Abstract
Primary autonomic failure is characterized by disabling orthostatic hypotension; but at least half of these patients have paradoxical supine hypertension. Renin-angiotensin mechanisms were not initially thought to contribute to this hypertension, as plasma renin activity is often undetectable in autonomic failure. Plasma aldosterone levels are normal, however, and we recently showed that plasma angiotensin II is elevated and acts at AT1 receptors to contribute to hypertension in these patients. Since aldosterone and angiotensin II can also bind mineralocorticoid receptors to elevate blood pressure, we hypothesized that mineralocorticoid receptor activation plays a role in the hypertension of autonomic failure. To test this hypothesis, we determined the acute effects of the mineralocorticoid receptor antagonist eplerenone (50 mg, oral) versus placebo on supine blood pressure in a randomized, double blind, crossover study. Medications were given at 8:00 PM with blood pressure recorded every 2 hours for 12 hours. Ten primary autonomic failure patients with supine hypertension completed this study (7 Pure Autonomic Failure, 2 Multiple System Atrophy, 1 Parkinson’s disease; 7 male; 70±2 years of age). Eplerenone maximally reduced supine systolic blood pressure by 32±6 mmHg at 8 hours after administration (vs. 8±10 mmHg placebo, p=0.016), with no effect on nocturia (12-hour urine volume: 985±134 placebo vs. 931±94 ml eplerenone, p=0.492; nocturnal weight loss: −1.19±0.15 placebo vs. −1.18±0.15 kg eplerenone, p=0.766). These findings suggest that inappropriate mineralocorticoid receptor activation contributes to the hypertension of autonomic failure, likely independent of canonical mineralocorticoid effects, and provides rationale for use of eplerenone in these patients.
Keywords: autonomic nervous system, hypertension, aldosterone, angiotensin
Introduction
Primary autonomic failure provides a unique human model to study the development of hypertension in the setting of chronic autonomic impairment. This is a neurodegenerative disorder characterized by cellular lesions rich in the protein α-synuclein in central or peripheral autonomic pathways.1 The clinical picture of autonomic failure is dominated by orthostatic hypotension and absence of baroreflex buffering. At least half of these patients also have supine hypertension, which can be severe and increases risk for cardiovascular end-organ damage.2, 3 Supine hypertension limits the use of pressor agents and increases nocturnal pressure natriuresis to drive volume depletion and subsequent worsening of morning orthostatic tolerance in these patients.2 Thus, there is an emerging need to identify molecular mechanisms and optimal treatment strategies for hypertension in autonomic failure.
Renin-angiotensin system (RAS) mechanisms were not initially thought to contribute to hypertension in autonomic failure. Plasma renin activity is often undetectable in autonomic failure, and is unresponsive to postural or pharmacologic stimuli.4–6 Plasma aldosterone levels, however, are normal in these patients.5 In addition, we showed that plasma angiotensin (Ang) II levels are elevated in autonomic failure, and that a single dose of the AT1 receptor antagonist losartan effectively lowered overnight blood pressure in these patients.7 These findings suggest that downstream RAS pathways are intact in autonomic failure, despite suppressed renin. Ang II and aldosterone also bind mineralocorticoid receptors (MR) to promote hypertension, through both genomic and more rapid non-genomic actions.8–10 In this study, we used a pharmacologic approach to determine if inappropriate MR activation contributes to supine hypertension in autonomic failure. We hypothesized that acute administration of the MR antagonist eplerenone would lower overnight blood pressure in these patients. To determine the therapeutic potential for management of hypertension in autonomic failure, we also measured eplerenone effects on nocturnal natriuresis and morning orthostatic tolerance.
Methods
The Vanderbilt Institutional Review Board approved this study. Written informed consent was obtained from each patient (http://clinicaltrials.gov identifier: NCT00223717).
Study Participants
We studied 10 patients diagnosed with severe primary autonomic failure (7 Pure Autonomic Failure, 2 Multiple System Atrophy, 1 Parkinson’s disease) by American Autonomic Society consensus criteria.11 All patients had neurogenic orthostatic hypotension and comorbid supine hypertension defined as systolic blood pressure (SBP) ≥150 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg.2 Patients with secondary causes of autonomic failure (e.g. diabetes mellitus, amyloidosis, autoimmune ganglionopathy) or renal failure were excluded.
General Protocol
Patients were studied at the Vanderbilt Clinical Research Center on an inpatient basis. Medications affecting the autonomic nervous system, blood pressure, or volume were withheld at least 5 half-lives before admission (e.g. fludrocortisone, midodrine, anti-hypertensives). All other medications were held constant during admission. Patients received a low monoamine, methylxanthine-free, fixed 150 mEq sodium and 60–80 mEq potassium diet. Screening consisted of a comprehensive physical examination and medical history, 12-lead ECG, and routine safety laboratory tests (e.g. CBC, CMP, urinalysis). All patients had an overnight screening study to examine for supine hypertension, which consisted of arm cuff blood pressure measurement (Dinamap ProCare, GE Healthcare) in duplicate at two-hour intervals from 8:00 PM to 8:00 AM.
Autonomic Function Testing
Standardized autonomic function testing was performed in all patients and included orthostatic stress, sinus arrhythmia, Valsalva maneuver, hyperventilation, isometric handgrip, and cold pressor tests.12 Blood pressure was measured intermittently using an automated oscillometric sphygmomanometer (Dinamap) and continuously using finger photoplethysmography (Nexfin, BMEYE). Heart rate (HR) was measured by continuous ECG. For orthostatic stress testing, supine blood pressure and HR were measured at 8:00 AM, after an overnight rest. Patients were then asked to stand for 10 minutes, with vitals measured at 1, 3, 5, and 10 minutes standing (or as long as tolerated). Fasting blood samples were collected at the end of the supine and standing periods via an intravenous catheter placed at least 30 minutes before testing. Plasma norepinephrine was measured by high-performance liquid chromatography with electrochemical detection.13 Plasma renin activity was measured as the conversion of angiotensinogen to Ang I using radioimmunoassay (GammaCoat, Diasorin). The lower detection limit is 0.2 ng/ml/hr, with an average 8% intra-assay and 7% inter-assay variability. This assay has 100% cross-reactivity for Ang I, with <0.3% cross-reactivity for other Ang II and Ang III. Plasma aldosterone was measured by chemiluminescent immunoassay (Liasion, Diasorin). The lower detection limit is 3.0 ng/dl, with approximately 8% intra- and inter-assay variability. For renin activity and aldosterone levels below detection limits, a value of one-half the detection limit was assigned for statistical analysis.
Overnight Medication Trials
We studied the effects of a single dose of the MR antagonist eplerenone (50 mg, oral; Greenstone LLC) versus placebo on overnight blood pressure in a randomized, double blind, crossover study. The Vanderbilt Investigational Pharmacy provided all medications. Prior to the study, possible treatment sequences were determined using computer-generated random numbers, with consecutive patients randomly assigned to a treatment sequence. Patients and investigators were blinded to the treatment assignment. We used the recommended starting dose of eplerenone for treatment of essential hypertension.14 Mean peak plasma concentrations of eplerenone are reached approximately 1.5 hours after oral administration, with a half-life of 4 to 6 hours. Thus, at least one washout day was allowed between treatments to avoid carryover effects. Medications were administered to patients at 8:00 PM with 50 ml tap water. Patients were instructed to remain supine throughout the night, and fluid intake was restricted. Supine blood pressure was measured twice in a row at two-hour intervals from 8:00 PM to 8:00 AM using an automated sphygmomanometer (Dinamap). Urine was collected during the 12-hour period following drug administration to measure sodium, potassium, and creatinine levels. Nocturnal sodium and potassium excretion were defined as the ratio of urinary sodium or potassium to creatinine, to correct for incomplete bladder emptying in these patients. Changes in body weight from 8:00 PM to 8:00 AM were used as another measure of nocturnal volume loss. To assess morning orthostatic tolerance, patients were asked to stand at 8:00 AM for 10 minutes or as long as tolerated, with blood pressure measured at 1, 3, 5, and 10 minutes of standing.
Statistical Analysis
For the primary endpoint, we compared the decrease in supine SBP produced by eplerenone versus placebo during the 12-hour overnight period. To evaluate for these changes in blood pressure, we used a two-way ANOVA to test effects of treatment, time, and their interaction. To summarize the overnight blood pressure changes, we also compared the peak change in SBP (at 8 hours after administration) following placebo versus eplerenone using paired Wilcoxon signed rank nonparametric tests. As secondary endpoints, we examined for differences in nocturnal natriuresis and morning orthostatic tolerance between treatments. For morning orthostatic tolerance, an area under the curve for the 4 standing measurements was calculated by the trapezoidal rule (AUCSBP=mean SBP*time), with comparisons made only for patients who could stand after both treatments. Urine volume, body weight, urinary sodium excretion, urinary potassium excretion, the sodium: potassium ratio, and the AUC for morning SBP were compared between treatments using Wilcoxon signed rank tests. Data are presented as mean ± SEM. A two-tailed p value <0.05 was defined as statistically significant. Analyses were performed with SPSS (Version 22.0, IBM).
Sample Size Calculations
Power calculations were based on preliminary data obtained in 4 patients. A study investigator not involved in the statistical analysis provided data to the first author (A.A.) for sample size calculations in a blinded manner (Treatment A versus B). These data showed an SBP difference of 22-mmHg between treatments at 8 hours after administration, with a 19-mmHg standard deviation of difference. Assuming similar effect size and variance, we estimated that 10 patients would have 90% power to detect a difference in SBP between treatments. These calculations used an alpha level of 0.05 and paired t-test analysis (PS Dupont, Version 3.0.34).15
Results
Patient Clinical Characteristics
The clinical characteristics of autonomic failure patients are shown in Table 1. A history of hypertension was present before diagnosis in 3 patients (30%), with the remaining patients exhibiting supine hypertension after the onset of autonomic failure. All patients exhibited a significant decrease in SBP upon standing (orthostatic hypotension), with an inadequate compensatory increase in HR, consistent with loss of baroreflex buffering (Tables 1 and 2). Plasma renin activity was undetectable in 9 patients (90%) in the supine position, and in 6 patients (60%) in the upright position. Plasma aldosterone levels were within a normal range in supine and upright positions. All patients exhibited severe impairment of autonomic reflexes (Table 2). Respiratory sinus arrhythmia was reduced suggesting parasympathetic dysfunction. Evidence of sympathetic dysfunction included blunted pressor responses to isometric handgrip and cold pressor tests, and an exaggerated decrease in SBP during Phase II and absence of blood pressure overshoot during Phase IV of the Valsalva maneuver.
Table 1.
Patient Clinical Characteristics
| Parameter, unit | Patients (n=10) | Normal* |
|---|---|---|
| Age, years | 70 ± 2 | 64 ± 3 |
| Gender, men/women | 7/3 | 6/4 |
| Body Mass Index, kg/m2 | 25 ± 1 | 25 ± 1 |
| Disease Duration, years | 8 ± 2 | |
| Supine | ||
| SBP, mmHg | 174 ± 7 | 116 ± 5 |
| DBP, mmHg | 92 ± 4 | 74 ± 4 |
| HR, bpm | 64 ± 3 | 66 ± 4 |
| Norepinephrine, pg/ml | 128 ± 54 | 352 ± 65 |
| Plasma Renin Activity, ng/ml/hr | 0.10 ± 0.01 | 0.34 ± 0.13 |
| Plasma Aldosterone, ng/dl | 4.2 ± 1.1 | 2.4 ± 0.7 |
| Upright | ||
| SBP, mmHg | 95 ± 13 | 121 ± 6 |
| DBP, mmHg | 55 ± 5 | 79 ± 3 |
| HR, bpm | 79 ± 6 | 76 ± 4 |
| Norepinephrine, pg/ml | 185 ± 51 | 689 ± 61 |
| Plasma Renin Activity, ng/ml/hr | 0.18 ± 0.05 | 0.73 ± 0.29 |
| Plasma Aldosterone, ng/dl | 7.8 ± 2.0 | 5.7 ± 1.0 |
Data are presented as mean ± SEM.
Normal values are for reference only, and were obtained from healthy age-matched volunteers obtained from the Vanderbilt Autonomic Dysfunction Center database. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Table 2.
Autonomic Function Testing
| Parameter, unit | Patients (n=10) | Normal* |
|---|---|---|
| Orthostatic Change in SBP, mmHg | −73 ± 11 | 4 ± 3 |
| Orthostatic Change in HR, bpm | 12 ± 2 | 10 ± 2 |
| Sinus Arrhythmia Ratio | 1.05 ± 0.02 | 1.33 ± 0.11 |
| Valsalva Ratio | 1.13 ± 0.04 | 1.51 ± 0.09 |
| Valsalva Phase II ΔSBP, mmHg | −80 ± 10 | −37 ± 6 |
| Valsalva Phase IV ΔSBP, mmHg † | −45 ± 6 | 13 ± 7 |
| Hyperventilation ΔSBP, mmHg | −36 ± 9 | −4 ± 3 |
| Handgrip ΔSBP, mmHg | 6 ± 3 | 22 ± 7 |
| Cold Pressor ΔSBP, mmHg | 8 ± 3 | 16 ± 3 |
Data are presented as mean ± SEM. SBP, systolic blood pressure; HR, heart rate; ΔSBP, change in systolic blood pressure compared with baseline.
Normal values are for reference only, and were obtained from healthy age-matched volunteers obtained from the Vanderbilt Autonomic Dysfunction Center database.
A negative value indicates that the blood pressure overshoot was absent.
Blood Pressure-Lowering Effects of Eplerenone
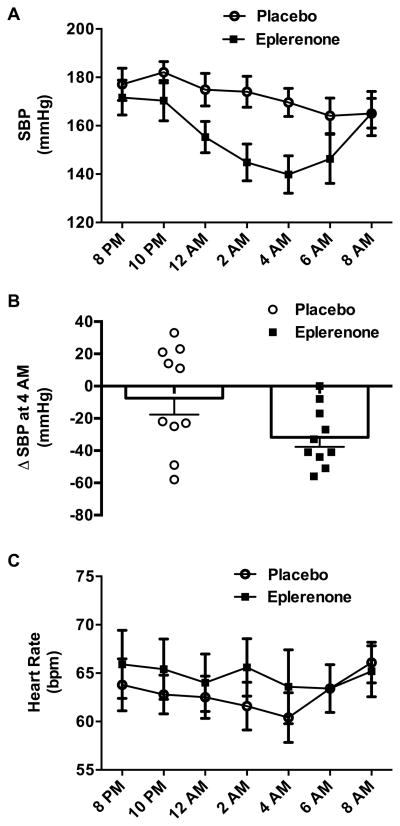
All patients completed both treatment arms, with no difference in baseline SBP between placebo and eplerenone study nights (177±7 and 172±7 mmHg, respectively; p=0.266). The main effect of eplerenone to decrease SBP was significant (p=0.048 for drug effect, p=0.001 for time effect, p=0.042 for interaction; two-way ANOVA; Figure 1A). Eplerenone maximally decreased SBP by 32±6 mmHg at 8 hours after administration (versus 8±10 mmHg placebo; p=0.016; Figure 1B), resulting in an average SBP of 140±8 mmHg at this 4:00 AM time point. Eplerenone similarly lowered mean blood pressure at 8 hours after administration (placebo: −7±7 mmHg; eplerenone: −21±3 mmHg; p=0.039), with no significant effect on DBP (placebo: −3±3 mmHg; eplerenone: −8±3 mmHg; p=0.164). There were no differences in HR following placebo versus eplerenone (p=0.625 for drug effect, p=0.081 for time effect, p=0.394 for interaction; two-way ANOVA; Figure 1C).
Figure 1. Blood Pressure Lowering Effect of Eplerenone in Autonomic Failure.
Effect of single dose eplerenone (50 mg, oral) versus placebo administered at 8:00 PM on overnight systolic blood pressure (SBP) and heart rate (HR) in autonomic failure patients with supine hypertension. Eplerenone produced a significant decrease in SBP compared with placebo as summarized by: (panel A) changes in SBP over time (p=0.048 for main treatment effect, two-way ANOVA); and (panel B) the peak change in SBP (ΔSBP) (p=0.016), which was determined by subtracting 4:00 AM versus baseline values. There were no differences in HR following placebo versus eplerenone (p=0.625 for main treatment effect; two-way ANOVA; panel C).
Effects of Eplerenone on Nocturnal Natriuresis and Morning Orthostatic Tolerance
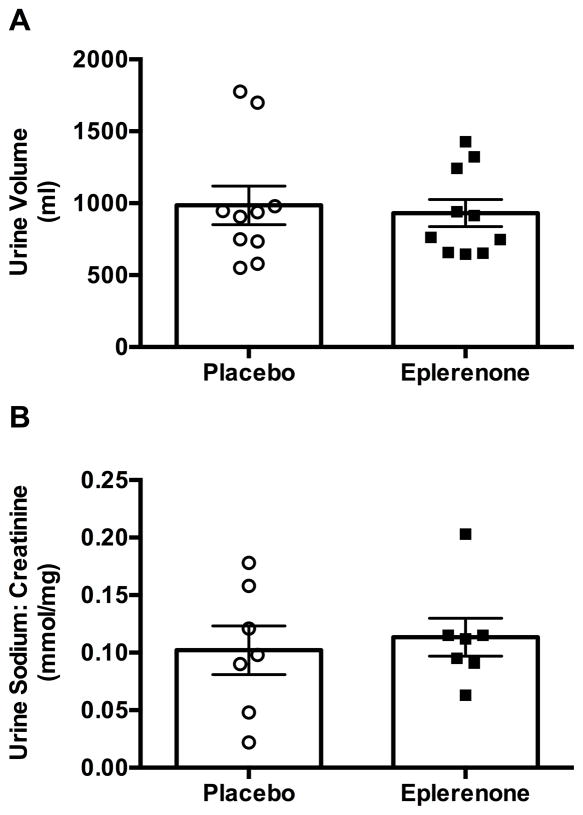
Eplerenone did not alter overnight body weight (placebo: −1.19±0.15 kg; eplerenone: −1.18±0.15 kg; p=0.766) or 12-hour urinary volume (Figure 2A; p=0.492). We were able to measure urinary sodium, potassium, and creatinine levels in 7 of these patients. There were no differences in urinary sodium excretion (Figure 2B; 0.938) or potassium excretion (0.033±0.003 placebo vs. 0.031±0.003 mmol/mg eplerenone; p=0.688) between treatments, despite a similar magnitude of blood pressure lowering with eplerenone (−31±8 vs. −12±13 mmHg placebo). The sodium: potassium ratio was also similar following placebo versus eplerenone (3.19±0.65 vs. 3.82±0.62, respectively; p=0.509). Five out of the 10 patients could not stand at 8:00 AM following both placebo and eplerenone treatment. Of the remaining 5 patients, the maximum standing time was similar between eplerenone and placebo (2±1 and 3±2 minutes, respectively; p=0.625). The morning orthostatic tolerance, estimated as the AUC for standing SBP during a 10-minute test, was also similar between treatments (placebo: 616±210; eplerenone: 668±251; p=0.688).
Figure 2. Effect of Eplerenone on Nocturnal Pressure Natriuresis.
There was no significant effect of eplerenone on nocturnal changes in urine volume (panel A; p=0.652) or urinary sodium excretion (panel B; p=0.938) in autonomic failure patients with supine hypertension.
Discussion
The main finding of this study is that acute administration of eplerenone lowered overnight blood pressure in autonomic failure patients with supine hypertension. This blood pressure lowering effect occurred independent of changes in nocturnal natriuresis or kaliuresis, perhaps suggesting independence from traditional genomic renal volume effects. Importantly, eplerenone did not worsen morning orthostatic tolerance. Taken together, these findings suggest that inappropriate MR activation contributes to the hypertension of autonomic failure, and provide new rationale for use of MR antagonists in the management of these patients.
An important caveat of this study is that the depressor effects of eplerenone could be due to multiple mechanisms including blockade of aldosterone, cortisol, Ang II, or hormone-independent ligands. Eplerenone increases plasma renin activity and aldosterone in hypertensive patients and animal models suggesting blockade of RAS actions.16, 17 Glucocorticoids can also activate the MR with high affinity, particularly in tissues with low co-expression of the 11β-hydroxysteroid dehydrogenase type 2 enzyme (which inactivates cortisol).18 Plasma aldosterone and cortisol are normal in autonomic failure5, 7, 19; however, even if the normal circadian rhythm is preserved in autonomic failure, with a nadir at night, these patients could still have increased sensitivity to these hormones. Plasma Ang II levels are also elevated in autonomic failure,7 which could independently activate MR to increase blood pressure. Unfortunately, specific pharmacologic probes (such as aldosterone synthase inhibitors) are not currently available for clinical research to dissect the precise ligand activating the MR. While cortisol synthesis can be blocked with metyrapone, this drug increases ACTH and 11-deoxycortisol, which can also activate the MR.
We chose eplerenone since it is a highly selective competitive MR antagonist, with reduced side effects compared with spironolactone and a more rapid time course to reach peak plasma concentrations.16 Spironolactone could also lower blood pressure in autonomic failure, but this was not tested. In mild to moderate essential hypertension, chronic eplerenone (50 mg) reduced seated SBP by 4–12 mmHg depending on frequency of daily dosing, without effects on HR.17 The magnified hypotensive effect of acute eplerenone in autonomic failure is likely due to lack of baroreflex buffering. The hemodynamic mechanisms in autonomic failure are unclear, but eplerenone decreases cardiac output and systemic vascular resistance with minor effects on HR in animal models. Interestingly, eplerenone did not significantly reduce DBP in autonomic failure. Chronic eplerenone reduced seated DBP in essential hypertension,17 but had no effect in hypertensive hemodialysis patients.20 Eplerenone is superior to losartan in lowering blood pressure in low renin essential hypertension.21 A similar magnitude of depressor responses is observed, however, with eplerenone and losartan in autonomic failure (>30 mmHg).7
Classical activation of the MR results in translocation of the ligand-receptor complex to the nucleus where it binds hormone response elements in the promoter region of target genes to stimulate transcription.22 More recently, activation of cell surface or cytosolic MR has been shown to elicit non-genomic rapid effects of aldosterone including vasoconstriction and sympathetic activation.9 In addition, Ang II can activate vascular MR, through direct binding or indirectly through AT1 receptor transactivation, to induce vasoconstriction, arterial stiffness, and oxidative stress.10, 23 The lack of effect on renal measures (e.g. volume, natriuresis, kaliuresis, sodium: potassium ratio) combined with the rapid time course for blood pressure lowering effects of eplerenone in autonomic failure may suggest non-canonical mechanisms are involved.
A stepwise approach is recommended for treatment of hypertension in autonomic failure, starting with non-pharmacologic methods such as head-up tilt, and only initiating pharmacologic treatment when needed. Several medications acutely lower overnight blood pressure in autonomic failure (e.g. nitroglycerin, sildenafil, clonidine, nebivolol, losartan).7, 24–26 of these, only clonidine and losartan reduced nocturnal sodium excretion, and none improved morning orthostatic tolerance. In essential hypertension, chronic eplerenone increases natriuresis and diuresis in essential hypertension by blocking renal MR.27 The lack of decreased sodium excretion in the face of a blood pressure reduction with eplerenone may indicate a compensatory natriuretic effect. We do not know the mechanism underlying this compensation but our data supports the interpretation that the blood pressure effect of eplerenone in autonomic failure is not medicated by canonical MR blockade at the renal tubule. Finally, eplerenone did not improve morning orthostatic tolerance, but we would not have expected it to, given that nocturnal volume loss was not lessened. On the other hand, there was no evidence of a residual hypotensive effect the morning after administration, as supine blood pressure recovered at this time point.
There are some limitations to this study. First, a relatively small number of patients were included in this study. Autonomic failure is a rare disease and our sample size is similar to other clinical studies for supine hypertension in these patients.7, 24–26 Second, we did not determine precise mechanisms involved in the blood pressure lowering effects of eplerenone in autonomic failure, and this is an active area of investigation in our laboratory. Third, this study may have been underpowered to detect differences in urinary measures. Unfortunately, we were not able to obtain samples from all patients due to urinary incontinence. We estimate based on the present data, however, that 567 patients would be required to have 80% power to detect significant differences in natriuresis, which suggest that if differences were present, they are likely not clinically relevant. Fourth, we examined the effect of acute eplerenone. Further studies are needed to determine optimal dosing and to assess safety and efficacy of chronic administration in autonomic failure, with caution exercised in patients with renal impairment. Since the magnitude of eplerenone response varied in this study, treatment plans also need to be individualized.
Perspectives
MR antagonists effectively lower blood pressure in essential hypertension, particularly in low renin and drug resistant forms of the disease.28 This study highlights the utility in studying patients with autonomic failure, in whom the lack of baroreflex buffering helps unmask effects that would be difficult to study in essential hypertension. In particular, the autonomic mechanisms involved, and the contribution of genomic versus non-genomic mechanisms to beneficial effects of these therapies has been difficult to assess. Autonomic failure provides a unique human model to examine these mechanistic aspects of MR antagonism. It is important to note that the supine hypertension is driven by different pathophysiologic mechanisms in these patients; by residual sympathetic tone in central autonomic failure (MSA) and by increased vascular resistance in peripheral autonomic failure (PAF).29 While underpowered in this study, comparisons of depressor responses to eplerenone in central versus peripheral autonomic failure patients may provide new insight into sympathetic effects of MR antagonism. Our findings also suggest that volume-independent mechanisms are involved in blood pressure lowering effects of eplerenone in autonomic failure. These findings may have particular relevance for understanding other low renin forms of hypertension. Autonomic failure is a devastating disease with few effective therapeutic options. MR antagonists may represent a previously unexplored treatment strategy for hypertension in autonomic failure.
Novelty and Significance.
1) What is New
Acute administration of the MR antagonist eplerenone effectively lowered overnight blood pressure in autonomic failure patients with supine hypertension, independent of renal volume effects, and without worsening morning orthostatic tolerance.
2) What is Relevant
These findings enhance our understanding of plasma renin-independent mechanisms for hypertension in autonomic failure. While eplerenone might be useful for treatment of hypertension in these patients, further safety and long-term efficacy studies are needed.
Autonomic failure provides a unique model to examine mechanisms involved in MR antagonism in low renin clinical hypertension. Thus, these results may impact our understanding of hypertension in general.
3) Summary
Eplerenone effectively reduced overnight blood pressure without worsening morning orthostatic tolerance in patients with primary autonomic failure. These findings provide new evidence that inappropriate mineralocorticoid receptor activation contributes to supine hypertension in these patients.
Acknowledgments
We acknowledge the patients who volunteered for these studies and the Vanderbilt Clinical Research Center nurses.
Sources of Funding
This work was supported by NIH grants P01 HL56693, U54 NS065736, and UL1 TR000445. Additional support was provided by American Heart Association grants 14SDG19710011 and 14CRP20380211.
Footnotes
Conflicts of Interest/Disclosures
There are no conflicts of interest or financial disclosures related to this study.
References
- 1.Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl 1):2–7. doi: 10.1007/s10286-007-1004-0. [DOI] [PubMed] [Google Scholar]
- 2.Biaggioni I, Robertson RM. Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin. 2002;20:291–301. vii. doi: 10.1016/s0733-8651(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 3.Milazzo V, Di Stefano C, Milan A, Ravera A, Sobrero G, Sabia L, Veglio F, Maule S. Cardiovascular complications in patients with autonomic failure. Clin Auton Res. 2015;25:133–140. doi: 10.1007/s10286-015-0275-0. [DOI] [PubMed] [Google Scholar]
- 4.Baser SM, Brown RT, Curras MT, Baucom CE, Hooper DR, Polinsky RJ. Beta-receptor sensitivity in autonomic failure. Neurology. 1991;41:1107–1112. doi: 10.1212/wnl.41.7.1107. [DOI] [PubMed] [Google Scholar]
- 5.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76:580–586. doi: 10.1210/jcem.76.3.7680352. [DOI] [PubMed] [Google Scholar]
- 6.Gordon RD, Kuchel O, Liddle GW, Island DP. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Invest. 1967;46:599–605. doi: 10.1172/JCI105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin ii, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension. 2013;61:701–706. doi: 10.1161/HYPERTENSIONAHA.111.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett KV, McCurley AT, Jaffe IZ. Direct contribution of vascular mineralocorticoid receptors to blood pressure regulation. Clin Exp Pharmacol Physiol. 2013;40:902–909. doi: 10.1111/1440-1681.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funder JW. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog Cardiovasc Dis. 2010;52:393–400. doi: 10.1016/j.pcad.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe IZ, Mendelsohn ME. Angiotensin ii and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 12.Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23:407–421. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Invest. 1988;81:213–220. doi: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft J. Eplerenone (inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure. Proc (Bayl Univ Med Cent) 2004;17:217–220. doi: 10.1080/08998280.2004.11927973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 16.Brown NJ. Eplerenone: Cardiovascular protection. Circulation. 2003;107:2512–2518. doi: 10.1161/01.CIR.0000071081.35693.9A. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–716. doi: 10.1016/s0895-7061(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto T, Hirata Y. Aldosterone as a cardiovascular risk hormone. Endocr J. 2007;54:359–370. doi: 10.1507/endocrj.kr-80. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox CS, Aminoff MJ, Penn W. Basis of nocturnal polyuria in patients with autonomic failure. J Neurol Neurosurg Psychiatry. 1974;37:677–684. doi: 10.1136/jnnp.37.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shavit L, Neykin D, Lifschitz M, Slotki I. Effect of eplerenone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric chronic hemodialysis patients - a pilot study. Clin Nephrol. 2011;76:388–395. doi: 10.5414/cn106973. [DOI] [PubMed] [Google Scholar]
- 21.Flack JM, Oparil S, Pratt JH, Roniker B, Garthwaite S, Kleiman JH, Yang Y, Krause SL, Workman D, Saunders E. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J Am Coll Cardiol. 2003;41:1148–1155. doi: 10.1016/s0735-1097(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 22.Fejes-Toth G, Pearce D, Naray-Fejes-Toth A. Subcellular localization of mineralocorticoid receptors in living cells: Effects of receptor agonists and antagonists. Proc Natl Acad Sci U S A. 1998;95:2973–2978. doi: 10.1073/pnas.95.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–839. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B, Raj SR, Robertson D, Biaggioni I. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension. 2008;51:1531–1536. doi: 10.1161/HYPERTENSIONAHA.107.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, Raj SR, Robertson D, Biaggioni I. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide-sensitive human hypertension. Hypertension. 2014;64:1241–1247. doi: 10.1161/HYPERTENSIONAHA.114.04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47:522–526. doi: 10.1161/01.HYP.0000199982.71858.11. [DOI] [PubMed] [Google Scholar]
- 27.Reyes AJ, Leary WP, Crippa G, Maranhao MF, Hernandez-Hernandez R. The aldosterone antagonist and facultative diuretic eplerenone: A critical review. Eur J Intern Med. 2005;16:3–11. doi: 10.1016/j.ejim.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Egan BM, Li J. Role of aldosterone blockade in resistant hypertension. Semin Nephrol. 2014;34:273–284. doi: 10.1016/j.semnephrol.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]