Abstract
Inflammation is an immune response triggered by microbial invasion and/or tissue injury. While acute inflammation is directed toward invading pathogens and injured cells, thus enabling tissue regeneration, chronic inflammation can lead to severe pathologies and tissue dysfunction. These processes are linked with macrophage polarization into specific inflammatory “M1-like” or regulatory “M2-like” subsets. Nitro-fatty acids (NO2-FAs), produced endogenously as byproducts of metabolism and oxidative inflammatory conditions, may be useful for treating diseases associated with dysregulated immune homeostasis. The goal of this study was to characterize the role of nitro-oleic acid (OA-NO2) in regulating the functional specialization of macrophages induced by bacterial lipopolysaccharide or interleukin-4, and to reveal specific signaling mechanisms which can account for OA-NO2-dependent modulation of inflammation and fibrotic responses.
Our results show that OA-NO2 inhibits lipopolysaccharide-stimulated production of both pro-inflammatory and immunoregulatory cytokines (including transforming growth factor-β) and inhibits nitric oxide and superoxide anion production. OA-NO2 also decreases interleukin-4-induced macrophage responses by inhibiting arginase-I expression and transforming growth factor-β production. These effects are mediated via downregulation of signal transducers and activators of transcription, mitogen-activated protein kinase and nuclear factor-κB signaling responses. Finally, OA-NO2 inhibits fibrotic processes in an in vivo model of angiotensin II-induced myocardial fibrosis by attenuating expression of α-smooth muscle actin, systemic transforming growth factor-β levels and infiltration of both “M1-“ and “M2-like” macrophage subsets into afflicted tissue. Overall, the electrophilic fatty acid derivative OA-NO2 modulates a broad range of “M1-” and “M2-like” macrophage functions and represents a potential therapeutic approach to target diseases associated with dysregulated macrophage subsets.
Keywords: nitro-fatty acids, nitro-oleic acid, macrophages, macrophage functional specialization, fibrosis, inflammation
Graphical Abstract
Introduction
A variety of phagocytic cells are the mainstays of the innate immune system, including neutrophils, monocytes and macrophages that accumulate and reside in the inflamed tissues. Macrophage subsets pursue distinct functions in both normative and disease states, and their lifespan may range from hours to months [1]. Specification of macrophage subsets and their association with pathophysiological processes are of significance in understanding mechanisms of tissue pathology and the design of new drug strategies. Macrophages stimulated by interferon-γ (IFN-γ) or Toll-like receptor (TLR) ligands (including bacterial lipopolysaccharide, LPS) undergo classical pro-inflammatory activation, resulting in formation of so-called “M1-like” macrophages [2]. Conversely, macrophages stimulated with interleukin-4 (IL-4) and/or interleukin-13 (reflecting the Th1 – Th2 polarization of T cells) undergo regulatory activation, resulting in formation of “M2-like” macrophages [2, 3]. Although these individual macrophage subsets share some similarities, “M1-like” macrophages are characterized by the production of high levels of reactive oxygen (ROS) and nitrogen oxide (RNS)-derived species (e.g. superoxide anion, O2.- and nitric oxide, NO) and pro-inflammatory cytokines (e.g. interleukin-6, IL-6; tumor necrosis factor-α, TNF-α; and interleukin-1β, IL-1β), yielding both potent microbicidal and tumoricidal activities. In contrast, “M2-like” macrophages are primarily involved in the control of wound healing, tissue repair, and remodeling. Producing mainly interleukin-10 (IL-10) and tumor growth factor-β (TGF-β), and expressing arginase-I, they are considered to have immunomodulatory functions [4-6]. Both macrophage subsets also contribute to the induction and progression of cardiovascular, chronic inflammatory, and autoimmune diseases [7-11].
Despite considerable insight into the mechanisms of macrophage involvement in different pathologies [7-11], only limited therapeutic strategies that modulate macrophage function are employed in clinical practice. Evidence suggests that nitro-fatty acids (NO2-FAs), generated as an adaptive response of organisms to oxidative stress, are pleiotropic signaling mediators that might also be pharmacologically useful [12]. Previous in vivo studies revealed that NO2-FAs in part exert anti-inflammatory actions by targeting “M1-like” macrophages, resulting in a mitigation of pathologies including pulmonary hypertension and atherosclerosis [13-15]. However, there is still only limited information about the effect of NO2-FAs on “M2-like” macrophages and the mechanisms whereby NO2-FA may modulate macrophage polarization towards “M1-“ or “M2-like” phenotypes.
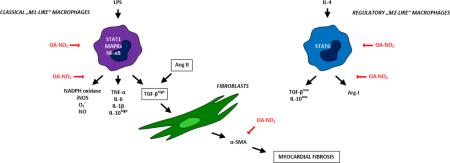
The present study defines the role of nitro-oleic acid (OA-NO2), an exemplary NO2-FA, in the activation of “M1-“ or “M2-like” macrophage subsets. Murine peritoneal macrophages RAW 264.7 and primary macrophages derived from mouse bone marrow (BMDMs) were exposed to different concentrations of OA-NO2 in the presence of LPS or IL-4. Significant emphasis was placed on the specification of signaling pathways hypothesized to be involved in macrophage functional specialization. Those include signal transducer and activator of transcription (STAT) proteins, mitogen activated protein kinases (MAPKs), and nuclear factor-κB (NF-κB) [2, 3], with focus placed on determining cytokine profiles of individual macrophage subsets. The effect of OA-NO2 was also tested in an in vivo model of angiotensin-II (Ang II)-induced myocardial fibrosis [16, 17] that is linked with dysregulation of different macrophage subsets [2, 18, 19]. This data supports that electrophilic lipid signaling mediators can modulate macrophage function in chronic inflammation.
Materials and Methods
Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). The OA-NO2 ((E)-9- and 10-nitro-octadec-9-enoic acids) (Suppl. Fig. 1) was kindly provided by Department of Pharmacology & Chemical Biology (University of Pittsburgh, Pittsburgh, PA, USA). The OA-NO2 was diluted up to 100 mM concentration in methanol and stored at −80°C. Before each experiment, 10 mM solution of OA-NO2 in methanol was prepared from 100 mM stock solution and diluted in Dulbecco's Modified Eagle's Medium (DMEM; PAN-Biotech, Aiden-bach, Germany) to obtain 100 μM of OA-NO2, which was used immediately for cell culture experiments. All stock solutions were prepared and stored in sterile, low-binding tubes.
Cell culture and treatment
Murine peritoneal RAW 264.7 macrophages (ATCC, Manassas, VA, USA) were grown in DMEM with 10% low endotoxin fetal bovine serum (FBS; PAA, Pasching, Austria) and 1% gentamycin.
RAW 264.7 macrophages were treated with different concentrations of OA-NO2 with or without LPS (100 ng/ml, E. coli serotype 026:B6) and IL-4 (20 ng/ml) for different time points. Before each experiment, macrophages were cultured in complete media as indicated above. Two hours before the start of experiments, the complete medium was replaced with serum free DMEM. Different concentrations of OA-NO2 (0.1, 0.25, 0.5, or 1.0 μM), based on their physiological relevance [20-24], were applied together with LPS or IL-4. Cell viability was measured by ATP Cell Viability test (BioThema, Handen, Sweden); no effect of OA-NO2 exposure was detected (data not shown).
Animal model and handling
Wild-type C57BL/6J mice were treated for 2 weeks with Ang II (1.5 ng/g/min) and OA-NO2 (6 mg/kg) solvated in polyethylene-glycol/ethanol (90:10, vol/vol) or vehicle (polyethyleneglycol/ ethanol, 90:10), via subcutaneously implanted osmotic minipumps (ALZET, Cupertino, CA, USA). The heart tissue was isolated immediately after the mice were sacrificed and stored for immunostaining and Western blot experiments. These animal studies were approved by the local authorities and Cologne Animal Care and Use Committees.
Detection of protein expression by Western blot technique
Expression and activation of proteins were detected in cell lysates or heart tissues as described previously [25]. Primary rabbit antibodies against STAT1, phospho-STAT1 (Tyr701), STAT3, phospho-STAT3 (Tyr705), STAT6, phospho-STAT6 (Tyr641), c-Jun N-terminal kinase (SAPK/JNK), phospho-SAPK/JNK (Thr183/Tyr185), extracellular signal-regulated kinase (ERK1/2), phospho-ERK1/2 (Thr202/Tyr204), p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), NF-kB p65, phospho-NF-kB p65 (Ser536), peroxisome proliferator-activated receptor-γ (PPARγ), receptor for macrophage-colony stimulating factor (M-CSFR), TGF-β, GAPDH; primary mouse antibodies against arginase-I, iNOS, β-actin, α-tubulin; and appropriate secondary IgG antibodies (Santa Cruz Biotechnology, Dallas, TX, USA and Cell Signaling Technology, Danvers, MA, USA) were used. Relative levels of proteins were quantified by scanning densitometry using the ImageJ™ program (National Institutes of Health, Bethesda, MD, USA), with the individual band density value expressed in arbitrary units (optical density, OD). The data in graphs represents the ratio between the individual values for OD of bands determined for phosphorylated (p-) or total (t-) form of protein, β-actin or GAPDH.
Determination of the relative cytokine and chemokine levels
Commercially available mouse cytokine ELISA kits (R&D Systems, Minneapolis, MN, USA; eBioscience, San Diego, CA, USA) were used for determination of cytokines in cell supernatants according to the supplier's instructions.
Determination of O2.- formation by cytochrome c reduction assay
The extracellular production of O2.- in macrophages was determined via spectrophotometric analysis of cytochrome c reduction [25]. The concentration of O2.- was calculated using the extinction coefficient of reduced cytochrome c.
Determination of NO production
Changes in NO production were measured indirectly as the accumulation of nitrites, the end product of NO metabolism, in medium using a spectrophotometric Griess assay [25].
Data analysis
Data was statistically analyzed using a Student's t-test or one-way analysis of variance (ANOVA), which was followed by Dunnett's test (Statistica for Windows 8.0/GraphPad Prism 5.01). All data is reported as means ± SEM. A *p value of less than 0.05 was considered significant.
Results
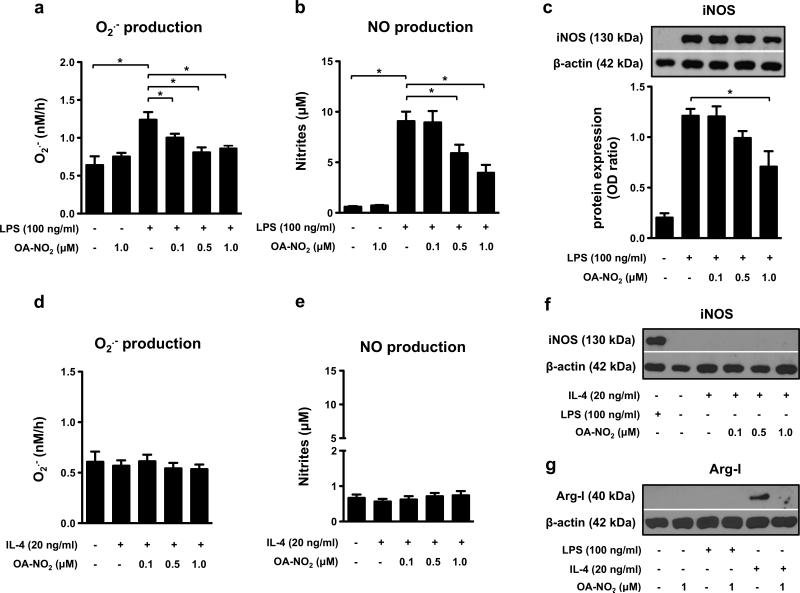
OA-NO2 inhibits O2.- and NO generation by “M1-like” RAW 264.7 macrophages
After activation with LPS (100 ng/ml), RAW 264.7 macrophages markedly increased production of O2.- (detected 4 h after cell stimulation, Fig. 1a), as well as NO production and iNOS expression (detected 24 h after cell stimulation, Fig. 1b, c). As anticipated, IL-4 (20 ng/ml) did not enhance either O2.- or NO production in RAW 264.7 cells treated for 4 and 24 h respectively (Fig. 1d, e). iNOS expression was also not elevated by IL-4 treatment (Fig. 1f). LPS-stimulated O2.- production was inhibited by all concentrations of OA-NO2 used (0.1, 0.5, 1.0 μM) (Fig. 1a). NO production was significantly reduced by 0.5 and 1 μM of OA-NO2 (Fig. 1b) and iNOS expression was inhibited by 1 μM OA-NO2 in RAW 264.7 macrophages (Fig. 1c).
Figure 1. OA-NO2 downregulates O2.- and NO production, iNOS and arginase-I expression in “M1-” and “M2-like” RAW 264.7 macrophages.
Cells were treated with different concentrations of OA-NO2 (0.1, 0.5, 1.0 μM) and stimulated with LPS (100 ng/ml) or IL-4 (20 ng/ml). O2.- production was monitored for 4 h (n=6) (a, d). Production of NO (n=6) (b, e), expression of iNOS (n=3) (c, f) and arginase-I (g) was detected in macrophages incubated for 24 h. The pictures represent one of three individual experiments. A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars and positive control (LPS- and IL-4-treated cells) or between two individual bars, respectively.
OA-NO2 blocks arginase-I expression in “M2-like” RAW 264.7 macrophages
IL-4 enhanced arginase-I production in RAW 264.7 cells treated for 24 h, an effect reversed by 1 μM OA-NO2 (Fig. 1g). Conversely, LPS did not induce arginase-I expression in RAW 264.7 macrophages incubated for 24 h (Fig. 1g).
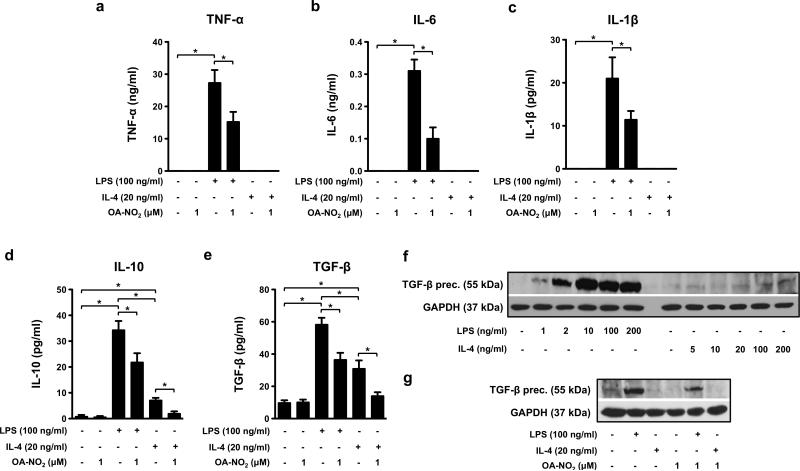
OA-NO2 modulates cytokine expression in “M1-” and “M2-like” RAW 264.7 macrophages
Both pro- and anti-inflammatory cytokine production was altered in RAW 264.7 cells stimulated by LPS (100 ng/ml) or IL-4 (20 ng/ml) and treated with OA-NO2 (1.0 μM) for 24 h. OA-NO2 decreased production of TNF-α, IL-6, and IL-1β in LPS-activated macrophages (Fig 2a-c). Furthermore, OA-NO2 inhibited IL-10 and TGF-β production that was enhanced by LPS and IL-4 (Fig 2d, e). LPS had a greater stimulatory effect on IL-10 and TGF-β expression compared with IL-4 treatment (Fig 2d, e). This response pattern was confirmed by dose-dependent LPS-induced (1, 2, 10, 100, 200 ng/ml) increases in the expression of a TGF-β precursor in cell lysates (Fig. 2f) and TGF-β levels in cell medium (Suppl. Fig. 2). In comparison, IL-4 (5, 10, 20, 100, 200 ng/ml) had only a slight effect on TGF-β precursor expression (Fig. 2f). The stimulatory effect of LPS on the TGF-β precursor was partially ameliorated by OA-NO2 (1.0 μM) (Fig. 2g).
Figure 2. OA-NO2 affects the cytokine profile in “M1-” and “M2-like” RAW 264.7 macrophages.
Cells were treated with OA-NO2 (1.0 μM) and stimulated with LPS (100 ng/ml) or IL-4 (20 ng/ml) for 24 h. TNF-α (a), IL-6 (b), IL-1β (c), IL-10 (d) and TGF-β (e) production was measured in cell supernatants (n=6-8). The expression of TGF-β precursor was monitored in RAW 264.7 macrophages treated with different concentrations of LPS (1-200 ng/ml), IL-4 (5-200 ng/ml), and/or OA-NO2 (1uM) (f). The pictures represent one of three individual experiments. A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars and positive control (LPS- and IL-4-treated cells) or between two individual bars, respectively.
OA-NO2 modulates the “M1-” and “M2-like” phenotype in BMDMs
The evaluation of primary isolated BMDMs confirmed the effects of OA-NO2 on RAW 264.7 macrophages. OA-NO2 (1.0 μM) significantly downregulated LPS-induced O2.- and NO formation (detected 4 and 24 h after cell stimulation, Suppl. Fig. 3a, b), expression of iNOS and arginase-I, as well as production of IL-10 and TGF-β (all measured 24 h after cell stimulation, Suppl. Fig. 3c, d, e) in BMDMs.
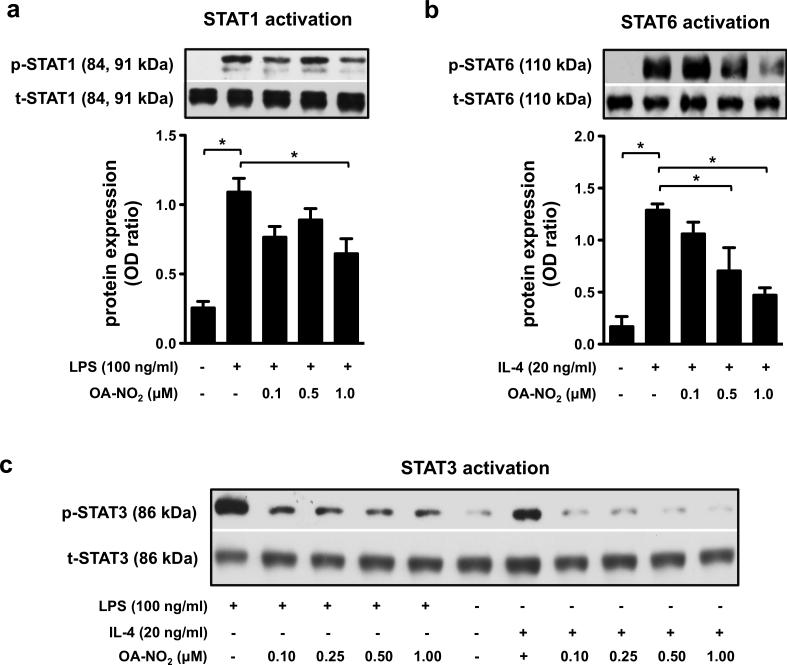
LPS- and IL-4-induced activation of STAT1, STAT3, and STAT6 is regulated by OA-NO2
RAW 264.7 macrophages were exposed to LPS (100 ng/ml) or IL-4 (20 ng/ml) in combination with OA-NO2 (0.1, 0.25, 0.5, 1.0 μM) for 1 h. STAT1 signaling was activated by LPS (Fig. 3a), but not by IL-4 (Suppl. Fig. 4a), in accordance with previous reports [3, 28, 29]. Conversely, STAT6 signaling was activated by IL-4 (Fig. 3b), but not by LPS (Suppl. Fig. 4b), while STAT3 was activated in both LPS- and IL-4-stimulated RAW 264.7 cells (Fig. 3c). Notably, the LPS- and IL-4-mediated phosphorylation of STATs was downregulated by OA-NO2 (Fig. 3a-c).
Figure 3. OA-NO2 downregulates the LPS- and IL-4-induced activation of STAT1, STAT3, and STAT6 in “M1-” and “M2-like” RAW 264.7 macrophages.
The expression and phosphorylation of STATs was detected in RAW 264.7 cells treated with different concentrations of OA-NO2 (0.1, 0.25, 0.5, 1.0 μM) and stimulated with LPS (100 ng/ml) (a, c) or IL-4 (20 ng/ml) (b, c) for 1 h. Expression of p-STAT1/t-STAT1 (a), p-STAT6/t-STAT6 (b), and p-STAT3/t-STAT3 (c) was detected in both “M1-” and “M2-like” macrophages (n=3). The pictures represent one of three individual experiments. A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars and positive control (LPS- and IL-4-treated cells) or between two individual bars, respectively.
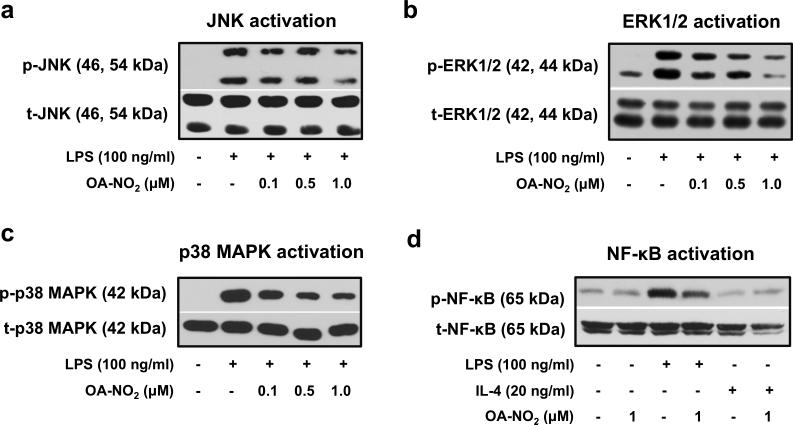
OA-NO2 downregulates activation of MAPKs and NF-κB in the “M1-like” RAW 264.7 macrophages
Phosphorylation of JNK, ERK1/2, p38 MAPK, and NF-κB (measured 1 h or 30 min after cell incubation) was induced by LPS (Fig. 4a-d), but not by IL-4 (Fig. 4d,, Suppl. Fig. 5a-c). LPS-mediated activation of ERK1/2, p38 MAPK, and NF-κB was significantly reduced by OA-NO2 (Fig. 4b-d). Partial (non-significant) inhibition was observed also in the case of JNK activation (Fig. 4a).
Figure 4. OA-NO2 regulates the activation of MAPKs and NF-κB in “M1-like” RAW 264.7 macrophages.
Cells were treated with OA-NO2 (0.1, 0.5, 1.0 μM) and exposed to LPS (100 ng/ml) or IL-4 (20 ng/ml). Phosphorylation of MAPKs (a-c) was detected 1 h after cell stimulation with LPS or IL-4. Expression and activation of NF-κB was monitored in both “M1-” and “M2-like” macrophage subpopulations treated for 30 min (d). The pictures represent one of three individual experiments.
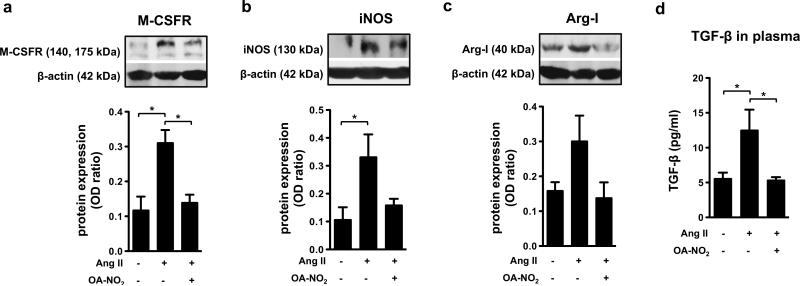
OA-NO2 inhibits Ang II-induced cardiac fibrosis in vivo
Chronic exposure to Ang II (1.5 ng/g/min) in vivo led to the induction of myocardial fibrosis, which is linked with a characteristic enhancement in α-smooth muscle actin (α-SMA) expression (Suppl. Fig. 6a). This fibrosis was also associated with increased expression of M-CSFR, reflecting the accumulation of macrophages in the myocardium (Fig. 5a). These processes were prevented by application of OA-NO2 (Fig. 5a and Suppl. Fig. 6a).
Figure 5. OA-NO2 decreases the Ang II-induced fibrotic processes in heart tissue.
The expression of M-CSFR (a), iNOS (b), and arginase-I (c) was detected in heart tissue of C57BL/6J mice treated for 2 weeks with Ang II (1.5 ng/g/min) and OA-NO2 (6 mg/kg) via subcutaneously implanted osmotic minipumps. The concentration of TGF-β was determined in plasma samples (n=6-9) (d). The pictures represent one of several individual experiments (n=6-9). A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars and positive control (Ang II-treated mice).
The inflammatory phenotype of the heart tissue included increased expression of iNOS and arginase-I, suggesting the presence of both “M1-“ and “M2-like” macrophage subsets (Fig. 5b, c). These changes were accompanied by indices of increased O2.- generation (Suppl. Fig. 6b). All of these parameters were decreased by OA-NO2 administration (Fig. 5b, c and Suppl. Fig. 6b). Additionally, Ang II-induced fibrosis was associated with elevated levels of systemic TGF-β, a response that was completely prevented in OA-NO2 treated mice (Fig. 5d).
OA-NO2 downregulates PPARγ activity but not expression in the “M2-like” RAW 264.7 macrophages
Expression of PPARγ was not significantly changed by LPS (100 ng/ml), IL-4 (20 ng/ml) nor OA-NO2 (1 μM) at 1h (Suppl. Fig. 7a) and 24h after cell stimulation (Suppl. Fig. 7b). On the other hand, the activity of PPARγ was significantly enhanced by IL-4 after 24h (but not 1h) of cell treatment, while this effect was totally reversed by OA-NO2 (Suppl. Fig. 7b). In determination of PPARγ activity, oleic acid (10 μM) was used as a positive control. No significant changes in PPARγ expression were detected in the heart tissue of Ang II- and OA-NO2-treated mice (Suppl. Fig. 7c).
Discussion
This study reveals the changes in functional polarization of “M1-” and “M2-like” macrophage subsets induced by electrophilic lipid signaling mediators such as OA-NO2 in an in vivo model of myocardial fibrosis, shown to be potentially impacted by both macrophage subpopulations [2, 18]. Macrophages exhibit remarkable plasticity by adopting both pro- and anti-inflammatory phenotypes in response to different stimuli and pathophysiological states [11]. During the early phase of an inflammatory response, “M1-like” macrophages produce pro-inflammatory mediators that can exert strong microbicidal and tumoricidal activities. During the later resolution phase of inflammation “M2-like” macrophages enter an anti-inflammatory and tissue-remodeling mode, whereupon they upregulate expression of mediators that resolve inflammation and support wound healing [2, 10]. Since the dysregulation of “M1-” as well as “M2-like” macrophage functions is associated with the development and progression of chronic inflammation and fibrosis [10], macrophages represent important targets for clinical treatment.
The present study reveals novel anti-inflammatory actions of electrophilic lipids such as OA-NO2 on the LPS-induced production of pro-inflammatory mediators and iNOS expression in “M1-like” macrophages. Specifically, OA-NO2 inhibited TNF-α, IL-6, IL-1β, NO, and O2.- formation in “M1-like” macrophages, events that lend mechanistic perspective to in vivo responses reported for lipid electrophiles [13, 14]. OA-NO2 also significantly reduced IL-4-induced “M2-like” phenotype manifestation in both RAW 264.7 macrophages and BMDMs, responses that were linked to decreased production of anti-inflammatory cytokines (IL-10 and TGF-β) and expression of arginase-I, a marker of regulatory “M2-like” macrophages [4, 32, 33]. This data was also unique in that LPS treatment enhanced levels of IL-10 and TGF-β in “M1-like” macrophages, although they are typically described as regulatory or even anti-inflammatory cytokines. The unexpected enhancement of IL-10 in “M1-like” macrophages can be accounted for the ability of IL-10 to downregulate excessive TGF-β action and α-SMA deposition in fibrotic tissue [19].
Since lipid electrophiles act as pleiotropic signaling mediators, defining the mechanisms underlying OA-NO2 actions in the functional polarization of macrophages toward “M1-” and “M2-like” phenotypes requires consideration of multiple signaling pathways that are centrally involved in these processes. This includes STAT, MAPK, NF-kB, PPARγ and ROS signaling [3, 5, 6, 21, 34-43]. STAT signaling is critical for macrophage polarization processes, for example LPS activates JNK-mediated tyrosine phosphorylation and the dimerization of STAT1 [3, 5]. The present data supports that OA-NO2-induced downregulation of NO production and iNOS expression in LPS-stimulated macrophages is mediated by inhibition of STAT1. Previous studies (employing up to 2.5 μM concentration of NO2-FAs) have shown OA-NO2-dependent downregulation of STAT1 activation [3, 20, 35]; and in our experiments even concentrations of 1.0 μM were effective. These concentrations are closer to those detected under in vivo conditions in blood and urine of healthy humans with total levels (free plus esterified form) in the high nM range once dissociated from nucleophilic binding partners [21-23]. Moreover, we report for the first time that OA-NO2 dose-dependently decreased IL-4-induced STAT6 phosphorylation. Both LPS- and IL-4-induced activation of STAT3 was almost completely ablated by OA-NO2, again affirming the broad scope of actions of these mediators. These results support that LPS- and IL-4-induced activation of macrophages triggers a complicated set of signaling networks, wherein both STAT1/6 and STAT3 signaling can cross-coordinate [34, 44]. Multiple actions of STAT3 (induced by LPS and IL-4) can link signaling events in both macrophage subsets, which could also explain enhanced levels of IL-10 and TGF-β detected in LPS-stimulated RAW 264.7 cells and BMDMs. Significantly, the effect of OA-NO2 on cytokine production could be the consequence of potent inhibition of STAT3 activation at all OA-NO2 concentrations.
The relationship between JAK/STAT and MAPK signaling [39] motivated the evaluation of OA-NO2 modulation of MAPK and NF-κB activation in “M1-” and “M2-like” macrophages. We discovered that increased phosphorylation of MAPKs and NF-κB was linked with an “M1-like” macrophage phenotype. OA-NO2 markedly inhibited LPS-induced ERK1/2, p38 MAPK (but not JNK), and NF-κB phosphorylation. These observations reveal for the first time the involvement of OA-NO2 in regulation of ERK1/2 and p38 MAPK activity in “M1-like” macrophages. Interestingly, a similar mechanism was previously found in human monocytes (THP-1), in which OA-NO2 prevented ERK1/2 phosphorylation elicited by phorbol 12-myristate 13-acetate [43]. The inhibitory action of OA-NO2 on NF-κB was recently described in macrophages and endothelial cells exposed to LPS [20, 52], where OA-NO2 reduced vascular inflammation via disruption of the TLR4 signalosome in lipid rafts, events that were upstream of any additional downstream modulation of NF-κB signaling [45]. All of these interactions could potentially modulate fibrogenic processes [46, 56]. It is anticipated that the OA-NO2-dependent reduction of p38 MAPK phosphorylation would also be associated with decreased formation of O2.-, that is dependent on activation of NADPH-oxidase [53]. It is likely that the reduced production of O2.- in “M1-like” macrophages was linked to disrupted assembly of NAPDH oxidase (p47phox and gp91phox subunits), an event reported for OA-NO2-treated neutrophils [30, 31].
PPARγ is a transcription factor associated with “M2-like” macrophage phenotypes [37, 38, 47], and has been identified as one of the molecular targets and mediators of OA-NO2 signaling [41, 42] [48, 49]. Related to this, OA-NO2 blocks phosphorylation and degradation of IκB and enhances the binding of PPARγ to NF-κB, further limiting pro-inflammatory actions of NF-κB, in models of allergic airway disease [50]. OA-NO2-dependent activation of PPARγ expression was also linked with the attenuation of experimental inflammatory bowel diseases [51]. Conversely, many signaling actions of thiol-reactive electrophilic NO2-FAs are mediated via PPARγ-independent mechanisms [20, 29, 43, 52]. In this study, we examined the activity and expression of PPARγ in “M1-” and “M2-like” macrophages and cardiac tissue obtained from mice treated with Ang II and OA-NO2. Only IL-4 significantly increased the activity of PPARγ after 24 h of macrophage stimulation, although it did not affect PPARγ expression. Surprisingly, the stimulatory effect of IL-4 on PPARγ in “M2-like” macrophages was decreased by OA-NO2. We speculate that IL-4- and OA-NO2-mediated regulation of PPARγ activity was dependent on the phosphorylation status of STAT6, a critical event in the activation of PPARγ in “M2-like” macrophages [40].
In macrophage differentiation, the inhibition of ROS production in turn inhibits macrophage-colony stimulating factor induced differentiation of “M2-like” macrophages, but not granulocytemonocyte colony stimulating factor induced “M1-like” subsets [6]. The mechanisms underlying this action involved the biphasic activation of ERK1/2, which is critical for macrophage differentiation [6]. In the present study design, one would not anticipate ROS mitigation of the early phase of macrophage polarization by LPS or IL-4 for multiple reasons. (1) Our experiments used differentiated macrophages (RAW 264.7 and BMDMs), which were treated in an absence of growth factors. The rates of O2.- generation in resting macrophages were low in comparison to extents of LPS-induced O2.- production detected in both subtypes of macrophages. (2) Additionally, there was not enhanced phosphorylation of ERK1/2 (or other MAPKs) in resting or IL-4-treated macrophages.
For in vivo studies, a murine model of fibrosis was selected in part because of the involvement of both “M1-” and “M2-like” macrophage subpopulations in the development of Ang II-induced myocardial fibrosis. Clinical studies have shown a strong and consistent relationship between myocardial fibrosis and chronic inflammation, which is associated with infiltration of heart tissue by “M1-like” macrophages and increased generation of ROS [2, 18]. Also, tissue fibrosis stems from an abnormal healing process regulated by “M2-like” macrophages which fail to respond to specific signals for its termination [19, 36, 54]. The process of fibrosis in general is characterized by increased production of TGF-β, which induces proliferation of tissue fibroblasts, transdifferentiation of fibroblasts to myofibroblasts, and subsequent deposition of excessive extracellular matrix [9-11, 19, 55].
There was a unique effect of OA-NO2 on the functional specialization of macrophages in myocardial fibrosis, that was associated with dysregulation of O2.- generation and TGF-β, α-SMA, M-CSFR, iNOS, and arginase-I expression. The increased concentration of TGF-β, detected in the plasma of Ang II-treated mice, was suppressed by OA-NO2. This response was predicted by in vitro studies showing OA-NO2 inhibition of TGF-β production in both “M1-” and “M2-like” macrophages. Based on these results, it is possible that LPS induces the same pathological enhancement of TGF-β production and signaling in “M1-like”macrophages as observed herein in Ang II-induced myocardial fibrosis. This precept is supported by the observation that TGF-β signaling is upregulated via a TLR4-NF-κB signaling axis, thus providing a link between pro-inflammatory and pro-fibrotic signals [56]. In this scenario, OA-NO2 will limit Ang II- and LPS-induced fibrosis and decrease IL-4-induced TGF-β production in “M2-like” macrophages, thus promoting the regulation of resolution and tissue remodeling. These remodeling processes are further dependent on arginase-I activation, which catalyzes conversion of L-arginine into ornithine and polyamines [2, 10]. We show that the elevated arginase-I expression in regulatory macrophages is attenuated by OA-NO2, further supporting the concept of OA-NO2 mediation of tissue repair.
Concerning the role of macrophages in the in vivo model of Ang II-induced atrial fibrosis, the increased expression of M-CSFR reflected the accumulation of macrophages in the fibrotic tissue. Enhanced iNOS, ROS generation and arginase-I expression implied the increased presence of both “M1-” and “M2-like” phenotypes in afflicted tissue. These markers were repressed by OA-NO2, in part via the decreased STAT3 activation, as described in our in vitro experiments. This assumption is reinforced by the observation that Ang II induces the phosphorylation and nuclear localization of STAT3 and is accompanied by increased levels of pro-fibrotic cytokines (TGF-β and IL-10) [57].
Conclusions
The pleiotropic signaling actions of reversibly-reactive electrophilic lipids such as NO2-FAs can mediate adaptive anti-inflammatory signaling responses that could be therapeutically useful. Physiologically-relevant concentrations of OA-NO2 significantly downregulated the production of reactive inflammatory mediators and pro-inflammatory cytokines (O2.-, NO, TNF-α, IL-6, IL-1β, TGF-β, IL-10) as well as iNOS and arginase-I expression in LPS- or IL-4-elicited “M1-” and “M2-like” macrophage subsets. This effect of OA-NO2 was linked with changes in the regulation of STAT1, STAT3 and STAT6, MAPKs and NF-κB activation. Through inhibition of TGF-β production, OA-NO2 suppressed the genesis and progression of fibrosis in an Ang II-induced model of myocardial fibrosis. These results indicate that NO2-FAs could play an important role in regulation of macrophage functional specialization toward “M1-” and “M2-like” subsets within different pathologies associated with chronic inflammation. It was also observed that “M1-“ and “M2-like” macrophage subpopulations are not strictly separated and can be induced to convert from one phenotype to another in response to the local biochemical milieu.
Supplementary Material
Highlights.
Nitro-oleic acid modulates both ”M1-“ and “M2-like” macrophage functions in vitro.
STATs, MAPKs and NF-κB are critical targets for nitro-oleic acid action.
Nitro-oleic acid decreases systemic TGF-β in angiotensin II-induced fibrosis in vivo.
Macrophage functions are affected by nitro-oleic acid in myocardial fibrosis.
Acknowledgements
We thank Lenka Vystrcilova for excellent technical assistance, Nikol Strakova and Jirina Hofmanova for great support with PPARγ experiments and Ronald M. Evans for providing us with the luciferase reporter construct. This work was supported by the Czech Science Foundation (no. 13-40824P). MP, HM, and AK were supported by the Academy of Sciences of the Czech Republic (no. M200041208) and Ministry of Education, Youth and Sports (no. LD15069). LK was supported by the European Regional Development Fund, project FNUSA-ICRC (no. CZ.1.05/1.1.00/ 02.0123) and BAF by NIH grants R01-HL-058115, R01-HL-64937, PO1-HL-103455.
Abbreviations
- α-SMA
α-smooth muscle actin
- Ang II
angiotensin II
- BMDM
bone marrow-derived macrophage
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-4
interleukin-4
- IL-6
interleukin-6
- IL-10
interleukin-10
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- M-CSFR
receptor for macrophage-colony stimulating factor
- NF-κB
nuclear factor-κ B
- NO2-FA
nitro-fatty acid
- O2.-
superoxide anion
- OA-NO2
nitro-oleic acid
- OD
optical density
- PPARγ
peroxisome proliferator-activated receptor γ
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- TGF-β
transforming growth factor-β
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
BAF and SRW acknowledge an interest in Complexa, Inc., as scientific founder/shareholder (BAF) and consultant (SRW). All other authors confirm that there are no conflicts of interest in this manuscript.
References
- 1.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circulation research. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunologic research. 2012;53:11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews. Immunology. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes. 2013;62:3394–3403. doi: 10.2337/db12-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. Journal of leukocyte biology. 2001;69:598–604. [PubMed] [Google Scholar]
- 6.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell research. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clinical & developmental immunology. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L, Marneros AG. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. The Journal of biological chemistry. 2014;289:8019–8028. doi: 10.1074/jbc.M113.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D. The immunology of fibrosis. Annual review of immunology. 2013;31:107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Seminars in liver disease. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews. Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira AM, Ferrari MI, Trostchansky A, Batthyany C, Souza JM, Alvarez MN, Lopez GV, Baker PR, Schopfer FJ, O'Donnell V, Freeman BA, Rubbo H. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. The Biochemical journal. 2009;417:223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinke A, Moller A, Pekarova M, Ravekes T, Friedrichs K, Berlin M, Scheu KM, Kubala L, Kolarova H, Ambrozova G, Schermuly RT, Woodcock SR, Freeman BA, Rosenkranz S, Baldus S, Rudolph V, Rudolph TK. Protective Effects of 10-Nitro-Oleic Acid in a Hypoxia-Induced Murine Model of Pulmonary Hypertension. Am J Resp Cell Mol. 2014;51:155–162. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:938–945. doi: 10.1161/ATVBAHA.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PRS, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovascular research. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrichs K, Adam M, Remane L, Mollenhauer M, Rudolph V, Rudolph TK, Andrie RP, Stockigt F, Schrickel JW, Ravekes T, Deuschl F, Nickenig G, Willems S, Baldus S, Klinke A. Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PloS one. 2014;9:e89307. doi: 10.1371/journal.pone.0089307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K, Didie M, Seniuk A, von Leitner EC, Szoecs K, Schrickel JW, Treede H, Wenzel U, Lewalter T, Nickenig G, Zimmermann WH, Meinertz T, Boger RH, Reichenspurner H, Freeman BA, Eschenhagen T, Ehmke H, Hazen SL, Willems S, Baldus S. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nature medicine. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covarrubias A, Byles V, Horng T. ROS sets the stage for macrophage differentiation. Cell research. 2013;23:984–985. doi: 10.1038/cr.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, Fang XB, Liu JQ, Tao K, Zhu XX, Tang CW, Hu DH. Protection against TGF-beta1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Archives of dermatological research. 2013;305:341–352. doi: 10.1007/s00403-013-1314-0. [DOI] [PubMed] [Google Scholar]
- 20.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. The Journal of biological chemistry. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisler AC, Rudolph TK. Nitroalkylation--a redox sensitive signaling pathway. Biochimica et biophysica acta. 2012;1820:777–784. doi: 10.1016/j.bbagen.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, Freeman BA, Schopfer FJ. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. Journal of lipid research. 2013;54:1998–2009. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsikas D, Zoerner A, Mitschke A, Homsi Y, Gutzki FM, Jordan J. Specific GC-MS/MS stable-isotope dilution methodology for free 9- and 10-nitro-oleic acid in human plasma challenges previous LC-MS/MS reports. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2009;877:2895–2908. doi: 10.1016/j.jchromb.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. American journal of physiology. Renal physiology. 2010;298:F754–762. doi: 10.1152/ajprenal.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekarova M, Kubala L, Martiskova H, Bino L, Twarogova M, Klinke A, Rudolph TK, Kuchtova Z, Kolarova H, Ambrozova G, Kuchta R, Kadlec J, Lojek A. Asymmetric dimethylarginine regulates the lipopolysaccharide-induced nitric oxide production in macrophages by suppressing the activation of NF-kappaB and iNOS expression. Eur J Pharmacol. 2013;713:68–77. doi: 10.1016/j.ejphar.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Viackova D, Pekarova M, Crhak T, Bucsaiova M, Matiasovic J, Lojek A, Kubala L. Redox-sensitive regulation of macrophage-inducible nitric oxide synthase expression in vitro does not correlate with the failure of apocynin to prevent lung inflammation induced by endotoxin. Immunobiology. 2011;216:457–465. doi: 10.1016/j.imbio.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker PR, Schopfer FJ, O'Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free radical biology & medicine. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. The Journal of biological chemistry. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Jia Z, Soodvilai S, Guan G, Wang MH, Dong Z, Symons JD, Yang T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. American journal of physiology. Renal physiology. 2008;295:F942–949. doi: 10.1152/ajprenal.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circulation research. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 32.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ihle JN. The Stat family in cytokine signaling. Current opinion in cell biology. 2001;13:211–217. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, Freeman BA, Chen YE, Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–4094. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochimica et biophysica acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. Journal of cell science. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 40.Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, Barak Y, Schwabe J, Nagy L. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 2009;116:205–218. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. American journal of physiology. Heart and circulatory physiology. 2007;293:H770–776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G, Ji Y, Li Z, Han X, Guo N, Song Q, Quan L, Wang T, Han W, Pang D, Ouyang H, Tang X. Nitro-oleic acid downregulates lipoprotein-associated phospholipase A2 expression via the p42/p44 MAPK and NFkappaB pathways. Scientific reports. 2014;4:4905. doi: 10.1038/srep04905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed ST, Ivashkiv LB. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J Immunol. 2000;165:5227–5237. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- 45.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing research reviews. 2015 doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW, Henson PM. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol. 2008;181:3575–3585. doi: 10.4049/jimmunol.181.5.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, Xu HE. Molecular recognition of nitrated fatty acids by PPAR gamma. Nature structural & molecular biology. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NKH, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang JF, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PRS, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent Peroxisome Proliferator-activated Receptor gamma Adduction by Nitro-fatty Acids SELECTIVE LIG- AND ACTIVITY AND ANTI-DIABETIC SIGNALING ACTIONS. Journal of Biological Chemistry. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy AT, Lakshmi SP, Reddy RC. The Nitrated Fatty Acid 10-Nitro-oleate Diminishes Severity of LPS-Induced Acute Lung Injury in Mice. PPAR research. 2012;2012:617063. doi: 10.1155/2012/617063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free radical biology & medicine. 2010;48:499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovascular research. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen HL, Bai H, Hai CX. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free radical biology & medicine. 2011;51:539–551. doi: 10.1016/j.freeradbiomed.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Iwata Y, Yoshizaki A, Komura K, Shimizu K, Ogawa F, Hara T, Muroi E, Bae S, Takenaka M, Yukami T, Hasegawa M, Fujimoto M, Tomita Y, Tedder TF, Sato S. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. The American journal of pathology. 2009;175:649–660. doi: 10.2353/ajpath.2009.080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature medicine. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 57.Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, Cheng J, Du J. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.