Abstract
Functional magnetic resonance imaging (fMRI) has had an essential role in furthering our understanding of brain physiology and function. fMRI techniques are nowadays widely applied in neuroscience research, as well as in translational and clinical studies. The use of animal models in fMRI studies has been fundamental in helping elucidate the mechanisms of cerebral blood flow regulation, and in the exploration of basic neuroscience questions, such as the mechanisms of perception, behavior, and cognition. Because animals are inherently noncompliant, most fMRI performed to date have required the use of anesthesia, which interferes with brain function and compromises interpretability and applicability of results to our understanding of human brain function. An alternative approach that eliminates the need for anesthesia involves training the animal to tolerate physical restraint during the data acquisition. In the present work we review these two different approaches to obtaining fMRI data from animal models, with a specific focus on the acquisition of longitudinal data from the same subjects.
Keywords: anesthesia, awake, brain, BOLD, cerebral blood flow, cerebral blood volume, neurovascular coupling, non-human primates, rodents, songbirds
1. Introduction
Functional magnetic resonance imaging (fMRI) has made a remarkable impact on brain research, establishing itself as the most prominent research tool in cognitive neuroscience (1;2) and showing great promise in translational and clinical studies (3). fMRI relies on the neurovascular coupling, a tight relationship between changes in neural activity and local regulation of cerebral blood flow (CBF), volume (CBV) and oxygen consumption (CMRO2) (4). Functional MRI provides excellent contrast to noise ratio, sub-millimeter spatial resolution, coverage of the whole brain, and relative ease of implementation. On the other hand, the temporal resolution of fMRI is relatively low (particularly with respect to the time scale of neuronal events) and the underlying fMRI signal mechanism (5) and its functional specificity (6) are still a subject of research.
The use of animal models has been essential to the development of fMRI techniques. Rodent models were the subjects in the first studies employing the blood oxygenation level dependent (BOLD) contrast (7;8), as well as the first CBF measurements using exogenous MRI tracers, including deuterium (9;10), fluorine (11;12) and gadolinium chelates (13;14), which also allowed estimation of CBV in addition to CBF. The advantages of using an endogenous tracer led to the development of arterial spin labeling (ASL) techniques to measure and quantify CBF non-invasively, with the first demonstrations once again occurring in rodents (15;16). Animal models of functional brain activation have been widely employed to address issues related to spatial localization of the functional signals, the magnitude of signal changes as a function of stimulation parameters, as well as temporal aspects of the hemodynamic response, giving critical insight into the brain’s physiology and function (17). Furthermore, the use of animal models in fMRI has been particularly advantageous in preclinical and translational studies of various models of brain disease.
A major practical issue related to performing fMRI in animals relates to compliance. The MRI environment poses stringent restrictions on subject positioning and movement, and typical fMRI studies last from a few to several hours. Notwithstanding the duration of the experiments, because animals are inherently noncompliant, most fMRI performed to date have required the use of anesthesia, which offers the important advantages of ensuring compliance, minimizing movement, and alleviating stress, at the expense of requiring the investigator to monitor and control the systemic physiological status of the animal. Another major disadvantage of the use of anesthesia is that it interferes with both neural activity and neurovascular coupling, thus compromising interpretability and applicability of results to the understanding of human brain function. An alternative choice to the use of anesthesia is to acclimate, condition and train the animal to tolerate physical restraint during the data acquisition. This approach offers the advantage of minimizing the need for physiological monitoring and maintenance. On the other hand, it is difficult to dynamically monitor the level of stress in awake animals and virtually impossible to establish its absence, even with extensive training. Stress can be a significant confound in the study of brain function, particularly when hemodynamic variables are used as surrogate markers of neural activity. Indeed, continued research on training protocols and evaluation of stress indicators is a subject of intense research (18). In the present work we review these two different approaches to obtaining fMRI data from animal models, with a specific focus on the acquisition of longitudinal data from the same subjects.
2. fMRI of Anesthetized Animal Models: From Terminal to Longitudinal Preparations
2.1. Anesthetics for Terminal Experiments
Urethane (19–23) and α-chloralose (24–36) are the most widely anesthetics used in fMRI studies of rodents. A major advantage of either anesthetic is that they preserve the neurovascular coupling (37). However, both substances are toxic and thus not adequate for longitudinal use, being used only in terminal preparations (38;39).
The use of anesthesia makes the animal lose the ability to regulate its own physiology and body temperature, forcing the investigator to monitor and maintain the vital signs of the animal to ensure stable physiology throughout the entire experiment. Therefore, surgical preparation is required. This consists of: (a) induction of anesthesia; (b) oral intubation or tracheostomy; and (c) placement of intravenous, intra-arterial and intraperitoneal catheters. Figure 1 shows a typical surgical preparation station and all associated equipment.
Fig. 1.
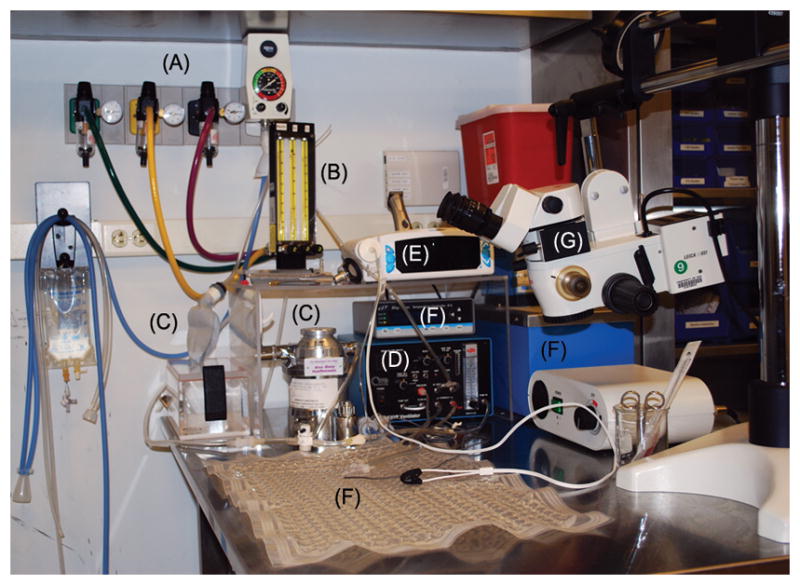
Typical surgical station for preparation of anesthetized animals. (A) Medical gases and vacuum scavenger; (B) Gas flowmeters and mixer; (C) Isoflurane vaporizer and compliance balloon; (D) Small animal ventilator; (E) Pulse oximeter/capnograph; (F) Temperature regulator, heated water bath and water mat; (G) Surgical microscope.
Fifteen to thirty minutes prior to induction of anesthesia it is useful to treat the animals with atropine sulfate (Vedco Inc, Saint Joseph, MO), given subcutaneously or intramuscularly at a single dose of 0.5 mg/kg, to decrease bronchial secretions and salivation during anesthesia, and as an anesthesia adjuvant. Anesthesia induction in rodents usually uses halogenated anesthetics (halothane, sevoflurane, and isoflurane). In contrast, intramuscular bolus of ketamine (either alone, or in combination with its potentiators xylazine, acepromazine or medetomidine hydrochloride) is frequently used for induction in both cats and non-human primates. Following the anesthesia induction, the animals are typically orally intubated or tracheostomized and mechanically ventilated to allow dynamic adjustments of respiratory parameters as to ensure stable physiology - under the effect of the anesthetic - throughout the experiment. It is extremely important to gain monitoring and control of the animals vital signs at this moment. The body temperature must be monitored via a rectal temperature probe, and a heated water pad or another suitable source of heat must be used to keep the body temperature at normal values (e.g., 37.5 ± 0.5 °C for rats; 38.5 ± 0.5 °C for cats and marmosets). Pulse oximetry and capnography, when available, can be started at this moment. Either technique alone or combined together provides a good assessment of the quality of the animal’s respiration, giving the investigator the necessary information to adjust the breathing gas mixture and the ventilator parameters to ensure good oxygenation and proper ventilation rate and volume to the animal. Following the start of mechanical ventilation, catheters are placed into the femoral artery, the femoral vein (or the lateral tail vein) and the intraperitoneal cavity. These catheters will allow the administration of anesthesia and other drugs that control the physiological status of the animal, the measurement of arterial blood pressure via a pressure sensor, and the sampling of arterial blood gases.
Once all surgical procedures are completed and all open wounds are sutured, the inhalational anesthetic can be discontinued and the anesthesia can be switched to either α-chloralose (α-chloralose, product #C8091, Sigma-Aldrich, St. Louis, MO) or urethane (urethane, product #U2500, Sigma-Aldrich). Typical dosage for α-chloralose is 80 mg/kg initial IV bolus (37) followed by 27 mg/kg·hr constant IV infusion (33). For urethane, the typical dosage is 1.25 g/kg single dose IP (23), as urethane is known to be a long-acting (8–10 hours) anesthetic. Both α-chloralose and urethane can be dissolved in slightly warmed phosphate-buffered saline (PBS) at a concentration of 10 mg/ml or 100 mg/ml, respectively. However, urethane is a known carcinogen and should only be manipulated in the hood using gloves. A muscular relaxant such as pancuronium bromide (Pavulon, 2 mg/ml, Teva Pharmaceuticals USA, North Wales, PA) may be periodically administered at a dose of 2 mg/kg/hr IV or IP to aid with immobilization.
2.2. Anesthetics for Longitudinal Experiments
Due to the extensive surgical preparation described above and also to the toxicity and adverse side effects of both α-chloralose and urethane, different anesthetics need to be used for longitudinal studies. These studies require minimal interventions on the animal so that its overall physiological state is stable throughout the duration of the study and especially each time the animal is tested. Because of this, inhalational compounds such as halothane (30) and isoflurane (40–48) are attractive in that they can be administered via a face mask to the animal, thus avoiding the need for catheterizations for vascular access. Furthermore, these agents provide stable physiology and allow for an easy control of the plane of anesthesia and a quick and smooth recovery of the animal upon withdrawal of the anesthetic. However, while both anesthetics are safe to use in repetitive studies in the same animal, they suppress neuronal activity (49) and cerebral metabolism (50) and affect both cerebrovascular tone as well as cerebrovascular reactivity (51), thus greatly influencing the cerebrovascular coupling (52) and making it necessary to redefine the proper stimulus parameters to produce robust activation (46). In fact, the influence of halogenated inhalational anesthetics on neurovascular coupling and cerebrovascular regulation can be long lasting. A recent study on the long term effects of a short hypoxic episode on CBF regulation in isoflurane anesthetized rats showed a dramatic reduction in the CBF response to hypoxia in animals that were exposed to isoflurane 5 days earlier (53).
As an alternative to inhalational anesthetics, injectable agents such as propofol and medetomidine are attractive in providing a satisfactory depth of anesthesia, a quick onset of action and a smooth recovery of the animals. Propofol is an injectable anesthetic with rapid mechanism of action that is increasingly used in fMRI experiments in animal models (40;54–57). The depth of anesthesia under propofol can be readily adjusted by varying the rate of infusion, and the animals quickly recover at the end of the experiment, thus facilitating longitudinal studies. Another agent that has been recently proposed as suitable for repetitive studies is the α2-adrenoreceptor agonist medetomidine hydrochloride (22;58–61), which has been shown to allow robust fMRI responses (58;59) and the measurement of resting state signal fluctuations in the brain (59–61). The sedative and analgesic effects of medetomidine can be quickly reversed with the application of atipamezole hydrochloride. However, some limitations of prolonged exposures to medetomidine include a gradual rise in blood pressure and heart rate, lower pO2 values, and a more difficult control of the plane of anesthesia (61).
When using injectable, intravenous anesthetics, special attention must be given to the total infusion volume in comparison with the total blood volume, especially in small animals such as rodents and small non-human primates, so that the infusion does not change blood chemistry. For example, the dose of propofol (10 mg/ml) required to maintain immobilization in rats and marmosets is 0.5 – 1.0 mg/kg/min (3 – 6 ml/kg/hr), which is about five times higher than required in humans (0.05 – 0.2 mg/kg/min). Yet the total blood volume of a 400g rat is ~25 ml (62), circa 1/200 of the total human blood volume. Thus, intravenous anesthetics have a much greater potential to disturb blood chemistry in small animals than in humans, and drug preparations should be chosen carefully. Propofol, as made by certain manufacturers, has a pH value as low as 4, posing a significant risk of inducing acidosis in small animals, especially in prolonged and/or longitudinal experiments. Like in clinical medicine, concurrent intravenous infusion of Lactated Ringer solution with the anesthetics may partially alleviate the detrimental effects of blood chemical disturbance.
2.3. Placement of the Animal in an MRI Compatible Bed
Magnetic resonance imaging poses stringent requirements on immobilization. After surgery, the animals are thus placed in a stereotaxic-like head holder and strapped to an MRI compatible bed. A number of beds are available from the major vendors of small animal MRI scanners, such as Bruker (Bruker-Biospin, Corp., Ettlingen, Germany) or Varian (Varian, Inc., Palo Alto, CA), or from third-party vendors (e.g., Rapid Biomedical, GmbH, Rimpar, Germany; Ekam Imaging, Inc., Shrewsbury, MA). Figure 2 shows a picture of the bed we use for fMRI of small animals. The bed is an essential part of the experiment, as it integrates, in a single platform, a resting place for the animal, the stereotaxic head holder, the physiological maintenance and monitoring devices, a stage for receive RF coils and preamplifiers, and another stage for the functional stimulation devices. Therefore, significant effort needs to be put on the design, fabrication and adaptation of an animal bed to include and integrate all the features necessary to the successful execution of the experiment.
Fig. 2.

(A) MRI-compatible animal bed, containing a stereotaxic head holder with ear pieces and a bite bar (B), to which the head of the animal is secured. Mechanical ventilation is provided by the gas lines. (C) Pulse oximetry sensor and rectal temperature probe. (D) Heated water mat (shown unfolded), which can be wrapped around the animal’s body to maintain temperature. (E) Multi-channel receive RF coils. (F) Multi-channel RF preamplifiers.
2.4. Physiological Monitoring
As mentioned above, the use of anesthesia makes the animal lose the ability to regulate its own physiology and body temperature, and it is the responsibility of the investigator to monitor and maintain the vital signs of the animal throughout the remainder of the experiment. As soon as the animal has been moved to the MRI-compatible bed, physiological monitoring must start – or resume if started during animal preparation – according to the available equipment. All essential physiological parameters, including, but not limited to the list below, should be monitored and maintained at normal values.
Rectal temperature: via temperature probe inserted in rectum. This is one of the most critical parameters to be monitored and maintained, as anesthesia impedes the animal’s own temperature regulation. Normal rectal temperature values for rats are 37.5 ± 0.5 °C, and 38.5 ± 0.5°C for cats and marmosets.
Mean arterial blood pressure (MABP): via pressure transducer connected to a femoral arterial line, if available, or via a pressure cuff wrapped around the tail or thigh. Typical MABP values in rats are 110–120 mmHg (63;64). Typical MABP values in conscious marmosets are 100–110 mmHg (65).
Pulse oximetry (SPO2): via a transducer placed on forelimb or hindlimb. Usually SPO2 values remain above 90 %.
Heart Rate (HR): derived either from MABP trace or from a pulse oximeter. For rats under α-chloralose, HR typically rises above 300 BPM, but stays below that value under other anesthetics. For marmosets under propofol, HR typically decreases from above 350 BPM in the conscious, awake condition to 150–250 BPM in anesthetized animals.
End-tidal CO2 (ETCO2): via a micro-capnometer hooked up to a face mask or to the respiratory line. The distance between the mechanical ventilator and the MRI magnet forces the use of long respiration lines, resulting in substantial mixing between expired and recirculated gas. Therefore, and especially for small animals, the micro-capnometer tends to display significantly attenuated values of ETCO2. Yet, these values are valid relatives and a significant correlation exists between ETCO2 and PaCO2, as shown in Fig. 3.
Respiratory pressure: monitored via a pressure transducer in mechanical ventilator. It is important to check the respiratory compliance of the intubated or tracheostomized animal throughout the experiment. The end-inspiratory pressure can be set by adjusting the flow of the air mixture in the ventilator. Typically, end-inspiratory pressures are in the range of 8 – 12 cm H2O.
Arterial blood gases, including pH, PaCO2 and PaO2: sampled from the femoral artery, when available. Even though arterial blood sampling in mice is restricted to much fewer samples than in rats, the latest-generation blood gas analyzers are able to work with samples as small as 30 μl of blood (typically 60 μl) (e.g., ABL80 FLEX, Radiometer America, Westlake, OH). Periodic sampling of arterial blood is also performed to yield information on arterial blood gases, hematocrit, and electrolytes. Data collected during unstable physiology is best discarded. Furthermore, large deviations in the arterial blood gases often call for administration of corrective pharmacological agents.
Fig. 3.

Pooled data plot of ETCO2 as measured with a capnograph versus PaCO2 sampled from arterial blood (n= 34 rats, average of 6 points per animal) in normocapnia and hypercapnia. The dashed line is the line of identity, and the correlation coefficient between ETCO2 and PaCO2 is 0.77 (r2 = 0.59). Even though the absolute value of ETCO2 is influenced by the size of the animal relative to the total flow and volume of air in the ventilator, the length of the gas lines, and the flow of expired air into the capnograph, the significant correlation between ETCO2 and PaCO2 allows ETCO2 values to be used as a relative index of changes in PaCO2.
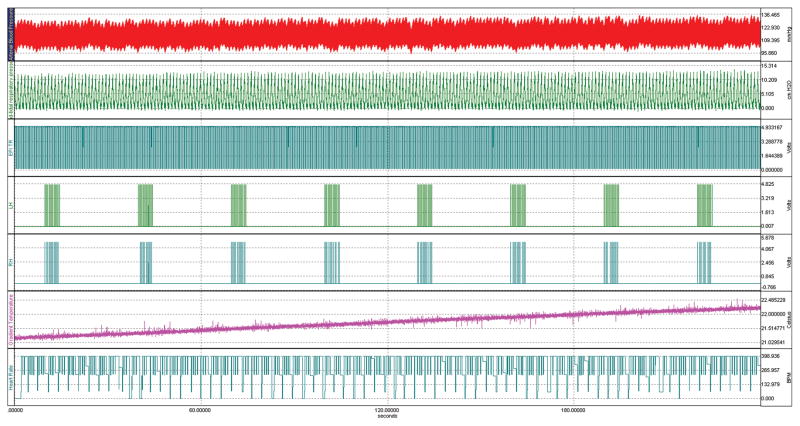
All the physiological parameters listed above can be recorded during the experiment using a multi-channel data acquisition system, such as the system MP150 (Biopac Systems, Inc., Goleta, CA). Figure 4 shows the physiological monitoring graph of a typical fMRI experiment to measure the BOLD and CBF response to electrical stimulation of the forepaws in a rat anesthetized with α-chloralose. Traces of the arterial blood pressure, respiratory pressure, and heart rate were recorded along with the forepaw stimulation epochs, the EPI acquisition times, and the gradient temperature. Monitoring of the animal’s physiology during the experiment assures that the data is acquired under stable conditions. For example, no changes in arterial blood pressure are noticed during the forepaw stimulation epochs. Another advantage of acquisition of physiological parameters is the possibility to perform retrospective correction of the influence of either the respiratory or cardiac cycles on the fMRI signal (66).
Fig. 4.
Physiological and fMRI data acquisition traces recorded during a typical fMRI experiment of electrical stimulation of both forepaws of a rat anesthetized with α-chloralose. The experiment lasted 4 minutes. From top to bottom, the traces show the arterial blood pressure (red), respiratory pressure (green), EPI acquisition tics (blue), stimulation of the left (green) and the right (blue) forepaws, the MRI gradient temperature (magenta) and the heart rate derived from the ABP trace (blue).
2.5. Recovery from Anesthesia
In longitudinal experiments in which artificial ventilation is used, the animal’s autonomous control of the respiration may be suppressed even after anesthetics are withdrawn. Thus, recovery from anesthesia needs to be closely monitored, and emergency procedures should be planned in advance. Throughout the recovery, artificial ventilation and rectal temperature monitoring should continue, and the intravenous infusion site should remain patent, until the animal is clearly able to breathe on his own after being disconnected from the breathing circuit. Antidotes for some anesthetics are available, such as naloxone for opioids and atipamezole for medetomidine chloride, and should be administered. In the event of respiratory and/or cardiovascular suspension after removal of the intubation tube, a respiratory stimulant (e.g. Doxapram) and sympathetic stimulant (e.g. Epinephrine) can be administered through the intravenous infusion tube, which should not be removed from the animal until she is fully alert.
3. fMRI of Conscious, Awake Animals
The use of anesthesia for MRI and fMRI studies in animal models has the advantages of effectively ensuring compliance and of minimizing stress via sedation. However, any anesthetic interferes, in different ways, with neural activity and cerebrovascular reactivity, representing a complex confound to the interpretability and applicability of the obtained data to the understanding of human brain function. The alternative to the use of anesthesia as a means to ensure compliance is to condition the conscious, awake animal, to tolerate the rigid head restraint required to ensure the acquisition of good quality data with acceptable levels of motion artifacts. The use of conscious, awake animals in the MRI setting is becoming increasingly popular, as exemplified by studies performed in rodents (18;29;42;55;67–71) and monkeys (71–84). To provide effective, yet relaxed restraint to the animals, the animal bed needs to be designed taking into account comfortable support for their head and body (77;85). Equally important is the development of acclimatization and training procedures to condition the animal to tolerate long periods of restraint with minimal stress (18), as a stressed out animal is as useful to data collection as an unresponsive one!
In this section, we describe our own experience with obtaining longitudinal fMRI data from conscious, awake marmosets in a 7T horizontal MRI scanner. Traditionally, the use of awake, conscious animals in the MRI requires the surgical implantation of head posts that can be rigidly secured by clamps to a specially designed frame (85). This approach allows maximum restraint of the animals, but has many disadvantages. The head implants typically generate susceptibility artifacts that degrade image quality by introducing geometric distortions and/or signal dropouts in the MR images. In addition, they require constant aseptic cleaning to prevent infections. Furthermore, if an infection appears, the animal needs to be treated with antibiotics and anti-inflammatory drugs that may interfere with neurovascular coupling and confound interpretation of the data. Moreover, the use of a head implant significantly detracts from a major advantage of MRI as a non-invasive technique. An alternative to the use of head implants is to secure the animal to a MRI-compatible stereotaxic head holder by means of ear pieces and a bite bar. To do this, the animals need to be sedated with a short-acting anesthetic, such as medetomidine, so that they can be attached to the stereotaxic head holder, after which they are allowed to wake up by reversal of the anesthesia (e.g., with atipamezole) (18;42;70;80). While it has been shown that animals can be successfully conditioned to not fight the head restraint upon regaining consciousness (18), this approach has the disadvantages of requiring the use of anesthesia and of utilizing a head holder that may potentially hurt the animal due to the presence of the ear bars. Our approach to restrain the head of the animals is different than either of the two above. We opted to eliminate the need for implanting head posts or to administer any anesthetics or sedatives altogether by acclimatizing the animals to be restrained by a custom-fit helmet that was specifically designed to match the contour of each individual head exactly, providing a comfortable, yet effective restraint.
3.1. Design of the Animal Bed and Restraint Device
As shown in Figure 5, the bed for fMRI of awake marmosets consists of a cylindrical tube of inner diameter 111 mm, made out of fiberglass impregnated with an epoxy resin (NEMA grade G-10, FPI Industries, Arnold, PA), cut in half length-wise, in which the marmoset lays in the prone, sphinx position. Two lateral support bars made out of Delrin are attached to either inner side of the bed to support the helmet and cover pieces. The bed attaches to the sliding mechanism on one end via the hanger (Figure 5), so as to float cantilevered inside the magnet without touching the gradients or the transmit RF coil. Prior to bringing the marmoset to lie in the cradle, a sleeveless jacket (Lomir Biomedical, Inc. Malone, NY) is placed on the marmoset. Next, a plastic semicylindrical cover made of Lexan is attached to the back of the marmoset’s jacket using plastic cable ties. The marmoset is then gently placed into the cradle and the cover is secured by screwing attached nylon thumb screws into the bars on the cradle. The animal is now loosely but effectively restrained from sliding out of the cradle anteriorly or posteriorly. The arms, legs and tail of the animal are free to move unimpededly. Additionally, the semi-cylindrical cover is a few centimeters from the marmoset’s back, allowing the animal to stretch and to adjust her body position as needed for comfort, as shown in Figure 5.
Fig 5.

Illustration of a restrained marmoset in the MRI-compatible bed. The body of the animal is loosely attached to a back cover via zip ties secured to a sleeveless jacket worn by the animal. The back cover is screwed to the side bars on the cradle, while the arms, legs and tail of the animal are free to move as she wishes. The head of the marmoset is secured to a two-piece, custom-built helmet made specifically for that individual alone. The chin piece on the bottom supports the chin of the animal, and the head piece on the top prevents head motion. Note that the helmet pieces are lined up with foam on the inside to provide a comfortable support to the entire head. The animal sits in the sphinx position looking out towards the back of the magnet. The bed is secured to the bed sliding mechanism on one end via the hanger.
Because the shape and size of marmosets’ skulls vary, individual custom-built helmets are designed for each animal. For this, a 3D spin-echo MRI of the entire head and neck is acquired from each animal. Next, a 3D surface rendering algorithm is applied to obtain the contour of the head, which is then fed into Rhinoceros 3.0(McNeel North America, Seattle, WA), a 3D modeling program, to design the top (head) and bottom (chin) helmet pieces. After the head and chin helmet pieces are designed (see Figure 6), they are sent to a 3D printer (ProJet HD3000, 3D Systems Corp., Rock Hill, SC), which builds the helmets, layer by layer, from liquid ABS plastic that hardens during the manufacturing process. After the helmet printing is complete, 3mm thick foam is glued onto the inside surface of both top and bottom pieces to provide greater comfort for the animal.
Fig 6.
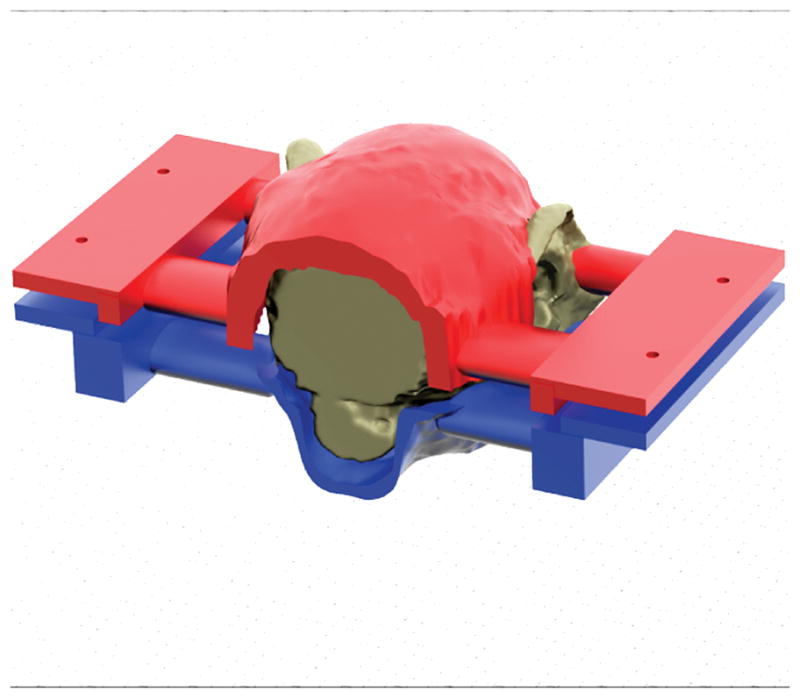
Detail of the construction of the custom-fit helmet. Based on a 3D MRI of the entire head of the marmoset, a 3D model of the helmet is created consisting of two pieces: the chin piece (blue) to support the chin of the animal, and the head piece (red) that supports the head and prevents motion. Once the model is created it is sent to a 3D plastic printer that creates the helmet.
Proper and perfect fitting of the pieces to each animal is guaranteed by design. Since the shape of each animal is used to produce a helmet manufactured specifically for its head, we can ensure that the animal is comfortable yet immobilized in. After the animal is lowered into the cradle and secured by the cover piece, the helmet top and bottom pieces are carefully placed around the animal’s head and screwed into the bars.
3.2. Acclimatization of the Animal to Body and Head Restraint
Once the individualized helmet pieces are built, the animal is needs to be acclimatized to the bed and to tolerate physical restraint during the MRI sessions. This acclimatization procedure consists of three phases, as illustrated in Figure 7:
Fig. 7.
Illustration of the three phases of training. In Phase 1 (top), the body of the animal is loosely attached to the MRI bed, and the animal is conditioned to staying in the bed for increasing periods of time. In phase 2 (middle), reinforcement of the training in phase continues while the animal gets used to MRI sounds. In phase 3 (bottom), the individualized helmet is introduced to restrain the head of the animal.
-
Phase 1, Acclimatization of Awake Marmosets to Being Contained in the Bed. In this phase, the animals are dressed with the jackets, attached to the back cover by zip ties and lowered into the bed. The cover is secured to the bed by nylon thumb screws. The bed is then inserted into a mock MRI tube and the animal is observed from a distance via a webcam. For phase 1, training begins with 15 minutes on day 1, and progresses to an hour by day 4 (see Table 1). As a reward, 3–6 cc of infant milk formula (Pediasure) and 3–5 mini-marshmallows are given at the beginning and end of each training session.
Prior to, during and after each of the acclimatization phases, a behavioral assessment takes place to provide a measure of the tolerance of the animals to the acclimatization procedures. The scoring of each animal is performed using the Behavioral Assessment Scale shown in Table 2 (86). It is our experience that all animals successfully complete the training in phase 1, starting with an average score of 4 on the Behavioral Assessment Scale and acclimatizing to an average score of 2 by the end of phase 1.
Phase 2: Acclimatization of Awake Marmosets to Being Contained in the Bed in the Presence of MRI Sounds. In spite of being entirely non-invasive, MRI is, unfortunately, a loud technique. Therefore, it is necessary that the animals get properly acclimatized to the sounds generated by the MRI scanner during imaging. In phase 2, the animals are restrained as in phase 1 for increasing periods (Table 1). While in the mock MRI tube, they are allowed to hear the sounds produced by the MRI scanner, played out at a softer level of loudness than in a real MRI session. This schedule reinforces the adaptation to the body restraint initiated in phase 1, and conditions the animals to ignore the MRI sounds produced by the scanner. As in phase 1, Pediasure and mini-marshmallows are used as rewards at the beginning and at the end of each training session. The same Assessment Scale for Behavioral Responses (Table 2) is used to assess the response of the marmosets to the restraint device in the presence of MRI sounds. It has been our experience that all animals successfully complete this phase of the training, starting again with an average score between 3 or 4 on the first day and moving down to an average score of 2 or better by the end of phase 2. At this point, if an animal proves difficult to train by systematically scoring above the mean score, the investigator may choose to adapt the exact timeline of the acclimatization procedures to the idiosyncrasies of that individual, or may choose to drop the animal out of the study.
Phase 3, Acclimatization of Awake Marmosets to Head Restraint in the Presence of MRI Sounds. In phase 3, marmosets are restrained to the bed by the body cover, as in the previous phases, and then fitted with their custom-built helmets, which are attached to the MRI bed as shown in Figs. 5–7. The use of the helmet effectively – yet comfortably – restrains head motion. The ears will be fitted with earplugs made of silicon jelly (Insta-Putty, Insta-Mold Products, Inc., Oaks, PA), which are pressed gently into the ear canals by foam pads from both sides of the head. This further restrains head motion and protects the animals from the MRI scanner’s noise. In phase 3, the animals are conditioned for increasing periods, as in previous phases (Table 1). While in the mock MRI tube, they allowed to hear the sounds produced by the MRI scanner, played out at the same level of loudness as in a real MRI session (Fig. 7). This schedule reinforces the adaptation to the body restraint of the previous phases, and further conditions the animals to ignore the MRI sounds produced by the scanner, while enforcing full head fixation. As in previous phases, the animals’ response to training will be evaluated by the Behavioral Response Scale shown in Table 2. It has been our experience that all animals are able to successfully complete this last phase of the training, starting again with an average score between 3 or 4 on the first day and moving down to an average score of 2 or better by the end of phase 3.
Table 1.
Three-Week Acclimatization Schedule
| Phase | Monday | Wed | Friday | Sunday | Procedures |
|---|---|---|---|---|---|
| 1 | 0:15 | 0:30 | 0:45 | 1:00 | Jacket |
| 2 | 1:00 | 1:20 | 1:40 | 2:00 | Jacket + MRI sounds |
| 3 | 1:00 | 1:20 | 1:40 | 2:00 | Jacket + MRI sounds + helmet |
Table 2.
Behavioral Assessment Scale
| Score | Behavior |
|---|---|
| 1 | Quiet: marmoset calm and relaxed |
| 2 | Mostly quiet, agitated only initially |
| 3 | Mostly quiet, with brief, intermittent mild agitation |
| 4 | Quiet after initial struggle, increasingly agitated over time |
| 5 | Mild agitation for about half of the restraint period |
| 6 | Moderate agitation during half of the restraint period |
| 7 | Restless and agitated during most of the restraint period |
| 8 | Extremely agitated during most of the restraint period |
Out of 14 marmosets trained to date, we only dropped one animal out of the study due to increased agitation during phase 2 of the acclimatization. After the animals have successfully completed the three week training program, they proceed to undergoing the actual MRI studies. Continued exposure to the MRI scanner in actual studies fully consolidates the acclimatization, and all animals are tolerant of all procedures after a couple of MRI sessions. Overall, this new method of restraint is completely non-invasive, comfortable for the animals and of great scientific payoff in eliminating the need to either perform surgery for installation of head-post implants, or to use sedatives to restrain the animals, allowing any marmoset to be acclimatized to restraint and utilized indefinitely in longitudinal experiments.
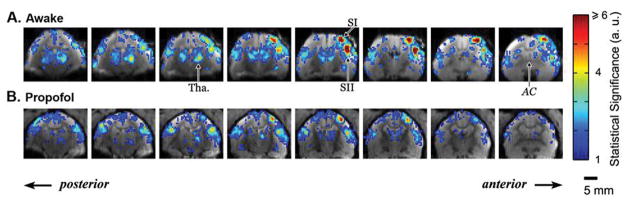
The advantages of using awake subjects can be fully appreciated by the high amplitudes and reproducibility of fMRI responses to somatosensory stimuli, as shown in Figure 8. In awake marmosets (Fig. 8A), electrical stimulation of one arm by repeated pulses (1.5 mA, 0.4 ms duration, 1–125 Hz) evoked fMRI responses in a series of brain regions including thalamus, caudate, putamen, primary (SI) and secondary (SII) somatosensory cortices. Responses were significant in contralateral thalamus, SI and SII, as well as in ipsilateral thalamus and SII. While the fMRI responses were much stronger in the contralateral side, occasionally robust ipsilateral responses were also detected in SI. In marmosets anesthetized by propofol (Fig. 8B), however, both the spatial extent as well as the fMRI amplitudes in responsive regions were significantly smaller compared to awake subjects. In particular, responses in ipsilateral thalamus and ipsilateral SI were insignificant in many anesthetized sessions, consistent with previous studies using anesthetized rats. Responses in caudate and putamen were also much weaker, although detectable. Furthermore, the response amplitudes in thalamus and cortical areas in anesthetized subjects were less than half the amplitudes in awake subjects.
Fig. 8.
Map of fMRI response evoked by electrical stimulation of the left arm of the marmoset. Eight contiguous coronal slices immediately posterior to the anterior commissure (AC) are shown in a representative session under awake (A) and propofol anesthesia (B). Robust BOLD responses can be detected in the thalamus (Tha.) and in the primary (SI) and secondary (SII) somatosensory cortex, although the responses are much more significant in the awake than in anesthetized animals.
Figure 9 shows the fMRI response obtained from the same animal in different MRI sessions in a time span of 10 months. Excellent reproducibility of the amplitude and spatial and temporal characteristics of the BOLD response were obtained (see Table 3), demonstrating that longitudinal fMRI studies can be successfully carried out in awake, behaving animals.
Fig. 9.
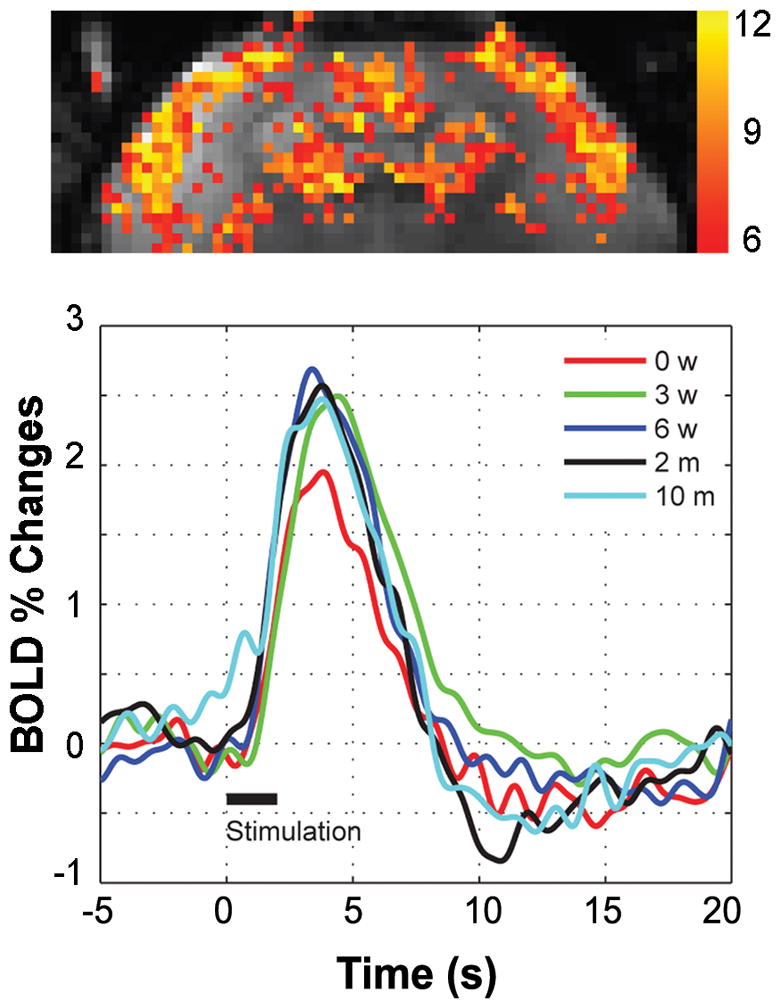
fMRI response obtained from the same animal in different MRI sessions in a time span of 10 months. (A) T-map demonstrating the main active areas of the brain, including SI, SII, and caudate putamen. (B)BOLD time-course in response to a 2 s electrical stimulus of both hands (2 mA, 0.3 ms, 64 Hz), obtained at five different times (0 weeks, 3 weeks, 6 weeks, 2 months and 10 months) post-acclimatization. Excellent reproducibility of the amplitude and temporal characteristics of the BOLD response is achieved.
Table 3.
The amplitude % signal changes, standard deviation (SD) of baseline, time-to-peak (TTP), and full width at half maximum (FWHM) of the BOLD response from a representative marmoset undergoing longitudinal fMRI (2 s stimulus duration, 2 mA, 0.3 ms pulse width, 64 Hz) of the somatosensory cortex.
| First scan | 3 weeks | 6 weeks | 2 months | 10 months | Mean ± SD | |
|---|---|---|---|---|---|---|
| Signal increase (%) | 1.95 | 2.50 | 2.69 | 2.57 | 2.47 | 2.44 ± 0.28 |
| SD of baseline (%) | 0.08 | 0.14 | 0.17 | 0.17 | 0.21 | 0.15 ± 0.08 |
| TTP (s) | 3.84 | 4.41 | 3.41 | 3.80 | 3.78 | 3.85 ± 0.36 |
| FWHM (s) | 3.92 | 4.70 | 4.34 | 4.14 | 4.55 | 4.33 ± 0.31 |
4. Conclusions
In the present chapter, we presented and reviewed different methodological approaches to obtaining fMRI data from animal models in longitudinal studies. When opting for which approach to follow, the reader is confronted with a balance of advantages and disadvantages that may not always point to the same choice, depending on the purpose of the study.
The use of anesthesia has the advantages of ensuring compliance, minimizing movement, and alleviating stress in an effect way. However, anesthesia suppresses many homeostatic pathways and requires the investigator to monitor and control the physiology of the animal. Another major disadvantage of the use of anesthesia is that it interferes with neural activity and neurovascular coupling, severely restricting the choice of the functional paradigm and compromising the interpretability and applicability of the data to the understanding of human brain function.
An alternative choice to the use of anesthesia is to acclimate, condition and train the animal to tolerate physical restraint during the data acquisition. This approach offers the advantage of minimizing the need for physiological monitoring and maintenance, but must be done in a well-devised and gradual manner so as to eliminate, as much as possible, stress of the animal to the forced restraint. Stress can introduce as much confound to the data as anesthesia, and thus the task of acclimatizing animals to awake studies cannot be taken lightly. Nevertheless, when performed consistently and correctly, acclimatization can be very effective in conditioning the animal to participate cooperatively in the study, producing results that, in principle, are experimentally as close to mimicking the setup in human studies as possible.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, NINDS (Alan P. Koretsky, Scientific Director).
References
- 1.Poldrack RA. The role of fMRI in cognitive neuroscience: where do we stand? Curr Opin Neurobiol. 2008;18:223–227. doi: 10.1016/j.conb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Dolan RJ. Neuroimaging of cognition: past, present, and future. Neuron. 2008;60:496–502. doi: 10.1016/j.neuron.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 4.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 5.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 9.Kim SG, Ackerman JJ. Quantification of regional blood flow by monitoring of exogenous tracer via nuclear magnetic resonance spectroscopy. Magn Reson Med. 1990;14:266–282. doi: 10.1002/mrm.1910140212. [DOI] [PubMed] [Google Scholar]
- 10.Detre JA, Eskey CJ, Koretsky AP. Measurement of cerebral blood flow in rat brain by 19F-NMR detection of trifluoromethane washout. Magn Reson Med. 1990;15:45–57. doi: 10.1002/mrm.1910150106. [DOI] [PubMed] [Google Scholar]
- 11.Detre JA, Williams DS, Koretsky AP. Nuclear magnetic resonance determination of flow, lactate, and phosphate metabolites during amphetamine stimulation of the rat brain. NMR Biomed. 1990;3:272–278. doi: 10.1002/nbm.1940030606. [DOI] [PubMed] [Google Scholar]
- 12.Barranco D, Sutton LN, Florin S, Greenberg J, Sinnwell T, Ligeti L, McLaughlin AC. Use of 19F NMR spectroscopy for measurement of cerebral blood flow: a comparative study using microspheres. J Cereb Blood Flow Metab. 1989;9:886–891. doi: 10.1038/jcbfm.1989.122. [DOI] [PubMed] [Google Scholar]
- 13.Villringer A, Rosen BR, Belliveau JW, Ackerman JL, Lauffer RB, Buxton RB, Chao YS, Wedeen VJ, Brady TJ. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn Reson Med. 1988;6:164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- 14.Rosen BR, Belliveau JW, Buchbinder BR, McKinstry RC, Porkka LM, Kennedy DN, Neuder MS, Fisel CR, Aronen HJ, Kwong KK. Contrast agents and cerebral hemodynamics. Magn Reson Med. 1991;19:285–292. doi: 10.1002/mrm.1910190216. [DOI] [PubMed] [Google Scholar]
- 15.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 17.Van der Linden A, Van CN, Ramos-Cabrer P, Hoehn M. Current status of functional MRI on small animals: application to physiology, pathophysiology, and cognition. NMR Biomed. 2007;20:522–545. doi: 10.1002/nbm.1131. [DOI] [PubMed] [Google Scholar]
- 18.King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe AS, Williams SC, Symms MR, Stolerman IP, Shoaib M. Functional magnetic resonance neuroimaging of drug dependence: naloxone-precipitated morphine withdrawal. Neuroimage. 2002;17:902–910. [PubMed] [Google Scholar]
- 20.Wu G, Luo F, Li Z, Zhao X, Li SJ. Transient relationships among BOLD, CBV, and CBF changes in rat brain as detected by functional MRI. Magn Reson Med. 2002;48:987–993. doi: 10.1002/mrm.10317. [DOI] [PubMed] [Google Scholar]
- 21.Kannurpatti SS, Biswal BB. Effect of anesthesia on CBF, MAP and fMRI-BOLD signal in response to apnea. Brain Res. 2004;1011:141–147. doi: 10.1016/j.brainres.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 22.Boumans T, Theunissen FE, Poirier C, Van der Linden A. Neural representation of spectral and temporal features of song in the auditory forebrain of zebra finches as revealed by functional MRI. Eur J Neurosci. 2007;26:2613–2626. doi: 10.1111/j.1460-9568.2007.05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Hyder F, Behar KL, Martin MA, Blamire AM, Shulman RG. Dynamic magnetic resonance imaging of the rat brain during forepaw stimulation. J Cereb Blood Flow Metab. 1994;14:649–655. doi: 10.1038/jcbfm.1994.81. [DOI] [PubMed] [Google Scholar]
- 25.Kerskens CM, Hoehn-Berlage M, Schmitz B, Busch E, Bock C, Gyngell ML, Hossmann KA. Ultrafast perfusion-weighted MRI of functional brain activation in rats during forepaw stimulation: comparison with T2 -weighted MRI. NMR Biomed 1996. 1996 Feb;9(1):20–23. doi: 10.1002/(SICI)1099-1492(199602)9:1<20::AID-NBM381>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Gyngell ML, Bock C, Schmitz B, Hoehn-Berlage M, Hossmann KA. Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha-chloralose anesthetized rats. Magn Reson Med. 1996;36:13–15. doi: 10.1002/mrm.1910360104. [DOI] [PubMed] [Google Scholar]
- 27.Bock C, Krep H, Brinker G, Hoehn-Berlage M. Brainmapping of alpha-chloralose anesthetized rats with T2*-weighted imaging: distinction between the representation of the forepaw and hindpaw in the somatosensory cortex. NMR Biomed. 1998;11:115–119. doi: 10.1002/(sici)1099-1492(199805)11:3<115::aid-nbm526>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Peeters RR, Tindemans I, De Schutter E, Van der LA. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19:821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 30.Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. BOLD and CBV-weighted functional magnetic resonance imaging of the rat somatosensory system. Magn Reson Med. 2006;55:316–324. doi: 10.1002/mrm.20744. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Shen J. Increased oxygen consumption in the somatosensory cortex of alpha-chloralose anesthetized rats during forepaw stimulation determined using MRS at 11.7 Tesla. Neuroimage. 2006;32:1317–1325. doi: 10.1016/j.neuroimage.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Stefanovic B, Bosetti F, Silva AC. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage. 2006;32:23–32. doi: 10.1016/j.neuroimage.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Stefanovic B, Schwindt W, Hoehn M, Silva AC. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J Cereb Blood Flow Metab. 2007;27:741–754. doi: 10.1038/sj.jcbfm.9600377. [DOI] [PubMed] [Google Scholar]
- 35.Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21:410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman P, Sanganahalli BG, Hyder F. Multimodal measurements of blood plasma and red blood cell volumes during functional brain activation. J Cereb Blood Flow Metab. 2009;29:19–24. doi: 10.1038/jcbfm.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 38.Soma LR. Anesthetic and analgesic considerations in the experimental animal. Ann N Y Acad Sci. 1983;406:32–47. doi: 10.1111/j.1749-6632.1983.tb53483.x. [DOI] [PubMed] [Google Scholar]
- 39.Silverman J, Muir WW., III A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci. 1993;43:210–216. [PubMed] [Google Scholar]
- 40.Willis CK, Quinn RP, McDonell WM, Gati J, Parent J, Nicolle D. Functional MRI as a tool to assess vision in dogs: the optimal anesthetic. Vet Ophthalmol. 2001;4:243–253. doi: 10.1046/j.1463-5216.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 41.Heinke W, Schwarzbauer C. Subanesthetic isoflurane affects task-induced brain activation in a highly specific manner: a functional magnetic resonance imaging study. Anesthesiology. 2001;94:973–981. doi: 10.1097/00000542-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abo M, Suzuki M, Senoo A, Miyano S, Yamauchi H, Yonemoto K, Watanabe S, Edstrom L. Influence of isoflurane concentration and hypoxia on functional magnetic resonance imaging for the detection of bicuculline-induced neuronal activation. Neurosignals. 2004;13:144–149. doi: 10.1159/000076568. [DOI] [PubMed] [Google Scholar]
- 45.Dashti M, Geso M, Williams J. The effects of anaesthesia on cortical stimulation in rats: a functional MRI study. Australas Phys Eng Sci Med. 2005;28:21–25. doi: 10.1007/BF03178860. [DOI] [PubMed] [Google Scholar]
- 46.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 47.Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135:186–194. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommers MG, van EJ, Booij LH, Heerschap A. Isoflurane anesthesia is a valuable alternative for alpha-chloralose anesthesia in the forepaw stimulation model in rats. NMR Biomed. 2009;22:414–418. doi: 10.1002/nbm.1351. [DOI] [PubMed] [Google Scholar]
- 49.Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 50.Todd MM, Drummond JC. A comparison of the cerebrovascular and metabolic effects of halothane and isoflurane in the cat. Anesthesiology. 1984;60:276–282. doi: 10.1097/00000542-198404000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Drummond JC, Todd MM, Scheller MS, Shapiro HM. A comparison of the direct cerebral vasodilating potencies of halothane and isoflurane in the New Zealand white rabbit. Anesthesiology. 1986;65:462–467. doi: 10.1097/00000542-198611000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wegener S, Wong EC. Longitudinal MRI studies in the isoflurane-anesthetized rat: long-term effects of a short hypoxic episode on regulation of cerebral blood flow as assessed by pulsed arterial spin labelling. NMR Biomed. 2008;21:696–703. doi: 10.1002/nbm.1243. [DOI] [PubMed] [Google Scholar]
- 54.Scanley BE, Kennan RP, Cannan S, Skudlarski P, Innis RB, Gore JC. Functional magnetic resonance imaging of median nerve stimulation in rats at 2.0 T. Magn Reson Med. 1997;37:969–972. doi: 10.1002/mrm.1910370625. [DOI] [PubMed] [Google Scholar]
- 55.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41:412–416. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Kalisch R, Elbel GK, Gossl C, Czisch M, Auer DP. Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 Tesla: implications for pharmacologic mri. Neuroimage. 2001;14:891–898. doi: 10.1006/nimg.2001.0890. [DOI] [PubMed] [Google Scholar]
- 57.Makiranta MJ, Lehtinen S, Jauhiainen JP, Oikarinen JT, Pyhtinen J, Tervonen O. MR perfusion, diffusion and BOLD imaging of methotrexate-exposed swine brain. J Magn Reson Imaging. 2002;15:511–519. doi: 10.1002/jmri.10103. [DOI] [PubMed] [Google Scholar]
- 58.Weber R, Ramos-Cabrer P, Wiedermann D, Van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46:1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 63.Hinojosa-Laborde C, Greene AS, Cowley AW., Jr Autoregulation of the systemic circulation in conscious rats. Hypertension. 1988;11:685–691. doi: 10.1161/01.hyp.11.6.685. [DOI] [PubMed] [Google Scholar]
- 64.Skarlatos S, Brand PH, Metting PJ, Britton SL. Spontaneous changes in arterial blood pressure and renal interstitial hydrostatic pressure in conscious rats. J Physiol. 1994;481(Pt 3):743–752. doi: 10.1113/jphysiol.1994.sp020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnell CR, Wood JM. Measurement of blood pressure and heart rate by telemetry in conscious, unrestrained marmosets. Am J Physiol. 1993;264:H1509–H1516. doi: 10.1152/ajpheart.1993.264.5.H1509. [DOI] [PubMed] [Google Scholar]
- 66.Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- 67.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Imaging brain activity in conscious animals using functional MRI. J Neurosci Methods. 1998;82:75–83. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 68.Khubchandani M, Mallick HN, Jagannathan NR, Mohan KV. Stereotaxic assembly and procedures for simultaneous electrophysiological and MRI study of conscious rat. Magn Reson Med. 2003;49:962–967. doi: 10.1002/mrm.10441. [DOI] [PubMed] [Google Scholar]
- 69.Tenney JR, Duong TQ, King JA, Ferris CF. FMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. 2004;45:576–582. doi: 10.1111/j.0013-9580.2004.39303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferris CF, Febo M, Luo F, Schmidt K, Brevard M, Harder JA, Kulkarni P, Messenger T, King JA. Functional magnetic resonance imaging in conscious animals: a new tool in behavioural neuroscience research. J Neuroendocrinol. 2006;18:307–318. doi: 10.1111/j.1365-2826.2006.01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–193. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubowitz DJ, Chen DY, Atkinson DJ, Grieve KL, Gillikin B, Bradley WG, Jr, Andersen RA. Functional magnetic resonance imaging in macaque cortex. Neuroreport. 1998;9:2213–2218. doi: 10.1097/00001756-199807130-00012. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Andersen AH, Avison MJ, Gerhardt GA, Gash DM. Functional MRI of apomorphine activation of the basal ganglia in awake rhesus monkeys. Brain Res. 2000;852:290–296. doi: 10.1016/s0006-8993(99)02243-x. [DOI] [PubMed] [Google Scholar]
- 74.Dubowitz DJ, Bernheim KA, Chen DY, Bradley WG, Jr, Andersen RA. Enhancing fMRI contrast in awake-behaving primates using intravascular magnetite dextran nanopartieles. Neuroreport. 2001;12:2335–2340. doi: 10.1097/00001756-200108080-00011. [DOI] [PubMed] [Google Scholar]
- 75.Ferris CF, Snowdon CT, King JA, Duong TQ, Ziegler TE, Ugurbil K, Ludwig R, Schultz-Darken NJ, Wu Z, Olson DP, Sullivan JM, Jr, Tannenbaum PL, Vaughan JT. Functional imaging of brain activity in conscious monkeys responding to sexually arousing cues. Neuroreport. 2001;12:2231–2236. doi: 10.1097/00001756-200107200-00037. [DOI] [PubMed] [Google Scholar]
- 76.Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 77.Andersen AH, Zhang Z, Barber T, Rayens WS, Zhang J, Grondin R, Hardy P, Gerhardt GA, Gash DM. Functional MRI studies in awake rhesus monkeys: methodological and analytical strategies. J Neurosci Methods. 2002;118:141–152. doi: 10.1016/s0165-0270(02)00123-1. [DOI] [PubMed] [Google Scholar]
- 78.Orban GA. Functional MRI in the awake monkey: the missing link. J Cogn Neurosci. 2002;14:965–969. doi: 10.1162/089892902760191171. [DOI] [PubMed] [Google Scholar]
- 79.Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 80.Ferris CF, Snowdon CT, King JA, Sullivan JM, Jr, Ziegler TE, Olson DP, Schultz-Darken NJ, Tannenbaum PL, Ludwig R, Wu Z, Einspanier A, Vaughan JT, Duong TQ. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004;19:168–175. doi: 10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfeuffer J, Shmuel A, Keliris GA, Steudel T, Merkle H, Logothetis NK. Functional MR imaging in the awake monkey: effects of motion on dynamic off-resonance and processing strategies. Magn Reson Imaging. 2007;25:869–882. doi: 10.1016/j.mri.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Goense JB, Ku SP, Merkle H, Tolias AS, Logothetis NK. fMRI of the temporal lobe of the awake monkey at 7 T. Neuroimage. 2008;39:1081–1093. doi: 10.1016/j.neuroimage.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 83.Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145:120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stefanacci L, Reber P, Costanza J, Wong E, Buxton R, Zola S, Squire L, Albright T. fMRI of monkey visual cortex. Neuron 1998. 1998 Jun;20:1051–1057. doi: 10.1016/s0896-6273(00)80485-7. [DOI] [PubMed] [Google Scholar]
- 86.Schultz-Darken NJ, Pape RM, Tannenbaum PL, Saltzman W, Abbott DH. Novel restraint system for neuroendocrine studies of socially living common marmoset monkeys. Lab Anim. 2004;38:393–405. doi: 10.1258/0023677041958918. [DOI] [PubMed] [Google Scholar]