Abstract
Background and Aims
New methods to measure visceral adipose tissue (VAT) by DEXA may help discern sex, race and phenotype differences in the role of VAT in cardiometabolic risk. This study was designed to: a) compare relationships between cardiometabolic risk factors and DEXA-VAT, anthropometric and body composition measures; b) determine thresholds for DEXA-VAT by race; and c) determine the most robust predictors of impaired glucose tolerance (IGT) and metabolic syndrome (MetSx) in obese women.
Methods
VAT area (cm2) was measured using Lunar iDXA scanner in 229 obese (BMI 30-49.9) women age 21–69 years of European American (EA = 123) and African American (AA = 106) descent. Linear regression modeling and areas under the curve (AUC) compared relationships with cardiometabolic risk. Bootstrapping with LASSO regression modeling determined thresholds and predictors of IGT and MetSx.
Results
DEXA-VAT explained more of the variance in triglycerides, blood pressure, glucose and HOMA-IR compared to anthropometric and body composition variables. DEXA-VAT had the highest AUC for IGT (0.767) and MetSx (0.749). Including race and interactionXrace terms in modeling did not significantly change results. Thresholds at which probability was ≥ 50% for IGT or MetSx were lower in AA women (IGT: 2120cm2 AA vs 2550cm2 EA; MetSx: 1320cm2 AA vs 1713cm2 EA). The odds for IGT or MetSx was 3-fold greater with each standard deviation increase in DEXA-VAT.
Conclusion
DEXA-VAT provides robust clinical information regarding cardiometabolic risk in AA and EA women and has great potential in risk reduction efforts.
Keywords: obesity, visceral, DEXA, body composition, cardiovascular, metabolic syndrome
INTRODUCTION
The accumulation of body fat in the visceral depot is mechanistically related to insulin resistance and development of type 2 diabetes, hypertension, dyslipidemia and cardiovascular disease (1-3). More specifically, intra-abdominal or visceral adipose tissue (VAT) promotes hepatic inflammation, steatosis, insulin resistance and dyslipidemia by releasing free fatty acids, hormones, and inflammatory chemokines and cytokines into the portal circulation that functionally impair insulin sensitivity and action (4, 5). While VAT is particularly pathogenic (6-8) and independently predicts all-cause mortality (9), mounting evidence suggests abdominal subcutaneous adipose tissue (SAT) also impacts the development of obesity related insulin resistance in certain phenotypes by first releasing fatty acids into the venous circulation and later into the portal vein (10, 11). With the epidemic prevalence of overweight and obesity (BMI ≥ 25) reaching 70% of U.S. adults (12), there is need to reliably differentiate and quantify VAT and SAT to discern differences in the role and function of VAT and SAT by sex, race and metabolically at risk phenotypes.
Although the prevalence of abdominal adiposity has increased two-fold in U.S. women over the past four decades (13), men tend to have greater VAT accumulation than (pre-menopausal) women (9, 14). In fact, women have about five times more SAT than VAT in the intra-abdominal compartment (15). This sexual dimorphism is apparent in other cardiovascular risk biomarkers (16) as well as relationships between abdominal fat and cardiometabolic risk. Racial and ethnic differences are also evident as people of south Asian descent have a greater proportion of VAT compared to those of European descent (17, 18) and European Americans have more VAT than African Americans, even when controlled for total body fat (19, 20). Despite less VAT, African Americans have greater prevalence of insulin resistance, type 2 diabetes and cardiovascular disease than other population subgroups (21-23). While prior investigations indicate functional roles for both VAT and SAT in the disparities associated with genotype (24), more information is needed further elucidating sex and race differences in the relationship between VAT and SAT and cardiometabolic risk to enable design of subgroup specific interventions.
In clinical practice and public health settings, anthropometric measures such as body mass index (BMI), waist circumference (WC), hip circumference (HC), waist-hip ratio (WHR), and more recently waist-height ratio (WHtR), are used as surrogates for intra-abdominal adiposity since they require little expense, time or technical expertise. Notably, these indicators are predictive of cardiometabolic outcomes in large population groups (25). However, anthropometric measures are unable to distinguish fat versus lean mass or the amount, type and distribution of adipose tissue. For example, BMI, WC, WHR and WHtR do not discriminate whether a higher value is due to increased total abdominal fat or the relative proportion of VAT to SAT. Hence, such measures cannot advance science regarding the particular roles of VAT versus SAT in specific genotypes or phenotypes such as the “metabolically healthy” obese or “metabolically unhealthy” lean (26-28). Moreover, manual measures are subject to high inter-rater variability (29). Considering these limitations, computed tomography scan (CT) and magnetic resonance imaging (MRI) are the recognized gold standards for quantifying and comparing regional fat amount, type and distribution in research settings. As such, these imaging techniques have contributed greatly to understanding metabolic phenotypes as well as disparities in vulnerability for cardiometabolic disease (30, 31). However, the complex and costly technology of CT and MRI limits their general clinical utility (32). Thus, there remains need for more practical, accessible and economical methods to evaluate adiposity and determine its role in cardiometabolic risk, disease and treatment.
In contrast to CT and MRI, dual energy X-ray absorptiometry (DEXA), originally designed to assess bone mineral density, allows estimation of whole body composition from a two-dimensional X-ray with low radiation exposure, short-scanning time, high precision and low cost (33). Most often, DEXA estimated intra-abdominal adipose tissue has derived from formulas based on manual manipulation of the abdominal region of interest using anatomical landmarks at the level of the 2nd to 5th lumbar vertebrae (34-36). However, such estimation has not distinguished VAT from SAT and the most reliable estimates have come from non-obese persons (35, 37, 38). Nevertheless, we previously showed strong correlations between DEXA and water-suppressed T1 weighted MRI measures of total and regional body composition, with coefficients of variation less than 2% for DEXA-derived adiposity measures (39). More recently, algorithms in updated versions of the software used on DEXA scanners have been developed to segment fat within the android region into VAT and SAT (40). This DEXA-VAT method has been validated against CT in subjects with BMI ranging from 18.5 to 40 kg/m2 and had a coefficient of determination (r2) of 0.959 for women and 0.949 for men (40). Yet, the relationship between DEXA-VAT and cardiometabolic risk factors has not been well established.
The purpose of this study was to test the hypothesis that DEXA-VAT is more robustly associated with cardiometabolic risk in obese women of European American and African American descent compared to other commonly acquired anthropometric and body composition indicators. Secondly, to test the hypothesis that relationships between DEXA-derived VAT and established cardiometabolic risk factors differ in obese women by race, and if so, determine thresholds by race that identify the amount of DEXA-VAT that elevates risk. Finally, to determine which anthropometric, body composition and clinical variables most robustly predicts impaired glucose tolerance and the metabolic syndrome in obese women.
METHODS
Subjects
This study is a cross-sectional analysis of DEXA acquired whole body scans from obese women enrolled in clinical trials conducted at the Vanderbilt Clinical Research Center (VCRC) between 2008 and 2013. Subjects were recruited from local media and electronic advertisements. Scans were included if female subjects were age ≥ 21 years, BMI was ≥ 30 kg/m2, and they were non-smokers. Race was self-identified. To be included in the analysis, subjects had anthropometric (height, weight, waist and hip circumferences), biochemical and clinical data obtained simultaneous with the DEXA scan using standardized protocols. Each subject provided written informed consent and study procedures were approved by the Vanderbilt University Institutional Review Board. Prior to analysis, subject records were de-identified and stored in a Vanderbilt REDCap database (41).
Anthropometry
Physical measures were obtained using standard methods to determine clinical trial eligibility and acquire baseline data. Height was measured to the nearest 0.1cm on a wall-mounted stadiometer. Weight was measured to the nearest 0.1kg on a calibrated digital platform scale without shoes, hats, outer clothing or pocket items. Waist and hip circumferences were measured via flexible measuring tape to the nearest 0.1cm above the right iliac crest and at the fullest extension of the buttocks, respectively. BMI, waist-hip and waist-height were calculated as ratios.
Dual Energy X-Ray Absorptiometry
Total and regional body composition was acquired by a certified densitometrist using a Lunar iDXA whole body scanner (GE Healthcare, Madison, WI) with enCore 2007 software (version 11.4). Before each acquisition, the scanner was phantom calibrated according to manufacturer instructions. Duplicate scans after repositioning 12 subjects showed coefficients of variation <2% for fat and lean total and trunk masses (39). Scans were imported into an updated version of the software (version 13.6) and reanalyzed using algorithms that provided automatic segmentation of VAT from total abdominal fat within the android region. VAT mass (g) was automatically transposed into area (cm2) using a constant correction factor (0.94 g/ml) that is consistent with the density of adipose tissue (40, 42).
Clinical and Biochemical Cardiometabolic Risk Factors
Systolic and diastolic blood pressure (SBP, DBP) and heart rate were obtained by VCRC research nurses with subjects in a supine resting state using a calibrated sphygmomanometer with a large size cuff. Fasting glucose, insulin, lipid profile (serum total cholesterol, LDL-cholesterol [LDL-C], HDL-cholesterol [HDL-C] and triglycerides [TG]) and high sensitivity C-reactive protein (hsCRP) were processed at the Vanderbilt Pathology Laboratory using standard procedures. Serum leptin was processed at the Vanderbilt Diabetes Hormone Core Laboratory. Insulin resistance (HOMA-IR) was scored using the HOMA2-IR model (43). Glucose tolerance was measured using a standard 75g oral glucose tolerance test or a frequently sampled intravenous glucose tolerance test (44, 45).
Statistical Analysis
Data were analyzed using R Statistical Software version 3.0.1 (http://www.r-project.org/). The level of significance was set at α = 0.05. Categorical variables are presented as frequency and percentages while continuous variables as mean ± standard deviation (SD). Data were checked for normality by visual inspection of histograms and stem and leaf plots. We used Wilcoxon signed-rank tests to assess the significance of inter-group differences. In order to determine whether DEXA-derived VAT is more robustly associated with cardiometabolic risk factors than anthropometric and other body composition variables, we fitted multiple linear regression models with VAT, anthropometric and body composition variables as outcome variables and cardiometabolic risk factors as independent variables. We then compared the coefficients of determination across the outcome variables while adding race and the interaction between race and the independent variable in the models to identify potential effects of race on these relationships. Next, we calculated areas under the curve (AUC) using binary logistic regression. Regression modeling was performed with VAT unadjusted and adjusted for body surface area. As no significant differences were detected between models, we present results only using unadjusted VAT. To identify variables from the binary regression models that most minimized sum of the squares of the errors we performed bootstrapping to resample the individual observations with replacement (N = 200) and least absolute shrinkage and selection operator (LASSO) regression modeling. This process allowed identification of significant predictors for two separate cardiometabolic outcomes: 1) impaired glucose tolerance (IGT) defined as having fasting glucose ≥ 100 mg/dl, 2-hour glucose between 140 and 199 mg/dl (46), and/or glucose disappearance constant (KG) < 1.5 (44, 47); and 2) the metabolic syndrome (MetSx) defined as having ≥ 3 of the 5 National Cholesterol Education Program's Adult Treatment Panel III criteria as modified by the American Heart Association (48). With anthropometric, body composition and cardiometabolic risk factors as independent variables, the residual deviance was treated as a chi square value to test the overall fit of each model. We also calculated the odds ratio for each independent variable in our final logistic regression models. For each race, we established a VAT threshold to determine at what point the probability of IGT and MetSx was at least 50% while other independent variables were fixed at their mean values.
RESULTS
Demographics and Baseline Characteristics
DEXA scans were acquired from 229 women who ranged in age from 21 to 69 years old and in BMI from 30.0 to 49.5 kg/m2. Of these 229, 123 (53.7%) reported being of European American (EA) descent and 106 (46.3%) reported being of African American (AA) descent. Total body fat ranged from 32.0 to 56.0% and VAT ranged from 173 to 5655cm2. Despite having similar mean age, height and % body fat (Table 1), the EA women had lower lean body mass, BMI, HC, WHtR, SBP, DBP, fasting insulin and HOMA-IR. Simultaneously, mean DEXA-VAT and serum TG levels were lower in the AA women.
Table 1.
Anthropometric, Body Composition and Clinical Cardiometabolic Risk Factors in 229 Obese Women
| Caucasian | African American | |
|---|---|---|
| n = 123 | n = 106 | |
| Age (y) | 38.6 ± 8.3 | 39.7 ± 9.1 |
| Height (cm) | 165.2 ± 9.2 | 163.6 ± 7.9 |
| Weight (kg) | 95.9 ± 13.7 | 100.3 ± 16.4 |
| Body Mass Index (kg/m2) | 35.1 ± 3.5 | 37.3 ± 4.8** |
| Waist Circumference (cm) | 104.8 ± 10.7 | 107.3 ± 11.4 |
| Hip Circumference (cm) | 118.2 ± 7.5 | 121.3 ± 10.3* |
| Waist/Hip ratio | 0.89 ± 0.08 | 0.89 ± 0.08 |
| Waist/Height ratio | 0.63 ± 0.05 | 0.65 ± 0.06* |
| Lean Mass (kg) | 48.8 ± 8.4 | 51.5 ± 8.3** |
| Fat Mass (kg) | 43.2 ± 7.8 | 44.7 ± 9.7 |
| Body Fat (%) | 46.9 ± 4.6 | 46.2 ± 4.5 |
| Trunk Fat (%) | 50.5 ± 4.6 | 49.6 ± 5.6 |
| Android Fat (%) | 53.5 ± 5.0 | 53.0 ± 6.3 |
| Android/Gynoid Ratio | 0.59 ± 0.17 | 0.57 ± 0.15 |
| VAT area (cm2) | 1646.4 ± 1007.5 | 1300.2 ± 661.7** |
| Systolic Blood Pressure (mm Hg) | 122.1 ± 11.8 | 126.8 ± 13.1** |
| Diastolic Blood Pressure (mm Hg) | 72.4 ± 8.1 | 76.6 ± 8.9** |
| Heart Rate (bpm) | 77.9 ± 11.8 | 77.5 ± 11.1 |
| Total cholesterol (mg/dL) | 170.3 ± 30.4 | 172.5 ± 34.7 |
| HDL-cholesterol (mg/dL) | 46.7 ± 13.6 | 47.5 ± 13.3 |
| LDL-cholesterol (mg/dL) | 102.1 ± 26.6 | 109.2 ± 31.7 |
| Triglycerides (mg/dL) | 107.1 ± 61.7 | 82.7 ± 48.6*** |
| hs-CRP (mg/L) | 5.1 ± 5.8 | 6.6 ± 7.2 |
| Leptin (ng/mL) | 33.0 ± 10.4 | 32.3 ± 11.9 |
| Glucose (mg/dL) | 103.1 ± 42.9 | 97.8 ± 25.9 |
| Insulin (mu/mL) | 11.4 ± 8.6 | 14.3 ± 10.9** |
| HOMA-IR (score) | 3.2 ± 3.9 | 3.6 ± 4.4** |
Wilcoxon signed rank test
P < 0.05
P < 0.01
P < 0.001
DEXA-VAT and Cardiometabolic Risk Factors
In both groups, DEXA-VAT was positively associated with TG, fasting glucose, fasting insulin and HOMA-IR, and negatively associated with HDL-C. DEXA-VAT was also positively associated with SBP and DBP in EA women, but not AA women. DEXA-VAT was positively associated with hs-CRP in AA women, but not EA women. We next assessed whether DEXA-VAT was more strongly associated with cardiometabolic risk factors compared to the other anthropometric and body composition variables (Table 2). Incorporating the interaction between DEXA-VAT and race, the strength of the relationships between SBP and DBP to DEXA-VAT, BMI, WC, HC, WHR and WHtR were similar. However, DEXA-VAT explained 10.2% more of the total variance in SBP and 11.7% more of the total variance in DBP than the other body composition variables (%body fat, %trunk fat, %android fat and android/gynoid ratio). WHR and android/gynoid ratio explained more of the total variance in HDL-C than DEXA-VAT or WC (+13.8%, +12.2%, +7.5% and +6.9%, respectively). Yet, DEXA-VAT explained 24.6% more of the total variance in TG than all other variables. Although WC explained as much of the total variance in fasting insulin as DEXA-VAT (r2 = 21.5% vs 21.7%, respectively), DEXA-VAT explained 31.8% more of the total variance in fasting glucose and 25.9% more of the total variance in HOMA-IR than all other variables.
Table 2.
Relationships Between Anthropometric and Body Composition Independent Variables and Cardiometabolic Risk Factorsa
| Systolic Blood Pressure (mmHg) |
Diastolic Blood Pressure (mmHg) |
Heart Rate (bpm) |
Total Cholesterol (mg/dL) |
HDL Cholesterol (mg/dL) |
LDL Cholesterol (mg/dL) |
Triglycerides (mg/dL) |
Hs-CRP (mg/L) |
Glucose (mg/dL) |
Insulin (mU/mL) |
HOMA-IR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VAT volume (cm2) | 0.102** | 0.117** | 0.005 | 0.020 | 0.075*** | 0.041 | 0.246*** | 0.07 | 0.318*** | 0.220*** | 0.259*** |
| Body Mass Index (kg/m2) | 0.060** | 0.073** | 0.001 | 0.005 | 0.002 | 0.018 | 0.075 | 0.032* | 0.03 | 0.113*** | 0.060*** |
| Waist Circumference (cm) | 0.071** | 0.070* | 0.004 | 0.013 | 0.070*** | 0.038 | 0.179*** | 0.068** | 0.200*** | 0.215*** | 0.215*** |
| Hip Circumference (cm) | 0.056* | 0.088* | 0.008 | 0.008 | 0.002 | 0.027 | 0.045 | 0.016 | 0.016 | 0.060** | 0.033* |
| Waist/Hip Ratio | 0.045 | 0.087 | 0.006 | 0.005 | 0.138*** | 0.019 | 0.201*** | 0.065* | 0.218*** | 0.136*** | 0.144*** |
| Waist/Height Ratio | 0.059* | 0.071* | 0.009 | 0.004 | 0.033** | 0.021 | 0.141*** | 0.050** | 0.080*** | 0.143*** | 0.102*** |
| Bodyfat (%) | 0.065 | 0.093 | 0.006 | 0.007 | 0.043* | 0.026 | 0.063 | 0.052* | 0.112*** | 0.032 | 0.017 |
| Trunkfat (%) | 0.052 | 0.07 | 0.007 | 0.007 | 0.022 | 0.028 | 0.056 | 0.065** | 0.017 | 0.083*** | 0.015 |
| Android fat (%) | 0.045 | 0.067 | 0.005 | 0.011 | 0.016 | 0.034 | 0.059 | 0.058** | 0.014 | 0.090*** | 0.02 |
| Android/Gynoid fat Ratio | 0.056 | 0.081 | 0.003 | 0.018 | 0.122*** | 0.039 | 0.212*** | 0.091 | 0.216*** | 0.151*** | 0.160*** |
VAT = visceral adipose tissue, HDL-Cholesterol = high-density lipoprotein cholesterol, LDL-Cholesterol = low-density lipoprotein cholesterol,
Hs-CRP = high sensitivity c-reactive protein, HOMA-IR = homeostatic model assessment-insulin resistance
R2 values from Binary Logistic Regression Modelling Accounting for the Interaction between Independent Variable and Race
P < 0.05
P < 0.01
P < 0.001
DEXA-VAT and Impaired Glucose Tolerance
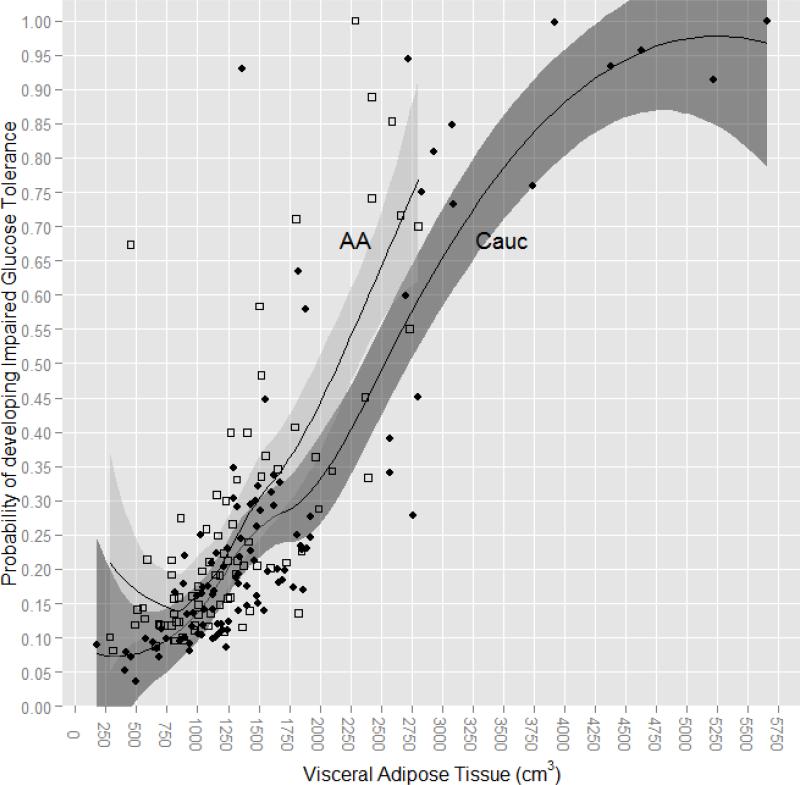
The proportion of EA and AA women with IGT did not differ (29.3 vs 29.2%, X2 (1) = 0.00, P = 0.100). To determine whether DEXA-VAT was a more robust predictor of IGT than the other anthropometric and body composition variables, we performed binary logistic regression. DEXA-VAT had the highest area under the curve (AUC = 0.766), with AUC values for anthropometric and other body composition variables ranging from 0.534 to 0.703 (Table 3). Adjusting the AUC for race (0.766) or incorporating the interaction between DEXA-VAT and race (0.759) did not change the significance of the relationship between DEXA-VAT and IGT. However, there was a difference by race in the amount of DEXA-VAT with regard to the probability of developing IGT; in EA women having DEXA-VAT of ≥ 2550cm2 increased the likelihood of developing IGT by ≥ 50% whereas in AA women having DEXA-VAT of ≥ 2120cm2 increased the likelihood of developing IGT by ≥ 50% (Figure 1).
Table 3.
Comparison of Areas Under the Curve For Relationships with Glucose Tolerance and Metabolic Syndrome
| Impaired Glucose Tolerance | Metabolic Syndrome | |||||
|---|---|---|---|---|---|---|
| AUC of the IV** | AUC × Race | AUC × Race x IV | AUC of the IV | AUC × Race | AUC × Race × IV | |
| VAT volume (cm2)* | 0.766 | 0.767 | 0.760 | 0.749 | 0.755 | 0.750 |
| Body Mass Index (kg/m2) | 0.604 | 0.767 | 0.760 | 0.640 | 0.629 | 0.622 |
| Waist Circumference (cm) | 0.670 | 0.656 | 0.668 | 0.674 | 0.682 | 0.680 |
| Hip Circumference (cm) | 0.518 | 0.532 | 0.541 | 0.538 | 0.556 | 0.543 |
| Waist/Hip Ratio | 0.699 | 0.687 | 0.689 | 0.676 | 0.683 | 0.683 |
| Waist/Height Ratio | 0.617 | 0.604 | 0.613 | 0.655 | 0.648 | 0.645 |
| Bodyfat (%) | 0.566 | 0.556 | 0.540 | 0.505 | 0.529 | 0.598 |
| Trunkfat (%) | 0.533 | 0.548 | 0.574 | 0.562 | 0.562 | 0.547 |
| Android fat (%) | 0.530 | 0.550 | 0.542 | 0.555 | 0.555 | 0.544 |
| Android/Gynoid fat Ratio | 0.703 | 0.695 | 0.695 | 0.712 | 0.720 | 0.708 |
VAT = visceral adipose tissue
IV = independent variable; AUC from bias corrected bootstrapping
Figure 1.
Probability of Developing Impaired Glucose Tolerance by Amount of VAT in Obese European American and African American Women
DEXA-VAT and Metabolic Syndrome
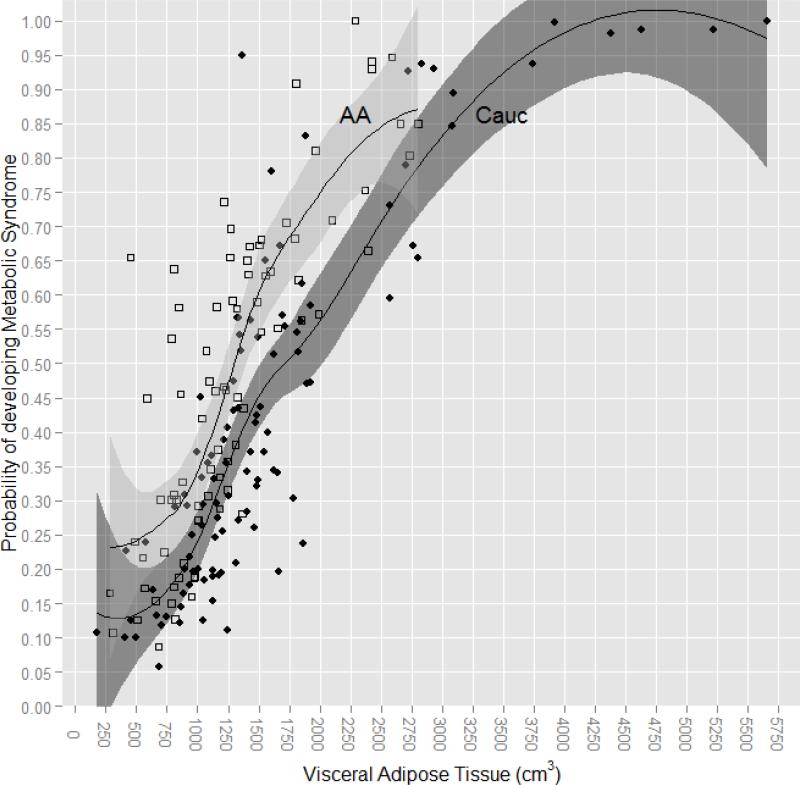
The proportion of EA and AA women with metabolic syndrome also did not differ significantly (39.0 vs 48.1%, X2 (1) = 1.92, P = 0.17). Again, we used binary logistic regression to determine whether DEXA-VAT was a more robust predictor of MetSx in comparison to the other anthropometric and body composition variables in these obese women. As with IGT, DEXA-VAT had the highest area under the curve (AUC = 0.749) for MetSx, with AUC values for anthropometric and body composition variables ranging from 0.506 to 0.712. Neither adjusting the AUC for race (0.755) nor incorporating the interaction between DEXA-VAT and race (0.749) altered the significance of the relationship between DEXA-VAT and MetSx. Also similar to IGT, there was a difference by race in the amount of DEXA-VAT with regard to the probability of developing MetSx; in EA women having DEXA-VAT of ≥ 1713cm2 increased the likelihood of developing MetSx by ≥ 50% whereas in AA women having DEXA-VAT of ≥ 1320cm2 increased the likelihood of developing MetSx by ≥ 50% (Figure 2).
Figure 2.
Probability of Developing Metabolic Syndrome by Amount of VAT in Obese European American and African American Women
Multivariate Models for Impaired Glucose Tolerance and Metabolic Syndrome
The final analyses were performed to determine the best fitting multivariate regression models to predict IGT and MetSx in these obese women, using the anthropometric, body composition and cardiometabolic variables that significantly improved the binary regression models. Bootstrapping with LASSO yielded DEXA-VAT, SBP, fasting insulin and hsCRP as the independent variables that accounted for most of the variability in the outcome of IGT (Table 4). DEXA-VAT most significantly predicted IGT (P < 0.001). Accounting for SBP, fasting insulin and hsCRP in the model, each standard deviation increase in VAT (SD = 880 cm2) increases the odds of having IGT by 3.04-fold. Comparing the residual deviance for the multivariate model with and without race, as well as the interaction between DEXA-VAT and race, did not alter the significance of the model (P = 0.49).
Table 4.
Multivariate Regression for Impaired Glucose Tolerance in 229 Obese Women
| Impaired Glucose Tolerance | OR | SE | z | p value | Lower | 95% CI Upper | |
|---|---|---|---|---|---|---|---|
| Model 1 | Fasting Insulin | 1.62 | 0.24 | 2.02 | 0.04 | 1.03 | 2.63 |
| VAT | 3.04 | 0.26 | 4.34 | < 0.001 | 1.91 | 5.23 | |
| SBP | 1.06 | 0.20 | 0.31 | 0.76 | 0.72 | 1.57 | |
| HsCRP | 1.11 | 0.17 | 0.59 | 0.55 | 0.77 | 1.56 | |
| Race | 1.34 | 0.40 | 0.74 | 0.46 | 0.61 | 2.99 | |
| Model 2 | Model 1 * Race | Deviance | Df | p value | |||
| 0.458 | 1 | 0.49 | |||||
With MetSx as the outcome variable (Table 5), bootstrapping with LASSO yielded DEXA-VAT, HOMA-IR and LDL-C as the independent variables most accounting for the variability in MetSx. Like IGT, DEXA-VAT most significantly predicted MetSx (P < 0.001). Accounting for HOMA-IR and LDL-C in the model, each standard deviation increase in VAT increases the odds of having MetSx by 3.28-fold. Again, comparing the residual deviance for the multivariate model with and without race, as well as the interaction between DEXA-VAT and race, did not alter the significance of the model (P = 0.31).
Table 5.
Multivariate Regression for Metabolic Syndrome in 229 Obese Women
| Metabolic Syndrome | OR | SE | z | p value | Lower | 95% CI Upper | |
|---|---|---|---|---|---|---|---|
| Model 1 | VAT | 3.28 | 0.29 | 3.96 | < 0.001 | 1.89 | 6.14 |
| HOMA-IR | 2.92 | 0.41 | 2.64 | 0.008 | 1.39 | 6.93 | |
| LDL | 1.54 | 0.19 | 2.22 | 0.03 | 1.06 | 2.29 | |
| Race | 2.02 | 0.37 | 1.89 | 0.05 | 0.98 | 4.22 | |
| Model 2 | Model 1 * Race | Deviance | Df | p value | |||
| 1.044 | 1 | 0.31 | |||||
DISCUSSION
We previously demonstrated that DEXA whole body scans are as reliable as whole body continuous MRI for measurement of total fat, total lean, trunk fat and trunk lean masses (39). The present study extends our prior findings by showing that estimating VAT directly from DEXA whole body scans is a more robust indicator of cardiometabolic risk in women with Class I and II obesity of European American and African American descent than other more commonly acquired anthropometric (weight, BMI, waist circumference, waist-hip ratio, waist-height ratio) and DEXA-derived surrogate measures of intra-abdominal adipose tissue (percent body fat, percent trunk fat, percent android fat, android/gynoid ratio). Simultaneous assessment of bivariate relationships with risk factors and comparison of the areas under the curve between VAT and each anthropometric and body compositon variable using binary logistic regression modeling confirmed the strength of the relationships between DEXA-VAT and risk factors in both groups of obese women.
Upon evaluating each anthropometric and body compositon biomarker individually, one detectable difference was that DEXA-VAT correlated with hsCRP levels only in AA women even though the high body mass and elevated serum concentrations of C-reactive protein suggest presence of systemic inflammation in all these women. Importantly, animal and humans studies confirm that systemic inflammation in obesity is mechanistically linked to insulin resistance and future development of type 2 diabetes and cardiovascular disease (49-51) as VAT secretes pro-inflammatory cytokines such as TNF-α and IL-6 along with chemokines such as macrophage migration inhibitory factor and the CC chemokine receptor 2 (52, 53). While one study showed higher TNF-α and soluble TNF receptors in EA women (54), others have reported greater levels of of inflammatory biomarkers such as serum CRP concentration in African Americans. The higher rates of cardiometabolic disease in AA women suggest greater sensitivity and/or consequences from existing in this state of chronic inflammation (55). While overall body fat or total trunk fat might explain this disparity, the present cohort were well matched in both total fat mass, percent total body fat, percent trunk fat and percent android fat. Future investigation of other physiological stress factors that may predispose differential response to inflammatory signals may help explain this phenomenon. It is also possible that differences in dietary intakes or physical activities play a role in the relationship between VAT and inflammaton. For example, some long chain fatty acids (i.e., palmitate (56, 57)), and dietary glycemic load (58, 59) have been shown to induce inflammation and peripheral insulin resistance.
Another detectable difference was that DEXA-VAT correlated with blood pressure (SBP and DBP) only in EA women. Neural mechanisms have been suggested as a possible link between excess adiposity and high blood pressure. In prior work, we found that increased sympathetic nerve activity as measured by direct recording of sympathetic nerves in skeletal muscle contributed to obesity related hypertension (60). Notably, VAT (measured by CT) correlates better with muscle sympathetic nerve activity than other body composition measures. Overall, little investigation has been conducted directly assessing racial differences in the association between VAT and blood pressure. One study has reported that the association between the sympathetic nervous system and obesity is less strong in AA women (61). Yet, findings based on the relationship between WC and SBP in women show conflicting results with regard to race differences (62, 63). With the high prevalence of hypertension in the African American population, 42% vs 28% in white adults (64), it may be that mechanisms unrelated to VAT byproducts better explain this profound disparity.
Importantly, we further extend current findings by showing that DEXA-derived VAT was the strongest predictor of having impaired glucose tolerance in both EA and AA women. Now recognized as a condition of prediabetes, IGT currently affects > 40 million U.S. adults, elevating risk for progression to full-blown type 2 diabetes (65). In fact, the odds of having IGT was 3-fold greater for each standard deviation increase in DEXA-VAT, underscoring the physiological role of VAT in the development of impaired insulin action. Having thresholds of DEXA-VAT for obese EA and AA women that elucidate at what point the probability of having impaired glucose tolerance is at least 50% provides clinically meaningful information as one-third of Americans who do develop type 2 diabetes remain undiagnosed (65, 66).
While other studies have reported VAT thresholds, most of the work has been performed in white adults and has focused solely on the construct of the metabolic syndrome. Two studies that reported VAT thresholds in African Americans used cross-sectional CT scans to identify VAT (67, 68). Shedding additional light on the importance of VAT in the ethnic disparities of cardiometabolic risk, the thresholds determined in the present study from DEXA-VAT were lower in AA women for both IGT and MetSx. While our finding for MetSx is in agreement with recent findings from a larger biracial sample where DEXA-VAT was assessed similarly using a Hologic brand scanner (69), our results suggest that in obese women VAT is highly pathogenic in both EA and AA women.
A considerable strength of this cohort of obese women was that the EA and AA women were not only similar in age but unusually homogeneous in height, waist circumference, total fat mass as well as percentage total body fat, trunk fat, android fat and android/gynoid ratio. Moreover, the proportion of EA and AA women in the cohort was similar which provided the power to make comparisons and detect inter-group differences. A relative limitation of this study was the cross-sectional design of our analyses which restricts inference with regard to causality and we included obese women only which restricts the generalizability of the findings. Another limitation was the inability to screen subjects for use of antihypertensive or lipid-lowering medications which might influence the biochemical results. Future work is needed with the newer DEXA algorithms using prospective and more representative population samples.
In conclusion, the present findings contribute to the accumulating body of evidence that show DEXA-VAT is a robust indicator of cardiometabolic risk. Specifically, this study shows that DEXA-VAT predicts having IGT and MetSx in women with Class I and II obesity who are of both EA and AA descent. By being able to determine cut-points in DEXA-VAT that identify at what level risk for having IGT and MetSx elevates substantially in each group, DEXA-VAT provides robust information regarding detrimental health consequences of accumulating intra-abdominal fat. Compared to other imaging techniques, DEXA scanners are widely available, radiation exposure and patient burden is low, and cost is modest. With VAT being a fundamentally meaningful measure of cardiometabolic risk and anthropometric measures being unable to discern fat versus lean mass or differentiatie type of fat, recently developed algorithms for assessing VAT by DEXA provide clinically useful information to determine cardiometabolic risk and aid in the design of phenotype specific interventions to reduce risk.
Acknowledgements
This study was supported by a grant from the Dr. Robert C. and Veronica Atkins Foundation to Dr. Silver, NIH K23 HL103976 PhRMA Foundation Career Development Award to Dr. Shibao, and resources from Vanderbilt CTSA award UL1TR000445 from the NIH National Center for Advancing Translational Sciences.
Footnotes
DISCLOSURES:
None of the authors have financial interests to disclose.
REFERENCES
- 1.Lebovitz HE. The relationship of obesity to the metabolic syndrome. International journal of clinical practice Supplement. 2003 Mar;(134):18–27. PubMed PMID: 12793594. [PubMed] [Google Scholar]
- 2.Canoy D. Distribution of body fat and risk of coronary heart disease in men and women. Current opinion in cardiology. 2008 Nov;23(6):591–8. doi: 10.1097/HCO.0b013e328313133a. PubMed PMID: 18830075. [DOI] [PubMed] [Google Scholar]
- 3.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008 Jul;61(7):646–53. doi: 10.1016/j.jclinepi.2007.08.012. PubMed PMID: 18359190. [DOI] [PubMed] [Google Scholar]
- 4.Girard J, Lafontan M. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: Visceral adipose tissue production and liver metabolism. Diabetes & metabolism. 2008 Nov;34(5):439–45. doi: 10.1016/j.diabet.2008.04.002. PubMed PMID: 18562233. [DOI] [PubMed] [Google Scholar]
- 5.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990 Jul-Aug;10(4):493–6. PubMed PMID: 2196039. [PubMed] [Google Scholar]
- 6.Liu J, Coady S, Carr JJ, Hoffmann U, Taylor HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans. Obesity (Silver Spring) 2014 Mar;22(3):811–8. doi: 10.1002/oby.20307. PubMed PMID: 23408700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007 Jul 3;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. PubMed PMID: 17576866. [DOI] [PubMed] [Google Scholar]
- 8.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013 Sep;21(9):E439–47. doi: 10.1002/oby.20135. PubMed PMID: 23687099. Pubmed Central PMCID: 3751977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006 Feb;14(2):336–41. doi: 10.1038/oby.2006.43. PubMed PMID: 16571861. [DOI] [PubMed] [Google Scholar]
- 10.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013 Jun;5(6):2019–27. doi: 10.3390/nu5062019. PubMed PMID: 23739143. Pubmed Central PMCID: 3725490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001 Apr;50(4):425–35. doi: 10.1053/meta.2001.21693. PubMed PMID: 11288037. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014 Feb 26;311(8):806–14. doi: 10.1001/jama.2014.732. PubMed PMID: 24570244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okosun IS, Chandra KM, Boev A, Boltri JM, Choi ST, Parish DC, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960-2000. Preventive medicine. 2004 Jul;39(1):197–206. doi: 10.1016/j.ypmed.2004.01.023. PubMed PMID: 15208003. [DOI] [PubMed] [Google Scholar]
- 14.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007 Dec;15(12):2984–93. doi: 10.1038/oby.2007.356. PubMed PMID: 18198307. Pubmed Central PMCID: 2883307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr. 1994 Jun;59(6):1277–85. doi: 10.1093/ajcn/59.6.1277. PubMed PMID: 8198051. [DOI] [PubMed] [Google Scholar]
- 16.Pascot A, Lemieux I, Prud'homme D, Tremblay A, Nadeau A, Couillard C, et al. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res. 2001 Dec;42(12):2007–14. PubMed PMID: 11734573. [PubMed] [Google Scholar]
- 17.Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004 Dec;27(12):2948–53. doi: 10.2337/diacare.27.12.2948. PubMed PMID: 15562212. [DOI] [PubMed] [Google Scholar]
- 18.Lear SA, Chockalingam A, Kohli S, Richardson CG, Humphries KH. Elevation in cardiovascular disease risk in South Asians is mediated by differences in visceral adipose tissue. Obesity (Silver Spring) 2012 Jun;20(6):1293–300. doi: 10.1038/oby.2011.395. PubMed PMID: 22282045. [DOI] [PubMed] [Google Scholar]
- 19.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr., Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010 Jan;91(1):7–15. doi: 10.3945/ajcn.2009.28136. PubMed PMID: 19828714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Coady S, Carr JJ, Md UH, Taylor HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between african and european americans. Obesity (Silver Spring) 2013 Feb 14; doi: 10.1002/oby.20307. PubMed PMID: 23408700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013 Jan;93(1):359–404. doi: 10.1152/physrev.00033.2011. PubMed PMID: 23303913. [DOI] [PubMed] [Google Scholar]
- 22.Black HR. The burden of cardiovascular disease: following the link from hypertension to myocardial infarction and heart failure. American journal of hypertension. 2003 Sep;16(9 Pt 2):4S–6S. doi: 10.1016/s0895-7061(03)00969-5. PubMed PMID: 14511895. [DOI] [PubMed] [Google Scholar]
- 23.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008 Mar;16(3):600–7. doi: 10.1038/oby.2007.92. PubMed PMID: 18239557. [DOI] [PubMed] [Google Scholar]
- 24.Barreira TV, Staiano AE, Harrington DM, Heymsfield SB, Smith SR, Bouchard C, et al. Anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clin Proc. 2012 May;87(5):452–60. doi: 10.1016/j.mayocp.2011.12.017. PubMed PMID: 22560524. Pubmed Central PMCID: 3498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004 Mar;79(3):379–84. doi: 10.1093/ajcn/79.3.379. PubMed PMID: 14985210. [DOI] [PubMed] [Google Scholar]
- 26.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008 Oct 11;372(9646):1281–3. doi: 10.1016/S0140-6736(08)61531-7. PubMed PMID: 18929889. [DOI] [PubMed] [Google Scholar]
- 27.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011 Jul;35(7):971–81. doi: 10.1038/ijo.2010.216. PubMed PMID: 20975726. [DOI] [PubMed] [Google Scholar]
- 28.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008 Aug 11;168(15):1617–24. doi: 10.1001/archinte.168.15.1617. PubMed PMID: 18695075. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003 Feb;77(2):379–84. doi: 10.1093/ajcn/77.2.379. PubMed PMID: 12540397. [DOI] [PubMed] [Google Scholar]
- 30.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. International journal of obesity. 2007 Mar;31(3):500–6. doi: 10.1038/sj.ijo.0803454. PubMed PMID: 16953256. [DOI] [PubMed] [Google Scholar]
- 31.Liou TH, Chan WP, Pan LC, Lin PW, Chou P, Chen CH. Fully automated large-scale assessment of visceral and subcutaneous abdominal adipose tissue by magnetic resonance imaging. Int J Obes (Lond) 2006 May;30(5):844–52. doi: 10.1038/sj.ijo.0803216. PubMed PMID: 16418756. [DOI] [PubMed] [Google Scholar]
- 32.Silver HJ, Welch EB, Avison MJ, Niswender KD. Imaging body composition in obesity and weight loss: challenges and opportunities. Diabetes, metabolic syndrome and obesity : targets and therapy. 2010;3:337–47. doi: 10.2147/DMSOTT.S9454. PubMed PMID: 21437103. Pubmed Central PMCID: 3047979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012 May;20(5):1109–14. doi: 10.1038/oby.2011.367. PubMed PMID: 22240726. Pubmed Central PMCID: 3343346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treuth MS, Hunter GR, Kekes-Szabo T. Estimating intraabdominal adipose tissue in women by dual-energy X-ray absorptiometry. Am J Clin Nutr. 1995 Sep;62(3):527–32. doi: 10.1093/ajcn/62.3.527. PubMed PMID: 7661113. [DOI] [PubMed] [Google Scholar]
- 35.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord. 2002 Jul;26(7):978–83. doi: 10.1038/sj.ijo.0801982. PubMed PMID: 12080453. [DOI] [PubMed] [Google Scholar]
- 36.Hill AM, LaForgia J, Coates AM, Buckley JD, Howe PR. Estimating abdominal adipose tissue with DXA and anthropometry. Obesity (Silver Spring) 2007 Feb;15(2):504–10. doi: 10.1038/oby.2007.629. PubMed PMID: 17299124. [DOI] [PubMed] [Google Scholar]
- 37.Scherzer R, Shen W, Bacchetti P, Kotler D, Lewis CE, Shlipak MG, et al. Comparison of dual-energy X-ray absorptiometry and magnetic resonance imaging-measured adipose tissue depots in HIV-infected and control subjects. Am J Clin Nutr. 2008 Oct;88(4):1088–96. doi: 10.1093/ajcn/88.4.1088. PubMed PMID: 18842798. Pubmed Central PMCID: 3156610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamel EG, McNeill G, Han TS, Smith FW, Avenell A, Davidson L, et al. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord. 1999 Jul;23(7):686–92. doi: 10.1038/sj.ijo.0800904. PubMed PMID: 10454101. [DOI] [PubMed] [Google Scholar]
- 39.Silver HJ, Niswender KD, Kullberg J, Berglund J, Johansson L, Bruvold M, et al. Comparison of gross body fat-water magnetic resonance imaging at 3 Tesla to dual-energy X-ray absorptiometry in obese women. Obesity (Silver Spring) 2013 Apr;21(4):765–74. doi: 10.1002/oby.20287. PubMed PMID: 23712980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012 Jun;20(6):1313–8. doi: 10.1038/oby.2011.393. PubMed PMID: 22282048. Pubmed Central PMCID: 3361068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. PubMed PMID: 18929686. Pubmed Central PMCID: 2700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 1994 Feb;18(2):79–83. PubMed PMID: 8148928. [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. PubMed PMID: 15161807. [DOI] [PubMed] [Google Scholar]
- 44.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Computer methods and programs in biomedicine. 1986 Oct;23(2):113–22. doi: 10.1016/0169-2607(86)90106-9. PubMed PMID: 3640682. [DOI] [PubMed] [Google Scholar]
- 45.Avignon A, Boegner C, Mariano-Goulart D, Colette C, Monnier L. Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes Relat Metab Disord. 1999 May;23(5):512–7. doi: 10.1038/sj.ijo.0800864. PubMed PMID: 10375055. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013 Jan;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. PubMed PMID: 23264425. Pubmed Central PMCID: 3537273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994 Apr;43(4):587–92. doi: 10.2337/diab.43.4.587. PubMed PMID: 8138065. [DOI] [PubMed] [Google Scholar]
- 48.American Heart A, National Heart L. Blood I, Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiology in review. 2005 Nov-Dec;13(6):322–7. PubMed PMID: 16708441. [PubMed] [Google Scholar]
- 49.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. The Proceedings of the Nutrition Society. 2011 Nov;70(4):408–17. doi: 10.1017/S0029665111000565. PubMed PMID: 21835098. [DOI] [PubMed] [Google Scholar]
- 50.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006 Mar;17(1):4–12. PubMed PMID: 16613757. [PubMed] [Google Scholar]
- 51.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010 Mar 25;316(2):129–39. doi: 10.1016/j.mce.2009.08.018. PubMed PMID: 19723556. [DOI] [PubMed] [Google Scholar]
- 52.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010 May;18(5):879–83. doi: 10.1038/oby.2010.22. PubMed PMID: 20186138. [DOI] [PubMed] [Google Scholar]
- 53.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007 Apr;56(4):1010–3. doi: 10.2337/db06-1656. PubMed PMID: 17287468. [DOI] [PubMed] [Google Scholar]
- 54.Hyatt TC, Phadke RP, Hunter GR, Bush NC, Munoz AJ, Gower BA. Insulin sensitivity in African-American and white women: association with inflammation. Obesity (Silver Spring) 2009 Feb;17(2):276–82. doi: 10.1038/oby.2008.549. PubMed PMID: 19039315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study). Am J Cardiol. 2004 May 15;93(10):1238–42. doi: 10.1016/j.amjcard.2004.01.067. PubMed PMID: 15135696. [DOI] [PubMed] [Google Scholar]
- 56.Coll T, Eyre E, Rodriguez-Calvo R, Palomer X, Sanchez RM, Merlos M, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008 Apr 25;283(17):11107–16. doi: 10.1074/jbc.M708700200. PubMed PMID: 18281277. [DOI] [PubMed] [Google Scholar]
- 57.Coll T, Palomer X, Blanco-Vaca F, Escola-Gil JC, Sanchez RM, Laguna JC, et al. Cyclooxygenase 2 inhibition exacerbates palmitate-induced inflammation and insulin resistance in skeletal muscle cells. Endocrinology. 2010 Feb;151(2):537–48. doi: 10.1210/en.2009-0874. PubMed PMID: 20022932. [DOI] [PubMed] [Google Scholar]
- 58.Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol. 2007 Feb;18(1):3–8. doi: 10.1097/MOL.0b013e328011c6e0. PubMed PMID: 17218824. [DOI] [PubMed] [Google Scholar]
- 59.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002 Mar;75(3):492–8. doi: 10.1093/ajcn/75.3.492. PubMed PMID: 11864854. [DOI] [PubMed] [Google Scholar]
- 60.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007 Jan;49(1):27–33. doi: 10.1161/01.HYP.0000251679.87348.05. PubMed PMID: 17116758. [DOI] [PubMed] [Google Scholar]
- 61.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, et al. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001 Sep;38(3):379–83. doi: 10.1161/01.hyp.38.3.379. PubMed PMID: 11566908. [DOI] [PubMed] [Google Scholar]
- 62.Tybor DJ, Lichtenstein AH, Dallal GE, Daniels SR, Must A. Independent effects of age-related changes in waist circumference and BMI z scores in predicting cardiovascular disease risk factors in a prospective cohort of adolescent females. Am J Clin Nutr. 2011 Feb;93(2):392–401. doi: 10.3945/ajcn.110.001719. PubMed PMID: 21147855. Pubmed Central PMCID: 3021431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brummett BH, Babyak MB, Siegler IC, Surwit R, Georgiades A, Boyle SH, et al. Systolic blood pressure and adiposity: examination by race and gender in a nationally representative sample of young adults. American journal of hypertension. 2012 Feb;25(2):140–4. doi: 10.1038/ajh.2011.177. PubMed PMID: 21976277. Pubmed Central PMCID: 3642773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajjar I, Kotchen JM, Kotchen TA. Hypertension: trends in prevalence, incidence, and control. Annual review of public health. 2006;27:465–90. doi: 10.1146/annurev.publhealth.27.021405.102132. PubMed PMID: 16533126. [DOI] [PubMed] [Google Scholar]
- 65.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006 Jun;29(6):1263–8. doi: 10.2337/dc06-0062. PubMed PMID: 16732006. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease C Prevention. Prevalence of diabetes and impaired fasting glucose in adults--United States, 1999-2000. MMWR Morbidity and mortality weekly report. 2003 Sep 5;52(35):833–7. PubMed PMID: 12966357. [PubMed] [Google Scholar]
- 67.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr. 2013 Mar;97(3):480–6. doi: 10.3945/ajcn.112.047787. PubMed PMID: 23364010. Pubmed Central PMCID: 3578400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003 May;26(5):1413–20. doi: 10.2337/diacare.26.5.1413. PubMed PMID: 12716798. [DOI] [PubMed] [Google Scholar]
- 69.Katzmarzyk PT, Greenway FL, Heymsfield SB, Bouchard C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in white and African American adults. Obesity (Silver Spring) 2013 Nov;21(11):2221–4. doi: 10.1002/oby.20519. PubMed PMID: 23794256. Pubmed Central PMCID: 3819404. [DOI] [PMC free article] [PubMed] [Google Scholar]