Abstract
Wheat stem rust, caused by Puccinia graminis f. sp. tritici (Pgt), is a devastating disease that can cause severe yield losses. A new Pgt race designated Ug99 has overcome most of the widely used resistance genes and is threatening major wheat production areas. Here we demonstrate that the Sr35 gene from Triticum monococcum is a coiled coil-nucleotide binding-leucine rich repeat gene that confers near-immunity to Ug99 and related races. This gene is absent in the A-genome diploid donor and in polyploid wheat, but is effective when transferred from T. monococcum to polyploid wheat. The cloning of Sr35 opens the door to the use of biotechnological approaches to control this devastating disease and to the analyses of the molecular interactions that define the wheat-rust pathosystem.
Puccinia graminis f. sp. tritici (henceforth Pgt) is the causal agent of wheat stem rust, a devastating disease responsible for major outbreaks and large losses of wheat yields in the past. The deployment of Pgt resistance genes, combined with the eradication of the alternative host (barberry) provided an effective control of this disease for the last fifty years (1). However, the widely deployed Pgt resistance gene Sr31 was overcome by a new race of Pgt identified in Uganda in 1999 designated Ug99 (or TTKSK according to the North American system for Pgt race nomenclature) (2). A decade later, six new Ug99-related Pgt races, some showing a broader virulence spectrum, have been detected and have spread to the wheat growing regions of Africa, Yemen and Iran (3). Roughly 90% of the wheat varieties grown worldwide are susceptible to Ug99 and related races, which represents a serious threat to global food security (3). The Borlaug Global Rust Initiative was launched in 2005 to coordinate international efforts to fight Ug99 (http://www.globalrust.org). The identification and characterization of Ug99 resistance genes Sr35 in this study and Sr33 in a companion paper (4) are part of these efforts.
The stem rust resistance gene Sr35 was identified in previous screens for resistance to Pgt in the diploid wheat species Triticum monococcum (5, 6). The genome of T. monococcum, designated Am, is closely related to the genome of T. urartu, the diploid donor of the A genome in tetraploid (T. turgidum, pasta wheat) and hexaploid wheat (T. aestivum, bread wheat) (7). Sr35 was prioritized for cloning because it confers near-immunity against Ug99, Ug99-realated races, and the TRTTF group of races from Africa, Yemen and Pakistan, which has a broad but different virulence profile from the Ug99 race group (3, 8). Sr35 was also selected because previous studies have confirmed that this gene is effective against the same virulent races when it is transferred to hexaploid wheat by crossing and recombination (5, 8).
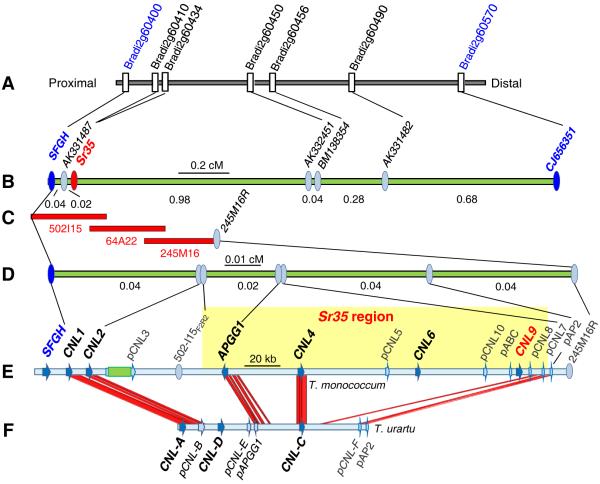
Sr35 was previously mapped on the long arm of chromosome 3Am in Triticum monococcum (8). In this study, we used 4,575 recombinant gametes and seven molecular markers derived from the colinear region in Brachypodium distachyon (Fig. 1A) to map Sr35 between markers AK331487 (0.02 cM) and AK332451 (0.98 cM, Fig. 1B). We then used the closest proximal markers AK331487 and SFGH (S-formylglutathione hydrolase-like) to screen a T. monococcum BAC library of the Sr35-resistant accession DV92 (9). The 23 selected BAC clones were assembled by fingerprinting into a single contig that spanned the Sr35 locus (Fig. 1C-D, Table S1, Fig. S1).
Fig. 1.
Genetic and physical maps of Sr35. (A) 174-kb collinear region of Brachypodium (8). Only genes for which a wheat orthologous gene was found in databases are represented. (B) Genetic map of Sr35 locus. (C) Screening the DV92 BAC library with proximal markers SFGH and AK331487 (only BACs from the minimum tilling path are shown). (D) High density map. (E) Graphical representation of the T. monococcum annotated sequences (KC573058). CNL= coiled coil-nucleotide binding-leucine rich repeat genes, ‘p’ before gene name = pseudogene (pCNL3 has an inserted retroelement). The Sr35 candidate gene region is highlighted in yellow. (F) Comparison of T. monococcum DV92 and T. urartu G1812 (KC816724) orthologous regions (92% identity threshold).
We sequenced three overlapping BACs covering the Sr35 region (10) and annotated the 307,519 bp sequence (KC573058). This sequence includes a cluster of coiled coil-nucleotide binding-leucine rich repeat (henceforth CNL) disease resistance genes including five intact genes (CNL1, CNL2, CNL4, CNL6 and CNL9), two pseudogenes (pCNL3 and pCNL10) and three small gene fragments (pCNL5, pCNL7 and pCNL8) (Fig. 1E). A phylogenetic tree of the complete CNL genes showed that CNL4 and CNL9 are the most closely related members of this cluster (Fig. S2). The annotated sequence also includes two unrelated genes (SFGH and APGG1) and two pseudogenes (pABC and pAP2) (Fig. 1E). Additional markers developed from this sequence were used to delimit the Sr35 candidate region to a 213 kb segment including candidate genes APGG1, CNL4, CNL6 and CNL9 (Table S1, Fig. 1E).
We sequenced these four candidate genes in a T. monococcum collection including 24 Ug99-resistant accessions carrying Sr35 and 25 susceptible accessions without Sr35. We identified two resistant (R1 and R2) and six susceptible haplotypes (S1-S6, Table S2, primers in Tables S3-S5). The two resistant haplotypes differ in a short CNL4 region with a 6-bp deletion and four single nucleotide polymorphisms (SNPs) but show no differences in APGG1, CNL6 and CNL9. All susceptible accessions have mutations in CNL9 and among them five have mutations only in CNL9, which suggests that this gene is necessary to confer resistance to Ug99. Among these five susceptible accessions, three share three close SNPs that result in amino acid changes at positions 854, 856 and 858 (RLWFT to HLRFS) in the C-terminal region of the leucine-rich repeat domain (Fig. 2A). The same three SNPs are present in the closely related CNL4 gene suggesting a conversion event.
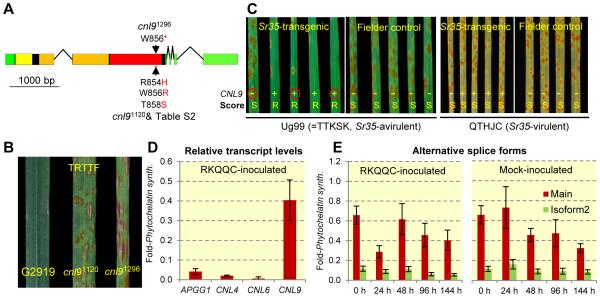
Fig. 2.
(A) CNL9 gene structure. Green=UTR, black= coding exons, yellow= coiled-coil domain, orange= nucleotide-binding domain, red= leucine-rich repeat domain, arrows= amino acid changes in susceptible induced mutants cnl91296 (W856*) and cnl91120 or natural mutants (Table S2, RLWFT to HLRFS) (B) Infection types produced on T. monococcum G2919 and CNL9 mutants cnl91120 and cnl91296 inoculated with Pgt race TRTTF. TRTTF is Sr35-avirulent and Sr21-virulent, which is required because of the presence of Sr21 in these lines. (C) Infection types on seedlings of T1 lines from event #1123 segregating for the CNL9 transgene. Plants carrying the CNL9 transgene (+) were resistant to Ug99 (R) and plants without the transgene (−) were susceptible (S) (Table S8). When inoculated with Sr35-virulent race QTHJC all plants were susceptible suggesting similar race specificity between the transgenic and the natural Sr35. Red circles indicate available progeny tests in Figure S5. (D) Relative transcript levels of candidate genes APPG1, CNL4, CNL6, and CNL9 (main isoform) in G2919 six days after inoculation with race RKQQC (E) Transcript levels of CNL9 main isoform (red) and isoform two (green, retained intron) in mock- or race RKQQC inoculated G2919 plants. Leaves were collected at 0, 24, 48, 96 and 144 h after inoculation. Transcript levels are expressed relative to the Phytochelatin synthase internal control using the 2−ΔCt method. Bars are standard errors of the means based on six biological and two technical replicates.
To validate the previous results, we mutagenized the Sr35-resistant accession G2919 with ethyl-methanesulfonate (10). Out of 1,087 M2 mutant families screened with race RKQQC we identified two mutant families segregating for susceptibility, which were validated with races Ug99 and TRTTF (Fig. 2B). Sequencing of the four candidate genes in these susceptible plants confirmed the presence of mutations only in CNL9. The first mutant (cnl91296) contained a G to A mutation that resulted in a premature stop codon at position 856 (Fig. 2A) and truncated the last 64 amino acids. In the progeny of a cross between cnl91296 and the resistant parental line G2919 (33 F2 plants), homozygocity for the mutation co-segregated with susceptibility to Ug99.
The second susceptible mutant (cnl91120) showed the same three SNPs detected in accession PI428167-2 (RLWFT to HLRFS, Table S2). To test if this was the result of seed contamination or crosspollination, we used genotyping-by-sequencing (11) to estimate the level of polymorphisms among cnl91296, cnl91120 and the non-mutagenized line G2919 (Table S6 and supplemental text). We show that cnl91120 has the level of mutations and the ratio of homozygous to heterozygous loci expected from a mutagenized plant. Therefore, a spontaneous gene conversion between CNL4 and CNL9 is the most parsimonious explanation for the three linked mutations in cnl91120. Two of these amino acid positions (856 and 858) overlap with 15 amino acids located in the C-terminal half of the leucine-rich repeat domain of CNL9 that show evidence of positive selection (Fig. S3A, S3B and Table S7). In summary, mutants cnl91296 and cnl91120 confirmed that CNL9 is necessary for the Sr35-mediated resistance and that the distal region of the leucine-rich repeat domain is critical for Sr35 function.
To determine if CNL9 is sufficient to confer resistance to Ug99, we generated transgenic hexaploid wheat plants expressing the CNL9 gene under the control of its native promoter (10). Out of four putative T0 transgenic plants only one, designated #1123, showed consistent expression of the transgene (Fig. S4) and co-segregation between the presence of the transgene and resistance to Ug99 and RKQQC in the T1 and T2 progeny (Fig. 2C, Fig. S5, Table S8). In contrast, all #1123 T1 and T2 plants were susceptible to the Sr35-virulent race QTHJC, regardless of the presence or absence of the transgene (Fig. 2C, Fig. S5). This result suggests that the CNL9 transgene has the same race specificity as Sr35. Taken together; the natural variation, mutant and transgenic results demonstrate that CNL9 is Sr35.
Using rapid amplification of cDNA ends (10) we found that the CNL9 transcripts have a 196 bp 5’UTR and a 1,526 bp 3’UTR that includes three introns (Fig. S6A). The three introns in the 3’ UTR were also detected in all the T. urartu, T. turgidum cv. durum, and T. aestivum related CNL genes for which we were able to obtain both genomic and transcript data (Table S9). Both CNL homologs from Brachypodium distachyon (Table S9) also have two introns in the 3’UTR, which indicates that this structural feature is conserved in this disease resistance cluster. Exons 3 and 4 from the B. distachyon CNL genes correspond to exons 4 and 5 from the T. monococcum CNL9 homolog.
Transcript levels of CNL9 in leaves from G2919 plants inoculated with Pgt race RKQQC (10) were 40-, 81- and 411-fold higher than those of candidate genes APGG1, CNL4, and CNL6, respectively (Fig. 2D); but not significantly different from mock-inoculated plants at different time points (Fig. 2E). Using isoform specific primers (Table S5, Fig. S6B), we found that roughly 8% of the T. monococcum CNL9 transcripts were represented by an alternative splicing variant that retained the second intron in the 3’UTR (Fig. 2E). We also detected transcripts with and without the same intron in T. turgidum (Table S9). The ratio between the two CNL9 transcript isoforms did not show changes in T. monococcum G2919 plants mock-inoculated and inoculated with Pgt race RKQQC (Fig. 2E). This suggests that the relative proportion of the two alternative splice forms is not affected by the presence of the pathogen. Previously reported alternative splicing events in CNL genes do not involve introns in the 3’ UTR (12-18), which might be a distinctive feature of this particular group of CNL genes.
Sr35 has not been reported so far in T. urartu or polyploid wheat species. To understand better the reasons for this absence we performed a comparative analysis of the T. monococcum (KC573058) and T. urartu (KC816724) colinear regions, which diverged less than one million years ago (19). The T. monococcum region encompassing genes CNL6 and CNL9 and pseudogenes pCNL5, pCNL8, pCNL10 and pABC is absent in T. urartu (Fig. 1F). Conversely, the T. urartu region including TuCNL-D and pseudogene pCNL-E is missing in T. monococcum. Large insertions and deletions have been found in other colinear intergenic regions of the T. monococcum and T. urartu genomes (19, 20). The large and repetitive genomes of wheat show higher rates of insertion and deletions than the human genome (19).
A screen of 41 T. urartu accessions and 19 wild tetraploid wheat T. turgidum ssp. dicoccoides accessions (Table S10) revealed no orthologues of TmCNL9 (Fig. S7). Gene TuCNL-H from T. urartu accession G1545 from Iran encoded the same RWT amino acids found in CNL9 at positions 854, 856 and 858, but the rest of the sequence was different and clustered with a separate set of CNL genes (Fig. S7). Since T. urartu is the donor of the A genome to the polyploid wheat species (7), it is not surprising that CNL9 homologues have not been detected in the genomic sequence of T. aestivum (http://www.wheatgenome.org/) or in the transcriptome of T. turgidum (http://wheat.pw.usda.gov/GG2/WheatTranscriptome/) (Fig. S2).
The absence of Sr35 in the tested pasta and bread wheat varieties highlights the value of wheat landraces and wild relatives as a reservoir of novel resistance specificities. It also suggests that Sr35 has the potential to improve stem rust resistance in a wide range of wheat germplasm. Our transgenic experiments also indicate that the transfer of CNL9-Sr35 to hexaploid wheat is sufficient to confer effective levels of resistance to Ug99. In contrast, some CNL genes, as for example wheat leaf rust resistance gene Lr10, require the presence of additional CNL genes to provide resistance (21).
CNL proteins mediate recognition of pathogen-derived effector molecules as well as host protein altered by the pathogen and subsequently activate host defenses. These proteins have an ancient origin and are encoded by one of the largest, most variable multigene families in plants (22). Members of this family confer resistance to a wide range of pathogens and pests. Remaining challenges are to identify which genes are responsible for resistance to a specific pathogen and to understand the signal transduction pathways involved in the plant resistance response. This information is particularly important in the case of Ug99, which now threatens the major wheat producing areas in Asia (3).
The identification of Sr35 and of Sr33 in a companion paper (4) opens the door to transgenic approaches to control this devastating pathogen. Sr35 shows a strong hypersensitive reaction to the TTKSK and TRTTF race groups when introgressed into hexaploid what, but is susceptible to some Pgt races and, therefore, should not be deployed alone. In contrast, Sr33 is resistant to all races tested so far (23, 24) but confers only moderate resistance to the Ug99 race group when introgressed alone in hexaploid wheat. Based on these complementary characteristics it might be beneficial to combine these two genes either by crossing and recombination or by transforming wheat with a cassette including both genes. The insertion of multiple resistance genes in a single locus can accelerate breeding efforts to pyramid multiple sources of resistance, which is a reasonable strategy to increase the durability of available resistance genes.
Supplementary Material
Acknowledgments
This project was supported by the National Research Initiative Competitive Grants 2011-68002-30029 (Triticeae-CAP) and 2012-67013-19401 from the USDA National Institute of Food and Agriculture, by the Borlaug Global Rust Initiative and by support to JD from the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation. We thank Jayaveeramuthu Nirmala, Sunshi Sehgal, Mariana Padilla, Shiaoman Chao, Katherine Jordan, Hyeonju Lee and Dehlia Burdan for excellent technical support, Bob Bowden, Jan Dvorak and Mike Pumphrey for providing critical materials, Ksenia Krasileva, Alina Akhunova, and Chengxia Li for valuable suggestions, and the UC-Davis and K-state Genomic facilities. Sequences have been deposited in GenBank under accession numbers KC573058, KC816724, KF113354-KF113357 and KC876115-KC876121.
Footnotes
Supplementary Materials
Materials and Methods
Figures S1-S7
Tables S1-S10
References (25-47)
Author contributions
Author contributions are listed in the Supplemental Online Materials.
References and Notes
- 1.McIntosh RA, Wellings CR, Park RF. In: Wheat rusts, an atlas of resistance genes. Jean K, editor. CSIRO; Melbourne, Australia: 1995. p. 200. [Google Scholar]
- 2.Pretorius ZA, Singh RP, Wagoire WW, Payne TS. Plant Dis. 2000;84:203. doi: 10.1094/PDIS.2000.84.2.203B. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, et al. Annu. Rev. Phytopathol. 2011;49:465. doi: 10.1146/annurev-phyto-072910-095423. [DOI] [PubMed] [Google Scholar]
- 4.Periyannan S, et al. Science. 2013 To be completed by Science. [Google Scholar]
- 5.McIntosh RA, Dyck PL, The TT, Cusick J, Milne DL. Plant Breeding. 1984;92:1. [Google Scholar]
- 6.Rouse MN, Jin Y. Plant Dis. 2011;95:941. doi: 10.1094/PDIS-04-10-0260. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak J, McGuire PE, Cassidy B. Genome. 1988;30:680. [Google Scholar]
- 8.Zhang W, et al. Crop Sci. 2010;50:2464. [Google Scholar]
- 9.Lijavetzky D, et al. Genome. 1999;42:1176. [PubMed] [Google Scholar]
- 10. SOM, Materials and methods are available as supplementary material on Science Online.
- 11.Saintenac C, Zhang D, Wang S, Akhunov E. G3 (Bethesda) 2013 doi: 10.1534/g3.113.005819. doi:10.1534/g3.113.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzo S, Jia YL. Plant Sci. 2009;177:468. [Google Scholar]
- 13.Ferrier-Cana E, et al. Theor. Appl. Genet. 2005;110:895. doi: 10.1007/s00122-004-1908-1. [DOI] [PubMed] [Google Scholar]
- 14.Tan XP, et al. BMC Plant Biol. 2007;7:56. doi: 10.1186/1471-2229-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sela H, et al. Mol. Plant. Pathol. 2012;13:276. doi: 10.1111/j.1364-3703.2011.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halterman DA, Wei FS, Wise RP. Plant Physiol. 2003;131:558. doi: 10.1104/pp.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halterman DA, Wise RP. Mol. Plant Pathol. 2006;7:167. doi: 10.1111/j.1364-3703.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 18.Gassmann W. Nuclear pre-mRNA processing in plants. 2008;326:219–233. [Google Scholar]
- 19.Dubcovsky J, Dvorak J. Science. 2007;316:1862. doi: 10.1126/science.1143986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wicker T, et al. The Plant Cell. 2003;15:1186. doi: 10.1105/tpc.011023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loutre C, et al. Plant J. 2009;60:1043. doi: 10.1111/j.1365-313X.2009.04024.x. [DOI] [PubMed] [Google Scholar]
- 22.Yue JX, Meyers BC, Chen JQ, Tian DC, Yang SH. New Phytol. 2012;193:1049. doi: 10.1111/j.1469-8137.2011.04006.x. [DOI] [PubMed] [Google Scholar]
- 23.Huerta-Espino J. University of Minnesota; 1992. [Google Scholar]
- 24.Rouse MN, Olson EL, Gill BS, Pumphrey MO, Jin Y. Crop Sci. 2011;51:2074. [Google Scholar]
- 25.Wilkinson PA, et al. BMC Bioinformatics. 2012;13:219. doi: 10.1186/1471-2105-13-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo MC, et al. Genomics. 2003;82:378. doi: 10.1016/s0888-7543(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 27.Soderlund C, Longden I, Mott R. Comput. Appl. Biosci. 1997;13:523. doi: 10.1093/bioinformatics/13.5.523. [DOI] [PubMed] [Google Scholar]
- 28.Akhunov ED, Akhunova AR, Dvorak J. Theor. Appl. Genet. 2005;111:1617. doi: 10.1007/s00122-005-0093-1. [DOI] [PubMed] [Google Scholar]
- 29.Dubcovsky J, et al. Genetics. 1996;143:983. doi: 10.1093/genetics/143.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carver TJ, et al. Bioinformatics. 2005;21:3422. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K, et al. Mol. Biol. Evol. 2011;28:2731. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu LM, Niu BF, Zhu ZW, Wu ST, Li WZ. Bioinformatics. 2012;28:3150. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langmead B, Trapnell C, Pop M, Salzberg SL. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, et al. Bioinformatics. 2009;25:2078. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosakovsky Pond SL, Frost SDW, Muse SV. Bioinformatics. 2005;21:676. doi: 10.1093/bioinformatics/bti079. [DOI] [PubMed] [Google Scholar]
- 36.Scheffler K, Martin DP, Seoighe C. Bioinformatics. 2006;22:2493. doi: 10.1093/bioinformatics/btl427. [DOI] [PubMed] [Google Scholar]
- 37.Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SDW. Mol. Biol. Evol. 2006;23:1891. doi: 10.1093/molbev/msl051. [DOI] [PubMed] [Google Scholar]
- 38.Christensen AH, Quail PH. Transgenic Res. 1996;5:213. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 39.Ayella AK, Trick HN, Wang WQ. Mol. Nutr. Food Res. 2007;51:1518. doi: 10.1002/mnfr.200700233. [DOI] [PubMed] [Google Scholar]
- 40.Klosterman SJ. Methods Mol. Biol. 2012;835:121. doi: 10.1007/978-1-61779-501-5_8. [DOI] [PubMed] [Google Scholar]
- 41.Long XY, et al. Plant Mol. Biol. 2010;74:307. doi: 10.1007/s11103-010-9666-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen A, Dubcovsky J. PLOS Genet. 2012;8:e1003134. doi: 10.1371/journal.pgen.1003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawat N, et al. BMC Plant Biol. 2012;12:205. doi: 10.1186/1471-2229-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilian B, et al. Mol Biol Evol. 2007;24:2657. doi: 10.1093/molbev/msm192. [DOI] [PubMed] [Google Scholar]
- 45.Takken FLW, Goverse A. Curr. Opin. Plant Biol. 2012;15:375. doi: 10.1016/j.pbi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Kelley LA, Sternberg MJE. Nat. Protoc. 2009;4:363. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 47.Stakman EC, Steward DM, Loegering WQ. Identification of physiologic races of Puccinia graminis var. tritici. 1962 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.