fair Lawn, NJ, USA). Rats that did not require PD98059 injection received injection of the vehicle for PD98059. Neurological scores were evaluated at 24 and 48 h after SAH (n=16). The SAH grades, brain water content (n=8), and BBB permeability (n=8) were evaluated at 48 h after SAH. Right Fr1 was harvested at 24 h after SAH to quantify phospho-ERK content by western blotting (n=8).
Subarachnoid haemorrhage model
Subarachnoid haemorrhage was induced by endovascular perforation as previously described by Garcia and colleagues.18 Briefly, rats were anaesthetized with 2% isoflurane, tracheally intubated with a 16-gauge catheter and mechanically ventilated. The animal was placed on a heat pad (Physitemp Instruments Inc., Clifton, NJ, USA) to maintain body temperature at 37°C. Subarachnoid haemorrhage was achieved by advancing a 4–0 sharp monofilament nylon suture (Beijing Cinontech Co. Ltd, Beijing, China) into the right internal carotid artery via the external carotid artery. The suture was pushed 5 mm further after resistance was felt in order to perforate the bifurcation of the anterior and middle cerebral arteries. The suture was then withdrawn and the blood flow in the internal carotid artery returned to produce SAH. Sham-operated rats underwent the same procedure except that the suture was withdrawn after feeling resistance. Heart rate and pulse oximeter oxygen saturation were monitored continuously and non-invasively using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA, USA). Animals received infiltration to the surgical wound with 0.25% bupivacaine.
Neurological scores
Neurological deficits were evaluated 24 and 48 h after SAH using an 18-point scoring system as described by Garcia and colleagues.19 The modified assessment consisted of six tests, including spontaneous activity, spontaneous movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to whisker stimulation (3–18 points).
Subarachnoid haemorrhage grade
An 18-point SAH severity grading system described previously20,21 was used. The basal cistern was divided into six segments that were scored from 0 to 3 according to the amount of subarachnoid blood clot. A total score was calculated by adding the scores from six segments (0–18 points). Animals receiving a score <8 were excluded from the study.
Assessment of brain water content
Brain water content was examined to assess the degree of brain oedema. At 48 h after SAH, brains were quickly separated into left and right cerebral hemispheres, cerebellum, and brainstem and weighed (wet weight). Brain tissue blocks were dried in an oven at 100°C for 72 h and weighed again (dry weight). The percentage of water content was calculated as follows: [(wet weight−dry weight)/wet weight]×100%.20,21
Measurement of blood–brain barrier permeability
Blood–brain barrier integrity was evaluated by Evans blue extravasation.22,23 At 48 h after SAH, Evans blue dye (2% in saline, 5 ml kg−1; Sigma Aldrich) was injected via the right femoral vein and allowed to circulate for 60 min. Under anaesthesia, rats were transcardially perfused with saline to remove the intravascular dye. Brains were then removed and divided into left and right cerebral hemispheres, cerebellum, and brainstem. The brain samples were weighed and homogenized in 2 ml of 50% trichloroacetic acid (Sigma-Aldrich), incubated overnight at 4°C, and centrifuged at 13 000g for 30 min. The amount of Evans blue in the supernatant was quantified by spectrophotometry at 620 nm. The results were expressed as micrograms per gram of brain tissue wet weight.
Immunoblot analysis
Rats were killed by deep isoflurane anaesthesia and transcardially perfused with normal saline at 24 h after SAH. The right Fr1 (perforation side) between bregma +2 and 0 mm was harvested for western immunoblot analysis. Tissues were homogenized in RIPA buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS; Thermo Scientific, Rockford, IL, USA) with protease inhibitors (10 mg ml−1 aprotinin, 5 mg ml−1 peptastin, 5 mg ml−1 leupeptin, and 1 mM phenylmethanesulfonylfluoride; catalogue no. 084M4156V; Sigma-Aldrich) and phosphatase inhibitors (PhosSTOP; Roche Diagnostics GmbH, Mannheim, Germany) on ice as described.24 Homogenates were centrifuged at 4°C at 13 000g for 25 min, and the supernatant was used for immunoblotting.
Equivalent total protein amounts (20 µg) were loaded into each lane of preformed SDS-PAGE gels (12%; Bio-Rad Laboratories Inc., Hercules, CA, USA). After gel electrophoresis, proteins were transferred onto a nitrocellulose membrane (Bio-Rad Laboratories Inc.) and shaken with blocking buffer (Thermo Scientific) for 2 h at room temperature. The following primary antibodies were incubated with the membrane under gentle agitation at 4°C overnight: mouse anti-phospho-ERK antibody (1:200, catalogue no. 11714; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-ERK antibody (1:200, catalogue no. F3014; Santa Cruz Biotechnology) and rabbit anti-β-actin antibody (1:4000, catalogue nuo. 4967S; Cell Signaling Technology, Danvers, MA, USA). Secondary horseradish peroxidase-conjugated goat anti-mouse antibody (1:5000, catalogue no. E3113; Santa Cruz Biotechnology) or goat anti-rabbit antibody (1:5000, catalogue no. H0614; Santa Cruz Biotechnology) was used. After visualization with enhanced chemiluminescence, quantitative analysis of the protein bands was performed using an Image-Quant 5.0 GE Healthcare Densitometer (GE Healthcare, Sunnyvale, CA, USA). Densities of phospho-ERK protein bands were normalized to those of ERK in the same sample to control for errors in protein sample loading and transferring.
Statistical analysis
Parametrical data are presented as the mean (sd). Results of brain water content, Evans blue extravasation and immunoblotting were analysed by one-way analysis of variance, followed by Tukey's test. Neurological scores and SAH grade were analysed by one-way analysis of variance on ranks, followed by Tukey's test. A value of P<0.05 was accepted as significant. All statistical analyses were performed using SigmaStat (SYSTAT Software Inc., Point Richmond, CA, USA).
Results
Dexmedetomidine neuroprotection
Three rats had SAH grades <8 in SAH and SAH+dexmedetomidine post-treatment groups in Experiment 1, and these animals were excluded in the final data analysis. Five and four rats in the SAH and SAH+dexmedetomidine post-treatment groups, respectively, died within 48 h after onset of SAH, corresponding to 22 and 18% for these groups. No rats in the sham-operated groups died within the 48 h observation time. Similar mortality rates were observed and numbers of rats excluded from the final analysis owing to low SAH grades occurring in the SAH and sham-operated groups in the other sets of experiments.
The body weights of rats in the sham-operated, SAH, and SAH+dexmedetomidine post-treatment groups were 344 (7), 344 (20), and 341 (19) g (P>0.05), respectively, before surgery and 331 (10), 306 (24), and 312 (15) g, respectively, for sham-operated, SAH, and SAH+dexmedetomidine post-treatment groups at 48 h after SAH surgery. The body weights of rats in the SAH group were significantly decreased after SAH compared with their weights before SAH (P=0.023).
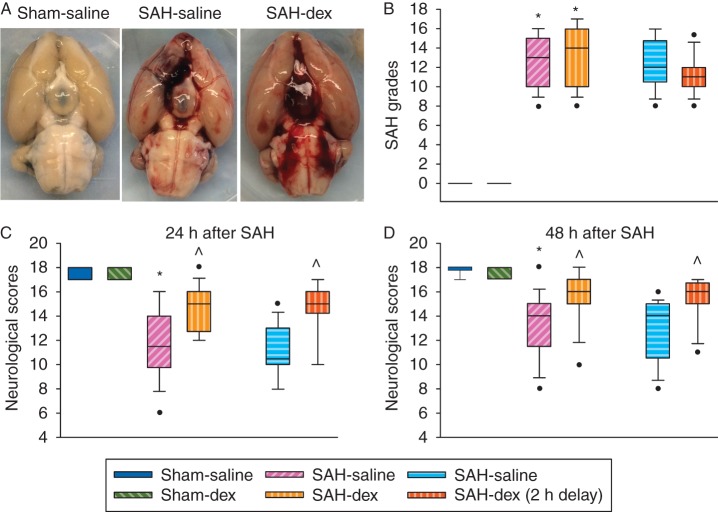
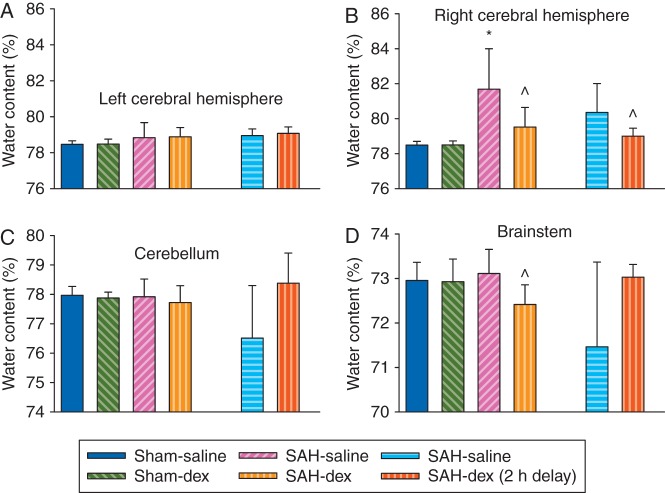
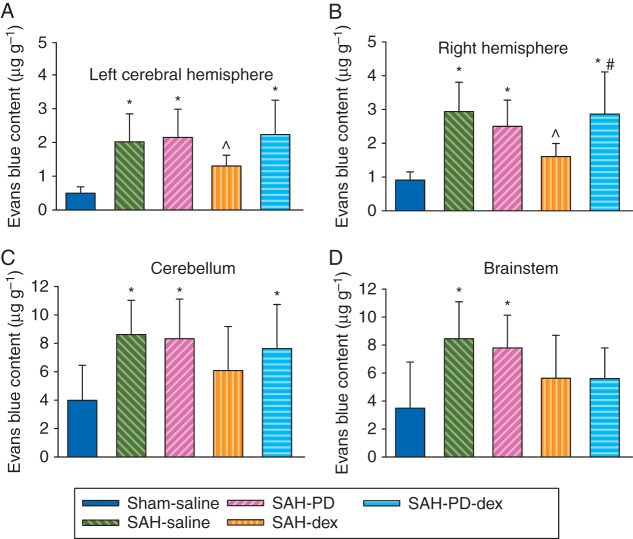
Subarachnoid haemorrhage grades were similar among the SAH groups with or without dexmedetomidine post-treatment. Neurological scores were significantly reduced by SAH [median (25th–75th percentile): 14 (12, 15) compared with 18 (18, 18) of sham-operated animals at 48 h after onset of SAH, P<0.001]. This reduction was reversed by dexmedetomidine, with the first dose applied immediately after onset of SAH [16 (15, 17), P=0.004 compared with SAH only]. This pattern existed when assessment of neurological scores was performed 24 or 48 h after the onset of SAH (Fig. 1). Likewise, application of dexmedetomidine at 2 h after onset of SAH (post-treatment) improved neurological outcome after SAH (Fig. 1). Consistent with these results, the right cerebral hemisphere in the SAH group contained more water than that in the sham-operated group (water content: 81.7 (2.3) and 78.5 (0.2)% in the SAH group and sham-operated group, respectively, P<0.001). This increase was attenuated by dexmedetomidine post-treatment [79.5 (1.1)%, P=0.007 compared with the SAH group; Fig. 2). Likewise, Evans blue content in brain tissue, which reflects the permeability of the BBB, was increased in the right cerebral hemisphere. This increase was also attenuated by dexmedetomidine post-treatment (Fig. 3). These results suggest that dexmedetomidine post-treatment provides neuroprotection after SAH. Interestingly, SAH from a right cerebral vessel also increased Evans blue content in the left cerebral hemisphere, cerebellum, and brainstem. This increase in the left cerebral hemisphere was significantly attenuated by dexmedetomidine applied immediately after SAH (Fig. 3). These results suggest that the effects of SAH on the brain can be diffuse.
Fig 1.
Dexmedetomidine post-treatment-induced neuroprotection. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately or 2 h after onset of SAH. (a) representative brain images. (b) Subarachnoid haemorrhage grades. (c) Neurological scores at 24 h after onset of SAH. (b) Neurological scores at 48 h after onset of SAH. Inside boxes: 25–75% interval, including the median; n=18 for the first four groups and n=16 for the last two groups. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. Dex, dexmedetomidine; SAH, subarachnoid haemorrhage.
Fig 2.
Dexmedetomidine post-treatment-induced reduction of brain oedema after SAH. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately or 2 h after onset of SAH. (a) Water content in left cerebral hemisphere. (b) Water content in right cerebral hemisphere. (c) Water content in cerebellum. (d) Water content in brainstem. Results are mean (sd); n=8. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. Dex, dexmedetomidine; SAH, subarachnoid haemorrhage.
Fig 3.
Dexmedetomidine post-treatment-induced reduction of blood–brain barrier permeability after SAH. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately or 2 h after the onset of SAH. (a) Evans blue content in left cerebral hemisphere. (b) Evans blue content in right cerebral hemisphere. (c) Evans blue content in cerebellum. (d) Evans blue content in brainstem. Results are mean (sd); n=10 for the first four groups and n=8 for the last two groups. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. Dex, dexmedetomidine; SAH, subarachnoid haemorrhage.
Role of extracellular signal-regulated kinase in dexmedetomidine neuroprotection
Phospho-ERK immunoreactivity in the right Fr1 was reduced by SAH, but this was significantly increased by dexmedetomidine post-treatment. This increase was abolished by PD98095, an ERK inhibitor (Fig. 4a and b). These results suggest that dexmedetomidine post-treatment can activate ERK in the brain after SAH.
Fig 4.
Dexmedetomidine post-treatment-induced activation of ERK and neuroprotection after SAH. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately after the onset of SAH. (a) Phospho-ERK immunoreactivity in the frontal cortex area 1. (b) Phospho-ERK immunoreactivity in the frontal cortex area 1 for animals treated with PD98095 at 30 min before dexmedetomidine application. (c) Subarachnoid haemorrhage grades. (d) Neurological scores. Results are mean (sd) [n=8 for panels (a) and (b)] or in box plot [n=16 for panels (c) and (d)]. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. #P<0.05 compared with SAH+dexmedetomidine group. Dex, dexmedetomidine; ERK, extracellular signal-regulated kinase; PD, PD98095; SAH, subarachnoid haemorrhage; Sal, saline.
There was no difference in the SAH grades between SAH groups treated with or without dexmedetomidine and PD98095. Consistent with these results, neurological scores were decreased by SAH when assessment was performed 24 or 48 h after SAH, which was attenuated by dexmedetomidine. PD98095 blocked the effect of dexmedetomidine (Fig. 4c and d). Water content in the right cerebral hemisphere was increased by SAH, and this was attenuated by dexmedetomidine. PD98095 abolished the effect of dexmedetomidine (Fig. 5). Similar effects occurred in Evans blue content in the right cerebral hemisphere. Evans blue content in the left cerebral hemisphere, cerebellum, and brainstem was increased. This increase was attenuated by dexmedetomidine in the left cerebral hemisphere, which was prevented by PD98095 (Fig. 6). PD98095 did not affect the neurological scores, water, and Evans blue content in the brain of animals with SAH only (Figs 4–6). These results suggest that the effects of dexmedetomidine are mediated by ERK.
Fig 5.
Attenuation of dexmedetomidine post-treatment effects on brain oedema after SAH by extracellular signal-regulated kinase inhibition. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately after onset of SAH. PD98095 was given 30 min before dexmedetomidine application. (a) Water content in left cerebral hemisphere. (b) Water content in right cerebral hemisphere. (c) Water content in cerebellum. (d) Water content in brainstem. Results are mean (sd); n=8. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. #P<0.05 compared with SAH+dexmedetomidine group. Dex, dexmedetomidine; PD, PD98095; SAH, subarachnoid haemorrhage.
Fig 6.
Attenuation of dexmedetomidine post-treatment effects on blood–brain barrier permeability after SAH by extracellular signal-regulated kinase inhibition. Adult male rats had SAH induced by endovascular perforation of the junction of right middle and anterior cerebral arteries. They were treated with dexmedetomidine, with the first dose given immediately after the onset of SAH. PD98095 was given 30 min before dexmedetomidine application. (a) Evans blue content in left cerebral hemisphere. (b) Evans blue content in right cerebral hemisphere. (c) Evans blue content in cerebellum. (d) Evans blue content in brainstem. Results are mean (sd); n=8. *P<0.05 compared with sham-operated animals. ^P<0.05 compared with SAH-only group. Dex, dexmedetomidine; SAH, subarachnoid haemorrhage.
Discussion
Our results show that SAH impairs neurological function and causes weight loss. Dexmedetomidine post-treatment improved the neurological outcome after SAH, and also reduced water content and BBB permeability. These effects were evident even when the first dose of dexmedetomidine was given 2 h after the onset of SAH. These results provide initial in vivo evidence for dexmedetomidine post-treatment-induced neuroprotection against SAH.
There are three studies that have investigated the neuroprotective effects of dexmedetomidine when administered after injury. Dexmedetomidine applied 3 h after transient global cerebral ischaemia did not provide neuroprotection in adult gerbils.9 In contrast, application of dexmedetomidine 2 h, but not 3 h, after a traumatic insult provided neuroprotection in mouse brain slices.12 In addition, incubation of mouse hippocampal slices with dexmedetomidine for 1 h at 1–2 h after oxygen and glucose deprivation reduced cell death.25 Our results show that dexmedetomidine applied 2 h after SAH was neuroprotective. Although the reason for the failure to show neuroprotection in the gerbil study is not known, the delay in application of dexmedetomidine or differences between SAH and the ischaemia model used could have contributed to it.
Dexmedetomidine post-treatment-induced neuroprotection against SAH appears to be mediated by ERK. Dexmedetomidine post-treatment increased the activated ERK. PD98095, an ERK inhibitor, inhibited this increase and also blocked the effects of dexmedetomidine on neurological scores, water content, and Evans blue content in brain. These results suggest a critical role of ERK in the dexmedetomidine post-treatment effects. Extracellular signal-regulated kinase has been suggested to be a protein kinase essential for cell survival.26 Dexmedetomidine might activate ERK to mediate its neuroprotection against ischaemic brain injury when it is applied during brain ischaemia in rats or after a traumatic injury in mouse hippocampal slices.11,12 Also, dexmedetomidine can activate ERK.27 Thus, ERK appears to be an important intracellular signaling molecule for dexmedetomidine-induced neuroprotection.
Two pathways have been described for dexmedetomidine to activate ERK. The first is via activation of α2ARs, which induces release of epidermal growth factor or epidermal growth factor-like growth factor, and then activates/phosphorylates ERK.28,29 The second pathway is α2AR independent. Dexmedetomidine could activate imidazoline I1 receptors that then increase protein kinase C activity to activate ERK.27 These two pathways might work together to enhance activation of ERK by dexmedetomidine.
Subarachnoid haemorrhage induces cerebral oedema by various mechanisms, such as autoregulatory breakdown in brain blood vessels because of hypertension, neuroinflammation, focal brain ischaemia, and increased intracellular water attributable to hyponatraemia.30 Many of these mechanisms result in increased BBB permeability as demonstrated in our study. Dexmedetomidine might inhibit these mechanisms via its neuroprotective effects, and might also work through α2AR to control the BBB permeability directly.31 These effects should contribute to dexmedetomidine-induced reduction of brain oedema after SAH as observed in the present study.
Our study could have significant translational implications. Subarachnoid haemorrhage is not uncommon and has a high mortality. If our findings in rats are confirmed in humans, translation of our finding to humans could easily be achieved. In addition, dexmedetomidine can be used to sedate patients with SAH in the intensive care unit. Thus, dexmedetomidine can be used for two purposes in this situation.
Our study has limitations. We did not perform a dose–response study and selected a dose that was protective against ischaemic stroke.11 Our SAH model simulates the clinical situation of intracranial aneurysm rupture. However, it is a difficult model and requires a large number of animals per group. Dose–response studies should be performed in the future to provide better characterization of the effects of dexmedetomidine on SAH. Also, mechanisms other than ERK activation could play a role in dexmedetomidine post-treatment against SAH. α2-Adrenergic receptors can regulate neurotransmitter release,32 but dexmedetomidine does not affect brain ischaemia–reperfusion-induced glutamate release.33–35 In addition, our study showed that PD98095 abolished dexmedetomidine post-treatment-induced neuroprotection. Thus, the effects of dexmedetomidine post-treatment on glutamate release might not be important for the effects observed in our study and have not been determined. Finally, we studied only male rats; therefore, potential sex differences were not determined.
In summary, our results suggest that dexmedetomidine post-treatment induces neuroprotection against SAH in rats, mediated by ERK activation.
Authors’ contributions
Conception of the project: Z.Z.
Design of the study: Y.W., R.H., Z.Z.
Performance of the experiments: Y.W.
Initial data analysis and drafting of the Methods section: Y.W.
Final data analysis and writing of the manuscript: Z.Z.
Declaration of interest
None declared.
Funding
National Institutes of Health, Bethesda, MD, USA (R01 GM098308 to Z.Z.); International Anaesthesia Research Society, Cleveland, OH, USA (2007 Frontiers in Anaesthesia Research Award to Z.Z.); American Heart Association Mid-Atlantic Affiliate by a Grant-in-Aid Baltimore, MD, USA (10GRNT3900019 to Z.Z.); University of Virginia, Charlottesville, VA, USA (Robert M. Epstein Professorship endowment).
References
- 1.Venti M. Subarachnoid and intraventricular hemorrhage. Front Neurol Neurosci 2012; 30: 149–53 [DOI] [PubMed] [Google Scholar]
- 2.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997; 28: 660–4 [DOI] [PubMed] [Google Scholar]
- 3.Ingall TJ, Whisnant JP, Wiebers DO, O'Fallon WM. Has there been a decline in subarachnoid hemorrhage mortality? Stroke 1989; 20: 718–24 [DOI] [PubMed] [Google Scholar]
- 4.Kamibayashi T, Maze M. Clinical uses of α2-adrenergic agonists. Anesthesiology 2000; 93: 1345–9 [DOI] [PubMed] [Google Scholar]
- 5.Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology 2005; 102: 1226–34 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Anesthesiology 1991; 75: 328–32 [DOI] [PubMed] [Google Scholar]
- 7.Maier C, Steinberg GK, Sun GH, Zhi GT, Maze M. Neuroprotection by the α2-adrenoreceptor agonist dexmedetomidine in a focal model of cerebral ischemia. Anesthesiology 1993; 79: 306–12 [DOI] [PubMed] [Google Scholar]
- 8.Cosar M, Eser O, Fidan H et al. The neuroprotective effect of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage. Surg Neurol 2009; 71: 54–9; discussion 9 [DOI] [PubMed] [Google Scholar]
- 9.Kuhmonen J, Pokorny J, Miettinen R et al. Neuroprotective effects of dexmedetomidine in the gerbil hippocampus after transient global ischemia. Anesthesiology 1997; 87: 371–7 [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Hossain M, Rajakumaraswamy N et al. Dexmedetomidine produces its neuroprotective effect via the α2A-adrenoceptor subtype. Eur J Pharmacol 2004; 502: 87–97 [DOI] [PubMed] [Google Scholar]
- 11.Zhu YM, Wang CC, Chen L et al. Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res 2013; 1494: 1–8 [DOI] [PubMed] [Google Scholar]
- 12.Schoeler M, Loetscher PD, Rossaint R et al. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol 2012; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1577–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zeng M, Chen W et al. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS ONE 2014; 9: e93639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrity AG, Botta S, Lazar SB et al. Dexmedetomidine-induced sedation does not mimic the neurobehavioral phenotypes of sleep in Sprague Dawley rat. Sleep 2015; 38: 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filbey WA, Sanford DT, Baghdoyan HA, Koch LG, Britton SL, Lydic R. Eszopiclone and dexmedetomidine depress ventilation in obese rats with features of metabolic syndrome. Sleep 2014; 37: 871–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS ONE 2010; 5: e13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Hu Q, Li B et al. Recombinant milk fat globule-EGF factor-8 reduces oxidative stress via integrin β3/nuclear factor erythroid 2-related factor 2/heme oxygenase pathway in subarachnoid hemorrhage rats. Stroke 2014; 45: 3691–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26: 627–34 [DOI] [PubMed] [Google Scholar]
- 20.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 2008; 167: 327–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii M, Sherchan P, Krafft PR, Rolland WB, Soejima Y, Zhang JH. Cannabinoid type 2 receptor stimulation attenuates brain edema by reducing cerebral leukocyte infiltration following subarachnoid hemorrhage in rats. J Neurol Sci 2014; 342: 101–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood–brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 2011; 31: 52–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Ayer R, Sugawara T et al. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med 2010; 38: 612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab 2011; 31: 1283–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahmani S, Rouelle D, Gressens P, Mantz J. Characterization of the postconditioning effect of dexmedetomidine in mouse organotypic hippocampal slice cultures exposed to oxygen and glucose deprivation. Anesthesiology 2010; 112: 373–83 [DOI] [PubMed] [Google Scholar]
- 26.Li L, Deng J, Zuo Z. Glutamate transporter type 3 mediates isoflurane preconditioning-induced acute phase of neuroprotection in mice. Brain Res Bull 2013; 98: 23–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahmani S, Paris A, Jannier V et al. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an α2-adrenoceptor-independent mechanism: evidence for the involvement of imidazoline I1 receptors. Anesthesiology 2008; 108: 457–66 [DOI] [PubMed] [Google Scholar]
- 28.Cussac D, Schaak S, Denis C, Paris H. α2B-Adrenergic receptor activates MAPK via a pathway involving arachidonic acid metabolism, matrix metalloproteinases, and epidermal growth factor receptor transactivation. J Biol Chem 2002; 277: 19882–8 [DOI] [PubMed] [Google Scholar]
- 29.Du T, Li B, Liu S et al. ERK phosphorylation in intact, adult brain by α2-adrenergic transactivation of EGF receptors. Neurochem Int 2009; 55: 593–600 [DOI] [PubMed] [Google Scholar]
- 30.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke 2002; 33: 1225–32 [DOI] [PubMed] [Google Scholar]
- 31.Borges N, Sarmento A, Azevedo I. Dynamics of experimental vasogenic brain oedema in the rat: changes induced by adrenergic drugs. J Auton Pharmacol 1999; 19: 209–17 [DOI] [PubMed] [Google Scholar]
- 32.Philipp M, Brede M, Hein L. Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol 2002; 283: R287–95 [DOI] [PubMed] [Google Scholar]
- 33.Kim HK, Zornow MH, Strnat MA, Maze M. Dexmedetomidine does not attenuate increases in excitatory amino acids after transient global ischemia in the rabbit. J Neurosurg Anesthesiol 1996; 8: 230–6 [DOI] [PubMed] [Google Scholar]
- 34.Engelhard K, Werner C, Kaspar S et al. Effect of the α2-agonist dexmedetomidine on cerebral neurotransmitter concentrations during cerebral ischemia in rats. Anesthesiology 2002; 96: 450–7 [DOI] [PubMed] [Google Scholar]
- 35.Goyagi T, Nishikawa T, Tobe Y, Masaki Y. The combined neuroprotective effects of lidocaine and dexmedetomidine after transient forebrain ischemia in rats. Acta Anaesthesiol Scand 2009; 53: 1176–83 [DOI] [PubMed] [Google Scholar]