Abstract
Very little prospective research investigates how cannabis withdrawal is associated with treatment outcomes, and this work has not used the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5) thresholds for cannabis withdrawal. The sample included 110 emerging adults entering outpatient substance use treatment who were heavy cannabis users with no other drug use and limited alcohol use. We used survival analyses to predict days to first use of cannabis and logistic regression to predict whether participants were abstinent and living in the community at 3 months. Those meeting criteria for cannabis withdrawal were more likely to return to use sooner than those not meeting criteria for cannabis withdrawal. However, the presence of cannabis withdrawal was not a significant predictor of 3-month abstinence. Emerging adults with DSM-5 cannabis withdrawal may have difficulty initiating abstinence in the days following their intake assessment, implying the need for strategies to mitigate their more rapid return to cannabis use.
Keywords: cannabis withdrawal, emerging adults, substance abuse treatment, cannabis dependence, relapse
Background
Cannabis use or dependence has been identified in more than 200 countries across the globe with 125 to 227 million people reporting use (Degenhardt et al., 2011; UNODC, 2014). Cannabis use continues to gain popularity across the world with countries like New Zealand showing similar prevalence rates (41.9%) as the United States (42.1%) and nearly no use in Asia, the Middle East, or Africa (Copeland & Swift, 2009; Degenhardt et al., 2011). Emerging adults (ages 18-25) in New Zealand, the United States, and the Netherlands have the highest prevalence rates with 62%, 54%, and 35% reporting use, respectively (Degenhardt et al., 2011). In the United States, cannabis is the most commonly used illicit substance, with 18.1 million persons 12 and older reporting use (Substance Abuse and Mental Health Services Administration [SAMHSA], 2012). Emerging adults have the highest past month cannabis prevalence (19%; SAMHSA, 2012), and heavy cannabis use renders individuals more susceptible (one in two chances) of becoming dependent (Hall & Pacula, 2003). Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-V; American Psychiatric Association [APA], 2013) is the first to include a diagnostic code for cannabis withdrawal (criteria for DSM-5 cannabis withdrawal appear in Table 1). This decision was based on findings that cannabis withdrawal was a distinct symptom of cannabis dependence (independent of tolerance) and a source of rapid relapse (use of cannabis to relieve withdrawal symptoms) in laboratory and outpatient studies (Agrawal, Pergadia, & Lynskey, 2008; Budney & Hughes, 2006; Copersino et al., 2006; Cornelius, Chung, Martin, Wood, & Clark, 2008; Kouri & Pope, 2000; Levin et al., 2010; Smith, 2002). Currently, the DSM-5 cites that a person would need to report experiencing three or more symptoms (including psychological, behavioral, and physiological) 1 week after cessation of heavy use to be diagnosed with cannabis withdrawal (Gorelick et al., 2012).
Table 1.
DSM-5 Cannabis Withdrawal Diagnosis Criteria.
| Criterion A | Cessation of cannabis use that has been heavy and prolonged |
| Criterion B | Three or more of the following develop within several days after Criterion A. (a) Irritability, anger, or aggression; (b) nervousness or anxiety; (c) sleep difficulty; (d) decreased appetite of weight loss; (e) restlessness; (f) depressed mood; and (g) at least one of the following physical symptoms causing significant distress: stomach pain, shakiness/tremors, sweating, fever, chills, and headache. |
| Criterion C | The symptoms in Criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| Criterion D | The symptoms are not due to a general medical condition and are not better accounted for by another disorder |
Source. American Psychiatric Association (2013).
Note. DSM-5 = Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
In a report by the World Health Organization, drug use, specifically cannabis use, was highest in the United States compared with other countries (Copeland & Swift, 2009; Degenhardt et al., 2008). Across the world, cannabis use rates are higher among younger adults compared with older adults (Degenhardt et al., 2008). Interestingly, the World Health Organization noted that the uneven distribution of drug use (i.e., countries with high prevalence rates compared with low rates) was not due to drug policy, given countries with more strict illicit drug policies did not have significantly lower use than countries with more liberal policies (Degenhardt et al., 2008). Advocates of liberal cannabis policies critique whether a withdrawal syndrome specific to cannabis use truly exists, leading some to question whether physiological dependence also exists (Budney, Hughes, Moore, & Vandrey, 2004). Therefore, the purpose of this study was to test whether the presence of a proxy for DSM-5 cannabis withdrawal at treatment intake assessment predicted fewer days to first cannabis use (e.g., the number of postassessment days before returning to use) or abstinence in the community (days of no use while living in the community) at 3 months.
Effects and Treatment of Cannabis Withdrawal
Cannabis withdrawal usually occurs 1 to 2 days after cessation of heavy use (Arendt, Rosenberg, Foldager, Sher, & Munk-Jørgensen, 2007) and can last between 7 and 14 days (Budney, Moore, Vandrey, & Hughes, 2003; Kouri & Pope, 2000). Some studies illustrate that heavy cannabis users exhibit withdrawal symptoms (Budney et al., 2004; Budney et al., 2003; Budney, Novy, & Hughes, 1999; Cornelius et al., 2008; Crowley, Macdonald, Whitmore, & Mikulich, 1998; Lee et al., 2013; Vandrey, Budney, Kamon, & Stanger, 2005), with more withdrawal symptoms being experienced by treatment seeking individuals or those diagnosed with dependence (Budney et al., 2004; Vandrey et al., 2005). Withdrawal symptoms are typically emotional and behavioral (e.g., anxiety, irritability, lowered concentration) with few studies finding evidence for pronounced physical symptoms of withdrawal (e.g., seizures, hypertension; Budney & Hughes, 2006; Budney et al., 2004; Kouri & Pope, 2000). Studies have shown that withdrawal symptoms, especially psychological ones, can last upward of 5 weeks (Copersino et al., 2006; Kouri & Pope, 2000).
Given the substantial impact withdrawal symptoms can have on an individual’s ability to remain abstinent, several studies have investigated the impact of medications (nefazodone, bupropion, and divalproex) on cannabis withdrawal (Allsop et al., 2014; Haney et al., 2008; Haney et al., 2004; Haney, Hart, Ward, & Foltin, 2003; Haney et al., 2001). Clinical trials have found the use of medications such as nefazodone, lofexidine, oral delta-9-tetrahydrocannabinol (THC), and nabiximols (combination of THC, cannabidiol [CBD], and other isoprenoids originating from the cannabis plant) to be effective in reducing cannabis withdrawal symptoms (Allsop et al., 2014; Haney et al., 2008; Haney et al., 2004; Haney et al., 2003). Findings from these studies indicated reductions in anxiety symptoms, muscle pain (Haney et al., 2003), feeling “miserable,” experiences of sleep trouble, chills, cravings, and depressed mood (Allsop et al., 2014; Haney et al., 2004). However, studies have also shown negative effects from medication usage such as worsening or increasing withdrawal symptoms such as irritability, depression, anxiety, and feeling tired (Haney et al., 2004; Haney et al., 2001). Allsop and colleagues (2014) also investigated longitudinal (1 month posttreatment) outcomes and found that, compared with placebo, those receiving nabiximols stayed in treatment longer. However, the use of nabiximols did not differ significantly in the frequency of cannabis use, number of reported cannabis-related problems, or number of self-reported cannabis dependence symptoms.
Other nonmedication studies have found cannabis withdrawal to be associated with functional impairment (e.g., impairment of normal, daily activities; Allsop et al., 2012; Allsop, Norberg, Copeland, Fu, & Budney, 2011). Specifically, functional impairment has been associated with increased withdrawal symptom severity, more severe cannabis dependence (i.e., more endorsed symptoms), more days of cannabis use at follow-up, and higher rates of relapse associated with specific withdrawal symptoms such as trouble sleeping, loss of appetite, feelings of anxiousness, mood swings, depressed mood, and feeling tense (Allsop et al., 2012). In an attempt to uncover the most appropriate definition (i.e., symptom count) of cannabis withdrawal, Chung, Martin, Cornelius, and Clark (2008) recruited 214 adolescents entering intensive outpatient treatment programs. Results indicated individuals with more severe withdrawal severity had more problems at 1-year follow-up; however, withdrawal was not predictive of cannabis use (Chung et al., 2008).
Cannabis Withdrawal and Abstinence Outcomes
Only four studies have been identified that investigated the impact of cannabis withdrawal on posttreatment abstinence. Although cannabis withdrawal was associated with difficulty achieving abstinence in two cross-sectional, retrospective-recall studies (Budney, Vandrey, Hughes, Thostenson, & Bursac, 2008; Cornelius et al., 2008), only two studies have prospectively investigated whether cannabis withdrawal predicts posttreatment abstinence (Arendt et al., 2007; Greene & Kelly, 2014).
Arendt et al. (2007) investigated the impact of cannabis withdrawal on abstinence outcomes among emerging adults treated for cannabis dependence 2 years after treatment intake (Arendt et al., 2007). Withdrawal symptoms did not predict abstinence, with approximately one third (33%) of the sample reporting abstinence at follow-up. This study was limited by a small sample size (n = 36) and a long interim period between assessments. It is possible that the long period (2-3 years) between baseline assessment and follow-up obscured any impact cannabis withdrawal may have had on abstinence outcomes. Our study addresses this methodological problem by using a larger sample size and looking at abstinence at 3 months following intake into treatment.
Greene and Kelly (2014) reported that adolescents endorsing one or more withdrawal symptom had equivalent posttreatment outcomes compared with those not experiencing withdrawal (Greene & Kelly, 2014). However, the presence of withdrawal interacted with problem recognition, with those reporting withdrawal and ability to acknowledge problems having better posttreatment outcomes. In essence, for some individuals having cannabis withdrawal could be a motivating factor for individuals to decide to quit using. This study, however, did not evaluate the DSM-5 cannabis withdrawal diagnostic threshold of three or more symptoms, and included a large number of covariates that could have reduced statistical power to test whether cannabis withdrawal predicted posttreatment abstinence.
The sparse prospective research on cannabis withdrawal in clinical samples has also been limited in two other key ways, both of which are addressed in this study. First, follow-up measures have not accounted for the time participants have spent in controlled environments (e.g., inpatient, jail). This is particularly relevant for emerging adults with cannabis problems, as nearly half of emerging adults (n = 1,149; 49%) engage in illegal behavior at intake to treatment, putting them at risk of incarceration (Dennis, White, & Ives, 2009; Smith, Godley, Godley, & Dennis, 2011). Second, many of the studies that have investigated cannabis withdrawal as a predictor of posttreatment abstinence have utilized polysubstance-using samples (e.g., cannabis and nicotine; Budney et al., 2008; Greene & Kelly, 2014). In Greene and Kelly’s (2014) study, almost 30% of those categorized as being in cannabis withdrawal had used cocaine or crack cocaine in the 90 days prior to treatment intake. This raises the possibility that the withdrawal symptoms reported at baseline were not really attributable to cessation of cannabis use, but rather due to the cessation of a number of different substances at the time of treatment intake. We address previous methodological limitations such as not limiting analyses to participants who only use cannabis, and not accounting for days spent in controlled environments during study follow-up periods.
Days to First Substance Use
The concept of days to first use typically refers to the length of time between a formal contact with a substance use disorder treatment professional and the next occurrence of substance use. In this article, days to first use refers to the number of days between receipt of a treatment intake assessment and the next reported use of cannabis. Researchers have found that days to first use for opiate users is a strong predictor of relapse and a return to pretreatment opiate levels (Gossop, Marsden, Stewart, & Treacy, 2001; Gossop, Stewart, Browne, & Marsden, 2002; Gruber, Delucchi, Kielstein, & Batki, 2008; Kertesz, Horton, Friedmann, Saitz, & Samet, 2003). Similar findings have been observed in other illicit drug use such as cocaine (Galloway, Singleton, & The Methamphetamine Treatment Project Corporate Authors, 2009; S. Weiss, 2010; R. D. Weiss et al., 1997). Several researchers have also found that adolescent and adult cannabis users have difficulty achieving initial periods of abstinence (Allsop et al., 2012; Budney, Higgins, Radonovich, & Novy, 2000; Budney & Hughes, 2006; Coffey et al., 2002) and attributed withdrawal symptoms as the reason they were unable to remain abstinent (Allsop et al., 2012; Budney &Hughes, 2006; Budney et al., 2004; Coffey et al., 2002; Copersino et al., 2006). Our study will extend this knowledge by investigating the impact DSM-5 cannabis withdrawal criteria have on days to first cannabis use among a heavy cannabis-using emerging adult sample.
Summary and Hypotheses
In general, more research is needed on emerging adults in substance use treatment (Arnett & Tanner, 2006; M. J. Mason & Luckey, 2003). Cannabis withdrawal symptoms are prevalent in clinical samples (Cornelius et al., 2008; Preuss, Watzke, Zimmermann, Wong, & Schmidt, 2010), and cannabis withdrawal is now a diagnostic code in the DSM-5 (APA, 2013). Although two studies have not found cannabis withdrawal to predict posttreatment abstinence (Arendt et al., 2007), it is possible that by addressing those study’s limitations, cannabis withdrawal at treatment intake will negatively predict abstinence (while residing) in the community (abstinent in the community) at 3 months. In addition, cannabis withdrawal is hypothesized to reduce the number of days to first cannabis use, with those meeting the criteria for DSM-5 cannabis withdrawal having a more rapid return to cannabis use following their treatment intake assessments. Given the few studies that have focused on cannabis withdrawal among emerging adults (Preuss et al., 2010), our study adds to the growing body of literature on emerging adults and also addresses important gaps in the literature regarding cannabis withdrawal and recovery among emerging adults.
Method
Participants and Procedures
Human participants approval was granted by the lead author’s Institutional Review Board (IRB) prior to all analyses. Data were obtained from 88 Center for Substance Abuse Treatment-SAMHSA-funded programs that provide community outpatient substance abuse treatment and whose records feed a national data set managed by the Global Appraisal of Individual Needs Coordinating Center (Dennis, Titus, White, Unsicker, & Hodgkins, 2003). Treatment sites were spread across the United States where agencies were trained to administer the Global Appraisal of Individual Needs (Dennis et al., 2003). Persons entering treatment came from a variety of referral sources, including the juvenile justice system, probation officers, parents, partners, spouses, or self-referral. At treatment entry, each person completed the initial Global Appraisal of Individuals Needs assessment, which covers a wide range of life domains. After the initial assessment, participants were referred to receive treatment (varies depending on site) and completed a follow-up assessment 3 months later.
We analyzed data for emerging adults entering treatment that either reported past year cannabis use or were diagnosed with DSM-IV-TR (4th edition; APA, 2000) cannabis abuse or dependence (N = 3,179 emerging adults). To be included in this study, we selected those that reported heavy and recent cannabis use (≥45 out of 90 days) who made a past week attempt to quit or cut down. To ensure that any reported withdrawal symptoms were attributable to cannabis and not another substance, we excluded individuals reporting other illicit drug use (e.g., cocaine, heroin, methamphetamine, inhalants, etc.), as well as those that binged on alcohol more than 13 days out of the past 90 days. Excluding participants who were frequent heavy episodic drinkers and including only heavy cannabis users allowed us to ensure we could identify participants that were primarily cannabis users and withdrawal symptoms could be uniquely attributed to cannabis use. Furthermore, individuals who used tobacco were included in the sample selection due to a large proportion of individuals who indicated they are current smokers. To adjust for potential confounds that smoking may have on withdrawal criteria, additional analyses were run (see below) to account for tobacco use. The final analysis sample included 110 emerging adults that met these criteria.
Measures
Global Appraisal of Individual Needs
The Global Appraisal of Individual Needs is a reliable and valid semistructured assessment tool (Buchan, Dennis, Tims, & Diamond, 2002; Dennis, Funk, Godley, Godley, & Waldron, 2004) with an empirically validated training and supervision process (Titus et al., 2012) that contains items consistent with the DSM-IV-TR (APA, 2000) criteria for substance use disorders and many common mental health diagnoses. Participants completed the initial Global Appraisal of Individual Needs assessment at baseline (i.e., treatment intake) and the follow-up version at 3 months. Timeline follow-back (Sobell & Sobell, 1992) methods were used to collect data at each time point. That is, during assessments, participants were asked to recall, for example, days of cannabis use in the previous 90 days. Protocol allows for research assistants to provide a calendar to participants and count the number of days of use in the previous 90 days. A recent systematic review on the validity of timeline follow-back methods for cannabis use found high overall agreement between reported levels of use and biological measures (e.g., urine sampling; Hjorthøj, Hjorthøj, & Nordentoft, 2012). These results suggest that when biological measures of reported use are not available, as is the case in this study, using timeline follow-back methods is an acceptable alternative.
Cannabis Withdrawal
Individuals reporting that they attempted to quit, cut down, or limit their use of alcohol and other drugs in the past week were asked about withdrawal symptoms due to stopping or reducing their use of cannabis. To classify individuals as meeting the criteria for DSM-5 cannabis withdrawal, we used the Current Withdrawal Scale, which is a count (22 items) of the psychological and physiological symptoms associated with stopping or cutting down use of alcohol or drugs in the past week. The Current Withdrawal Scale is internally consistent (α = .79; Conrad, Conrad, Riley, Funk, & Dennis, 2010). Although this scale predates the release of the DSM-5 criteria (APA, 2000), it contained all but one of these criteria (i.e., decreased appetite). For this study, we mapped the DSM-5 cannabis withdrawal criteria on to items of the Current Withdrawal Scale to create a proxy for individuals meeting and not meeting criteria. As a test of concurrent validity, we ran a chi-square test between individuals who were coded as meeting cannabis withdrawal criteria and a separate variable that asks participants when the last time the experienced withdrawal problems or used a substance to relieve physiological or psychological problems. Results indicated a significant chi-square test (χ2 = 18.39, p < .0001) with a larger proportion of individuals with cannabis withdrawal endorsing recent withdrawal and using substances to relieve withdrawal problems/physiological symptoms. Individuals who reported three or more symptoms (e.g., scores ≥ 3) were coded (cannabis withdrawal = 1) as having met criteria for a DSM-5 diagnosis of cannabis withdrawal.
Abstinent and in the Community
Participants were coded being abstinent and in the community if they (a) had not used any cannabis in the previous month and (b) reported living in the community versus in a controlled environment such as a jail or treatment facility. A value of 1 indicates that the emerging adult was abstinent in the community.
Days to First Cannabis Use
Days to first cannabis use is a single-item indicator of days to first use after the baseline (i.e., treatment intake) assessment. To construct this variable into an event variable, we used participants retrospective reporting of the number of days until first cannabis use after the baseline assessment. That is, at the 3-month follow-up, participants are asked, “After your last assessment [baseline], how long did you go before you used cannabis.” Values can range from 0 in which the participant used the day of the baseline assessment to 90, indicating that the participant did not use at all in the 3 months between assessments. Thus, lower values indicate more rapid return to cannabis use following the baseline assessment. A variable was created to represent the number of days each participant went before they met criteria for the event (days to first cannabis use). For example, if a participant reported not using cannabis for 2 weeks after the initial baseline assessment, their days to first cannabis use score would be 14.
Data Analysis
We used survival analysis (Singer & Willett, 1993) to address whether event occurrence (i.e., days to first cannabis use) differed between individuals meeting or not meeting criteria for cannabis withdrawal. Survival analysis accounts for the possibility that not all individuals in the sample will experience a particular event, or censored cases (i.e., use within a 90-day period between assessments). Censored cases can be thought of as having provided incomplete data, which survival analysis handles better than other traditional statistical methods (Singer & Willett, 2003).
Analysis Plan
To assess the pattern of days to first cannabis use in the 90 days following baseline assessments, we obtained life-table estimates, which aid in describing event occurrence data. Life-tables plot hazard probabilities of survival and failure. The former indicates not having experienced the event (i.e., no cannabis use) and the latter indicating event occurrence (i.e., cannabis use; Singer &Willett, 2003). Simply put, hazard ratios are ratios of event occurrence probabilities (i.e., cannabis use) for two different groups, such as those emerging adults with or without DSM-5-cannabis withdrawal. Survival probability refers to the probability that a random person will not experience an event (e.g., not use cannabis; Singer & Willett, 2003), and hazard probability is the probability of event occurrence. In this study, both survival and hazard probabilities were calculated separately for individuals who did and did not meet criteria for cannabis withdrawal. We assessed and plotted failure curves using PROC LIFETEST in SAS®. Furthermore, the Log-Rank (Mantel-Haenzel) test was used to assess significant differences between groups, as this test weights differences at all-time intervals equally. To test a multivariate model, we used Cox proportional hazards regression model using PROC PHREG in SAS® to assess hazard functions for individuals diagnosed with cannabis withdrawal while controlling for various demographic and treatment variables. We used −2 log likelihood (−2 Log L) model fit criteria to compare across models when adding covariates such as cannabis dependence diagnosis, gender, race, and days of treatment received in the 90 days prior to the intake assessment. As a secondary analysis, we added each of the withdrawal items into the Cox proportional hazards regression model while controlling for gender and race. This will allow us to determine what specific items may be more predictive of days to first use among individuals diagnosed and not diagnosed with cannabis withdrawal.
Our study also addressed the association between cannabis withdrawal at baseline and abstinence (while residing) in the community at 3-month follow-up. To test whether cannabis withdrawal at treatment intake negatively predicts whether emerging adults are abstinent in the community at 3 months, a logistic regression was conducted with the dichotomous outcome variable (abstinent in the community) and our dichotomized DSM-5-cannabis withdrawal variable as the predictor variable. All covariates used in the survival analysis were also used in the logistic regression analysis. Gender (female = 1) and race (non-White = 1) were entered as control variables. Non-White is defined as any person not solely identifying as “Caucasian” during the initial assessment. We also controlled for days of previous substance use treatment as those individuals who are recidivists (e.g., multiple treatment episodes) are more likely to drop out of treatment, experience continued problems related to substance use, and continue using substances (Mee-Lee, 2013). All parameter estimates are reported as the log odds of a positive response (achieved abstinent in the community status = 1) as a linear combination of the predictor variables. All data were analyzed using SAS® software (SAS Institute Inc. 2011. [Version 9.3], 2011).
Missing Data
To address missing data, we used multiple imputation (k = 20) methods and the PROC MI (EM) algorithm in SAS® software (SAS Institute Inc. 2011 [Version 9.3], 2011). At 3-month followup, 32% of data were missing. The expectation maximization iterates through two steps (expectation and maximization) until the change in estimates meets the convergence criterion. The end result of this procedure is a mean vector and covariance matrix that utilizes all information. Given the assumption that data are missing at random, the expectation maximization algorithm gives unbiased estimates of missing data (Allison, 2002; McLachlan, Krishnan, & Ng, 2008; Schafer & Graham, 2002).
Results
Participants
Participants were emerging adults ages 18 to 25 (M = 19.2, SD = 1.9) and were mostly male (n = 90, 81.8%). The sample was fairly diverse in terms of race and ethnicity with 21.8% (n = 24) White, 34.6% (n = 38) Hispanic, and 26.4% (n = 29) identifying as African American. Participants in this study were primary cannabis users averaging 70.09 (SD = 15.71) days of use in the past 90 days. Participants were not heavy drinkers, averaging 2.08 (SD = 2.98) days of binge drinking (e.g., five or more drinks in one sitting; Schulenberg, O’Malley, Bachman, Wadsworth, & Johnston, 1996; Vik, Carrello, Tate, & Field, 2000; Walters, Vader, & Harris, 2007; Wechsler & Nelson, 2001) and 7.54 (SD = 15.7) days of any alcohol use in the 90 days prior to their baseline assessment. At baseline, participants reported no days of using any other substance other than alcohol (see above) and cannabis. At baseline, 29.9% of individuals reported spending at least 1 day in a controlled environment, with 12% reporting 5 or more days. Similar results were found at 3-month follow-up, with 42.7% reporting at least 1 day in a controlled environment and 20.0% reporting 10 or more days in a controlled environment.
In terms of substance use diagnoses, 28.2% (n = 31) of participants were diagnosed with past year cannabis dependence, and 53.4% (n = 47) reported any lifetime cannabis use disorder. Demographic and substance use characteristics are reported for overall sample and broken out by cannabis withdrawal status in Table 2.
Table 2.
Demographic and Substance Use Characteristics by Total Sample (n = 110) and Withdrawal Status, M (SD) or n (%).
| Baseline |
Three-month follow-up |
||||
|---|---|---|---|---|---|
| Total sample | CW | No CW | CW | No CW | |
| Demographics | |||||
| Age, in years | 19.2 (1.9) | 19.5 (2.17) | 19.2 (1.81) | 19.4 (2.09) | 19.3 (1.83) |
| Female n (%)* | 20 (18.2) | 9 (8.18) | 11 (10.0) | ||
| White n (%)* | 24 (21.8) | 5 (4.55) | 19 (17.3) | ||
| Hispanic n (%)* | 38 (34.6) | 13 (11.8) | 25 (22.7) | ||
| African American n (%)* | 29 (26.4) | 2 (1.82) | 27 (24.6) | ||
| Other n (%) | 19 (17.3) | 7 (6.36) | 12 (10.9) | ||
| Employment n (%) | |||||
| Full-time | 11 (10.0) | 1 (0.91) | 10 (9.09) | 3 (4.48) | 12 (17.9) |
| Part-time | 17 (15.5) | 3 (2.73) | 14 (12.7) | 2 (2.99) | 9 (13.4) |
| Unemployed | 56 (50.9) | 13 (11.8) | 43 (39.1) | 7 (10.5) | 11 (16.4) |
| Marital status n (%) | |||||
| Married | 8 (7.27) | 1 (0.91) | 7 (6.36) | ||
| Education n (%) | |||||
| Enrolled full-time/part-time (college) | 26 (23.6) | 10 (9.09) | 16 (14.6) | 4 (6.00) | 16 (14.6) |
| Last grade completed | 10.9 (1.13) | 11.1 (.997) | 10.8 (1.17) | ||
| Psychiatric disorders | |||||
| Depressive symptom scale* | 3.69 (2.76) | 6.33 (1.86) | 2.83 (2.44) | 3.57 (3.74) | 2.04 (2.36) |
| Generalized anxiety n (%)* | 22.0 (20.0) | 12 (10.9) | 10 (90.9) | 2 (6.67) | 4 (13.3) |
| ADHDa,* | 8.58 (7.66) | 9.89 (5.21) | 5.00 (5.21) | 4.75 (7.09) | 1.60 (3.73) |
| Substance use diagnoses | |||||
| Cannabis dependence n (%) | 31.0 (28.2) | 7 (6.36) | 24 (21.8) | ||
| Lifetime cannabis use disorder n (%) | 47.0 (53.4) | 10 (11.36) | 37 (42.1) | ||
| Days of cannabis useb | 70.1 (15.7) | 73.3 (15.0) | 69.4 (15.0) | 30.0 (24.9) | 24.0 (23.8) |
| Binge drinkingc | 2.02 (2.98) | 2.30 (1.20) | 1.93 (3.06) | 3.82 (10.5) | 1.86 (5.97) |
| Days of alcohol used | 7.54 (12.7) | 8.56 (13.3) | 7.22 (12.5) | 6.35 (10.4) | 6.10 (10.0) |
| Days of crack/cocaine | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.005 (0.01) | 0.002 (0.02) |
| Days of heroin | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Days of methamphetamine | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Days of acid/LSD/hallucinogens | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.05 (0.242) | 0.00 (0.00) |
| Days of speed/uppers | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Days of downers/sedatives | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Days of PCP or angel dust | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Criminal justice | |||||
| Days on probation | 34.9 (41.9) | 27.8 (41.5) | 37.2 (42.0) | 26.2 (41.8) | 40.6 (44.7) |
| Days in a controlled environment | 3.17 (11.1) | 2.33 (7.80) | 3.44 (12.0) | 10.5 (27.7) | 7.04 (22.7) |
Note. CW = Cannabis withdrawal criteria met; ADHD = attention deficit hyperactivity disorder; LSD = lysergic acid diethylamide; PCP = phencyclidine. No CW = cannabis withdrawal criteria not met. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.).
Count of DSM-IV attention deficit hyperactivity disorder symptoms.
Mean days of cannabis use in past 90 days.
Days of drinking five or more drinks or to intoxication in the past 90 days.
Days of alcohol consumption in past 90 days.
p < .05 at baseline.
Prevalence and Withdrawal Severity
The average participant in this study endorsed 2.20 (SD = 2.15) withdrawal criteria. The three most commonly endorsed withdrawal symptoms (see Table 3, for withdrawal characteristics) were feeling sad, tense, or angry (48.2%), having trouble sleeping (40.0%), and restlessness (33.6%). The three least common withdrawal symptoms were having a fever (2.73%), shaky hands (tremors; 9.09%), and stomach ache (10.0%). Participants, on average, were nearly daily cannabis users (70 out of past 90 days) at baseline assessment, thus easily meeting the criterion for heavy use according to the DSM-5 cannabis withdrawal criteria. As our sample had no days of any other drug use at baseline and limited days of drinking (two binge drinking days and 7 days of alcohol use, on average, in the past 90 days), it appears that the withdrawal symptoms reported above are solely attributable to heavy cannabis use and recent cessation. Table 3 reports the percentages of emerging adults in this study endorsing each of the withdrawal criteria.
Table 3.
Endorsed Cannabis Withdrawal Symptoms.
| Variable | Total sample (n = 110): n (%) or M (SD) |
|---|---|
| Feel sad, tense, angry | 53 (48.2) |
| Feel really nervous | 29 (26.4) |
| Have trouble sleeping, too much/little | 44 (40.0) |
| Fidget, pace, have trouble sitting still | 37 (33.6) |
| Throw up or feel like throwing up (stomach) | 11 (10.0) |
| Shaky hands | 10 (9.09) |
| Sweat more/heart race/or goose bumps (chills) | 13 (11.8) |
| Have a fever | 3 (2.73) |
| Have muscle aches (headache) | 14 (12.7) |
| Mean withdrawal symptoms M (SD) | 2.20 (2.15) |
Furthermore, to increase confidence that participants were reporting withdrawal symptoms associated only with cannabis cessation and no other substances, we also examined associations between tobacco cessation and our withdrawal diagnosis variable. Nearly 72% of participants reported smoking tobacco in the past week. Furthermore, 67.3% reported smoking between one and five times per day, 20% between six and 10 times per day, and 11.8% between 11 and 20 times per day. Similar rates were found at 3-month follow-up. Rates of smoking were not significantly different between individuals who reported 3-month abstinence compared with those who were not abstinent. We created a dichotomous variable indicating whether participants reported smoking tobacco on 45 or more days out of the past 90 (on average smoking tobacco every other day), as well as no past week tobacco use (heavy use and recent cessation = 1; heavy use and no past week cessation = 0). Only 0.03% of the sample (n = 3) were coded as individuals that had a recent quit attempt after heavy past 90-day tobacco use. Further chi-square test was conducted between the heavy tobacco use variable and our dichotomous DSM-5 cannabis withdrawal variable. Results indicated a nonsignificant (χ2 = 3.55, df = 1, p > .05) association between heavy tobacco use and cannabis withdrawal. Therefore, we can assume that withdrawal symptoms are not better accounted for by alcohol use (average of 7 days in past 90) or tobacco use.
Days to First Cannabis Use: Hazard and Survivor Probabilities
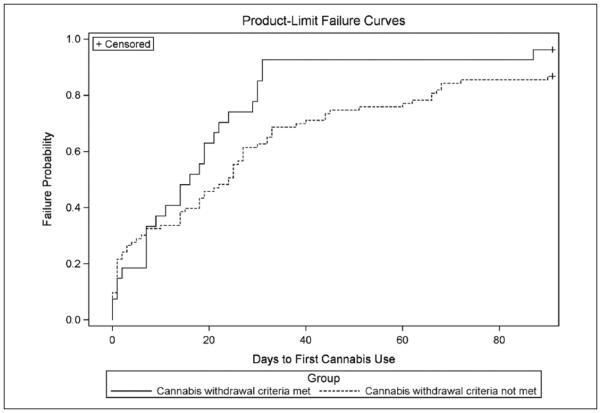
Figure 1 presents the estimated failure curves for days to first cannabis use for individuals meeting and not meeting criteria for cannabis withdrawal. Kaplan–Meier estimates were used for survival estimates. Figure 1 shows similar patterns between the two groups, but indicates that those who meet criteria for DSM-5 cannabis withdrawal have a much steeper incline in risk of using cannabis after an initial substance use assessment. For example, at 30 days postassessment, 85% of those meeting cannabis withdrawal criteria and 62% of those not meeting cannabis withdrawal criteria used cannabis at the 30-day mark. The median values of days to first cannabis use were 16 and 24 (days) for participants meeting and not meeting cannabis withdrawal criteria, respectively. Failure trends were significantly different between those meeting versus those not meeting DSM-5 cannabis withdrawal criteria (log-rank χ2 = 3.88, df = 1, p = .04). Log survival estimates were produced to determine whether an exponential model of survival was needed. Neither curve approximated a straight line through the origin, thus the exponential model was not appropriate.
Figure 1.
Failure probability curve for days to first cannabis use by participants meeting and not meeting DSM-5 cannabis withdrawal criteria.
Note. Time from baseline assessment to first marijuana use. Solid line indicates failure curve for individuals meeting DSM-5 cannabis withdrawal criteria. Dotted line indicates those who did not meet threshold criteria. DSM-5 = Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-V; APA, 2013).
We also found significant survival trends between those meeting and not meeting cannabis withdrawal criteria in Cox regression models that control for various demographic and treatment variables. To ensure we were using the best fitting model, we compared models using −2 log L estimates when covariates were added to the model. Our base model without entering any covariates resulted in a −2 log L of 786.720. After entering our variable of interest (cannabis withdrawal), the −2 log L value decreased to 782.349 (Δχ2 = 4.371, df = 1, p < .05). Demographic and treatment variables (e.g., gender, race, days of previous substance use treatment, and lifetime diagnosis of marijuana dependence) were entered in consecutive models. For parsimony, a binary cannabis dependence variable was removed as it did not provide a better overall fit after gender, race, and previous substance use treatment days were entered into the model (−2 log L = 770.470; Δχ2 = 11.879, df = 3, p < .05). Table 4 provides hazard ratio, confidence intervals, and significance testing results for the Cox regression analysis. Interpretation of our results indicate that individuals meeting criteria for cannabis withdrawal have approximately a 53% (hazard ratio = 1.71) increase in the hazard rate for earlier days to first use compared with those who did not meet criteria (χ2 = 4.34, df = 1, p = .03). Though previous days of substance use treatment was not significant (χ2 = 2.5, df = 1, p = .12), it should be noted that for each day of previous treatment, the hazard rate increased by 1.3%. Furthermore, females had approximately a 13% decrease in the hazard rate compared with males and non-White participants had approximately a 3% decrease in the hazard rate compared with White participants, although these finding were not significant.
Table 4.
Cox Proportional Hazards Regression.
| HR | df | 95% CI | |
|---|---|---|---|
| CW | 1.71* | 1 | [0.985, 2.68] |
| Female | 0.886 | 1 | [0.669, 2.19] |
| Non-White | 0.967 | 1 | [0.654, 1.78] |
| SU Tx | 1.01† | 1 | [0.993, 1.03] |
Note. HR = hazard ratio; CI = confidence interval; CW = DSM-5 cannabis withdrawal; SU Tx = previous substance use treatment days; DSM-5 = Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
p < .10.
p < .05.
Table 5 provides hazard ratios for each individual item of the withdrawal scale. Results indicate that individuals reporting having muscle aches (e.g., headache) have approximately a 77% (hazard ratio = 2.17) increase in the hazard rate for earlier days to first use compared with those who did not meet criteria (χ2 = 5.37, df = 1, p = .02). Furthermore, those who reported having trouble sitting still, pacing, or being fidgety had a 56% increase (hazard ratio = 1.77) in the hazard rate for earlier use in the 3-month follow-up period compared with individuals who did not report this withdrawal item (χ2 = 3.89, df = 1, p = .04). Finally, individuals who reported difficulty sleeping had approximately a 63% decrease in the hazard rate compared with those who did not have trouble sleeping (χ2 = 5.96, df = 1, p = .01).
Table 5.
Cox Proportional Hazards Regression for Individual Items.
| HR | df | 95% CI | |
|---|---|---|---|
| Female | 1.60 | 1 | [0.291, 1.34] |
| Non-White | 0.967 | 1 | [0.469, 1.49] |
| Muscle aches (headache) | 2.17* | 1 | [1.13, 4.12] |
| Shaky hands | 0.833 | 1 | [0.409, 1.70] |
| Sweat more/heart race/or goose bumps (chills) | 1.19 | 1 | [0.567, 2.48] |
| Have a fever | 0.399 | 1 | [0.081, 1.96] |
| Throw up or feel like throwing up (stomach) | 2.05† | 1 | [0.880, 4.79] |
| Have trouble sleeping, too much/little | 0.532* | 1 | [0.320, 0.883] |
| Feel sad, tense, angry | 0.968 | 1 | [0.595, 1.58] |
| Feel really nervous | 0.753 | 1 | [0.422, 1.34] |
| Fidget, pace, have trouble sitting still | 1.77* | 1 | [1.00, 3.11] |
Note. HR = hazard ratio; CI = confidence interval.
p < .10.
p < .05.
Predictors of Abstinence in the Community
Of the 110 participants, 28.2% (n = 31) reported being abstinent in the community at the 3-month follow-up assessment. Table 6 presents findings from the logistic regression models at 3 months. Gender, non-White, and previous days of substance use treatment were not significant predictors of abstinence in the community at 3 months. Though not significant, meeting DSM-5 cannabis withdrawal criteria at baseline (OR = 0.38, df = 1, p = .05) was associated with a lower likelihood of being abstinent in the community at 3 months. For ease of interpretation, we took the inverse of the odds ratio (1 / OR), which is 2.62. This indicates that relative to those meeting cannabis withdrawal criteria, those not meeting the cannabis withdrawal criteria have 2.6 times higher odds of being abstinent in the community at 3 months.
Table 6.
Logistic Regression Results.
| OR | df | 95% CI | |
|---|---|---|---|
| CW | 0.382* | 1 | [0.146, 1.00] |
| Female | 1.03 | 1 | [0.348, 3.04] |
| Non-White | 1.46 | 1 | [0.535, 3.99] |
| SU Tx | 0.989 | 1 | [0.953, 1.03] |
Note. OR = odds ratio; CI = confidence interval; CW = DSM-5 cannabis withdrawal; SU Tx = previous substance use treatment days. DSM-5 = Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
p = .05.
Discussion
The aims of this study were twofold. First, we wanted to extend the literature on the prevalence of cannabis withdrawal among emerging adults entering substance use treatment and improve methodology on the impact cannabis withdrawal has on abstinence 3 months following treatment entry. Second, we wanted to compare the days to first cannabis use after initial baseline assessment for those who met criteria for a proxy of DSM-5 cannabis withdrawal and those who did not.
In our sample, the most common withdrawal symptoms were psychological or behavioral with fewer emerging adults endorsing physiological symptoms. Our results are consistent with other studies that investigated prevalence and severity of withdrawal symptoms (Cornelius et al., 2008; Kouri & Pope, 2000). Specifically, some studies found chronic and current users of cannabis experienced significant increases in withdrawal symptoms such as anxiety, irritability, negative mood, and decreased appetite (Kouri & Pope, 2000).
Impact of Cannabis Withdrawal on Days to First Cannabis Use
The results of this study indicate that having met criteria for cannabis withdrawal does increase the risk of early cannabis use. That is, those individuals who met the threshold for cannabis withdrawal diagnosis were more likely to use cannabis earlier than those who did not meet the threshold. One of the more important findings from this study is that individuals who meet criteria for cannabis withdrawal diagnosis had a 53% increase in risk of using cannabis earlier than those who did not meet criteria. While some cross-sectional studies have shown that cannabis withdrawal symptoms are a reason for relapse (e.g., relief of symptoms; Budney & Hughes, 2006; Budney et al., 2003; Coffey et al., 2002; Copersino et al., 2006), our prospective study extends the literature on the difficulties in initiating abstinence following a treatment intake assessment. We measured cannabis withdrawal at baseline and days to next use at the 3-month assessment using reliable timeline follow-back methods. Thus, our study strengthens the inferences we can make about how withdrawal is associated with the number of days to next cannabis use.
This study underscores the need to carefully measure whether emerging adults meet the full criteria for DSM-5 cannabis withdrawal at the time they enter treatment, and then select appropriate strategies that can help them establish abstinence early in treatment. Additional research is needed to determine how to best extend the number of days that emerging adults with cannabis withdrawal go until their next use following a treatment intake assessment. There are many possible ways to achieve this, including the following: delivering brief motivational interventions immediately following an intake assessment to build motivation, dealing with immediate substance use risks (Carroll et al., 2006; Smith, Davis, Ureche, & Tabb, 2015; Smith, Ureche, Davis, &Walters, 2015), streamlining admissions processes so individuals have same day sessions during which they could make plans for addressing imminent substance use triggers (Garner, Godley, &Funk, 2002), providing immediate access to electronic interventions that focus on developing initial plans to maintain abstinence for a short time until one’s next appointment (Schaub et al., 2013; Walters et al., 2014), encouraging sedentary cannabis users to increase their physical activity during initial cessation attempts (Irons, Babson, Bergeria, & Bonn-Miller, 2014), or delivering pharmacological treatments for those at risk of cannabis withdrawal (Budney, Vandrey, Hughes, Moore, & Bahrenburg, 2007; B. J. Mason et al., 2012). This is especially pertinent as our analyses revealed large increased hazard ratios for individuals reporting physiological (muscle aches) and psychological (trouble sitting still) withdrawal items. In concert with our findings, pharmacological studies have found strong effects for symptom relief for individuals experiencing muscle aches, headaches, trouble sleeping, and feelings of anxiety (Haney et al., 2008; Haney et al., 2004; Haney et al., 2003). Furthermore, our findings regarding a decrease in hazard for individuals reporting sleep problems is puzzling as this is opposite of what previous research has found (Allsop et al., 2012). However, in a review on the effects of cannabis use and sleep patterns, the authors revealed mixed findings on sleep disturbances, especially for heavy or chronic cannabis users (Schierenbeck, Riemann, Berger, & Hornyak, 2008). It may be that heavy cannabis users do not view sleep problems as a result of cannabis use cessation, or it may not be a strong enough symptom to result in earlier days of use. Individuals may be able to find other ways to cope with sleeping problems (e.g., sleep aids, melatonin) better than physiological problems (e.g., muscle aches) and psychological problems (nervous, anxious mood). Furthermore, some studies suggest that the desirable effects of sleep from cannabis use diminish after chronic use (Halikas, Weller, Morse, & Hoffmann, 1985; Schierenbeck et al., 2008). It may be useful for physicians and practitioners to consider both psychotherapy and psychopharmacology when treating heavy cannabis users who are considering immediate cessation of use.
As length of abstinence is negatively associated with cannabis use relapse longitudinally (Flórez-Salamanca et al., 2013), it is important to find ways to prolong the days to first cannabis use early in treatment. Some studies have shown that length of abstinence is a strong predictor of relapse and a return to pretreatment levels of use for illicit drugs such as opiates (Gossop et al., 2001; Gossop et al., 2002; Gruber et al., 2008) and cocaine (R. D. Weiss et al., 1997). However, future research would benefit from producing similar studies for heavy cannabis users.
Impact of Cannabis Withdrawal on Abstinence in the Community Status
Here, we found that a proxy for DSM-5 cannabis withdrawal was not a significant (p = .05) predictor of abstinence in the community at 3 months for heavy cannabis users. It is unclear why cannabis withdrawal symptoms did not predict abstinence in the community at follow-up. It may have been due to a variety of factors that may influence risk of relapse such as psychiatric disorders, type of treatment received, personality, peer influence, other drug use, or age of onset (Andrews, Tildesley, Hops, & Li, 2002; Fergusson, Boden, & Horwood, 2006; Fergusson, Horwood, & Swain-Campbell, 2002; Fergusson, Swain-Campbell, & Horwood, 2002; Grekin, Sher, & Wood, 2006; Moore & Budney, 2002, 2003; Sher, Bartholow, & Wood, 2000; Swift, Hall, & Copeland, 2000). Furthermore, cannabis withdrawal has also been associated with family history of substance problems (Agrawal et al., 2008) indicating individuals that have a history of familial substance use are at increased risk of experiencing cannabis withdrawal and potentially more difficulty achieving abstinence. Second, these results are in concert with similar findings in which cannabis withdrawal did not predict abstinence among emerging adults in treatment that used longer follow-up periods (Arendt et al., 2007). Third, our findings may have been due to how we conceptualized our dependent variable. Previous studies used simple dichotomous measures of abstinence at follow-up. In this study, our abstinence in the community measure corrected for the common problem of days that participants spent in a controlled environment (e.g., jail, hospital). Our participants reported, on average, spending 3.1 (SD = 11.06) and 7.93 (SD = 23.9) days in a controlled environment at baseline and 3 months, respectively. Furthermore, one analysis that may have added to our understanding would be differentiating between a lapse and full relapse. That is, some individuals may have used immediately following the initial baseline assessment, rebounded, and not used for the rest of the follow-up period (lapse), whereas others may have used immediately following the baseline assessment and continued to use (relapse). Being able to differentiate between these two types of “slips” may help explain why cannabis withdrawal did not predict abstinence in the community.
In addition, like other studies, differentiating cannabis withdrawal symptoms from psychopathology creates difficulties measuring the actual impact of cannabis withdrawal. Furthermore, some studies have found that tobacco withdrawal is difficult to distinguish from cannabis withdrawal.
Limitations
The results of this study should be interpreted with caution. As the study only consisted of emerging adults, results may not generalize to older adults or adolescents. We were also unable to control for the quality of treatment received, which could have accounted for longer time to first cannabis use. Furthermore, our study did not control for multiple predictors of posttreatment abstinence, as statistical power would have been reduced with increasingly more predictor variables. For example, variations in treatment quality across sites, mental health severity, tobacco withdrawal, or other indicators of baseline severity known to predict posttreatment abstinence may have better accounted for posttreatment outcomes than simply the presence of withdrawal (Budney et al., 2008). Our study was also only able to use a proxy for DSM-5 cannabis withdrawal as our measure was missing one item (decreased appetite) needed for full criteria. This may affect the outcomes such that upward of 13% of individuals report changes in appetite as a symptom associated with cessation of cannabis use (Chung et al., 2008). Unfortunately, this study is limited in that tobacco withdrawal symptoms were not measured and thus this limitation cannot be tested. However, associations between cannabis withdrawal and days of tobacco use, times smoked per day, past month tobacco dependence, and past week quit attempts were not significant indicating that cannabis withdrawal symptoms were likely not accounted for by use of tobacco. Nevertheless, we did control for gender, race, cannabis withdrawal, and days of previous substance use treatment in all models. In addition, the low percentage of emerging adults with cannabis dependence precluded testing for interaction effects, which may have shed light on whether the number of withdrawal symptoms interacts with other substance-related problems in predicting abstinence in the community status. We were also unable to utilize biological measures (e.g., urine sampling) to ensure accurate reporting of cannabis use. However, recent studies have found that using timeline follow-back methods have high levels of agreement with biological measures when reporting illicit drug use (Hjorthøj et al., 2012). Finally, our exploratory analysis on the hazard ratios for each of the individual withdrawal items would benefit from replication using a larger sample.
In summary, notwithstanding the above-mentioned limitations, this study provides novel data about how the DSM-5 threshold predicts earlier days to first cannabis use. Cannabis withdrawal was a significant predictor of days to first cannabis use at 3-month follow-up; however, aspects of our study such as the impact, tobacco withdrawal, and psychiatric comorbidity warrant further investigation. It appears critical to identify strategies that may assist individuals suffering from cannabis withdrawal to achieve initial abstinence.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (K23AA017702) to D.C.S.
Biographies
Jordan P. Davis is a PhD student in the School of Social Work at the University of Illinois at Urbana– Champaign. His research interests include substance use treatment outcomes among emerging adults and adolescents, impact of treatment on biological markers of stress and genetic expression change, developmental changes following treatment entry, and quantitative methods.
Douglas C. Smith is an associate professor at the University of Illinois at Urbana–Champaign. His research interests include treatment development and the impact of peers on treatment outcomes among emerging adults.
Jason W. Morphew is a PhD student in educational psychology at the University of Illinois at Urbana– Champaign. His research interests include the impact of interventions on cognitive processing and educational outcomes and quantitative methods.
Xinrong Lei is a research specialist in the Child and Family Research Center at the University of Illinois at Urbana–Champaign. Her research interests include child maltreatment and quantitative methods. She is also a SAS programmer and statistical consultant.
Saijun Zhang is a research specialist in the Child and Family Research Center at the University of Illinois at Urbana–Champaign. His research interests include child maltreatment and quantitative methods. He is also a SAS programmer and statistical consultant.
Footnotes
Authors’ Note
The National Institute on Alcohol Abuse and Alcoholism had no role in the study design, collection, analysis or interpretation of results, writing the manuscript, or decisions on publication outlet.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Agrawal A, Pergadia ML, Lynskey MT. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? The American Journal on Addictions. 2008;17:199–208. doi: 10.1080/10550490802019519. [DOI] [PubMed] [Google Scholar]
- Allison PD. Missing data: Quantitative applications in the social sciences. British Journal of Mathematical and Statistical Psychology. 2002;55:193–196. [Google Scholar]
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Norberg MM. Nabiximols as an agonist replacement therapy during cannabis withdrawal: A randomized clinical trial. JAMA Psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, Budney AJ. Quantifying the clinical significance of cannabis withdrawal. PLoS ONE. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: Patterns and predictors of cannabis withdrawal and distress. Drug and Alcohol Dependence. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Author; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th. Author; Washington, DC: 2000. text rev. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Andrews JA, Tildesley E, Hops H, Li F. The influence of peers on young adult substance use. Health Psychology. 2002;21:349–357. doi: 10.1037//0278-6133.21.4.349. [DOI] [PubMed] [Google Scholar]
- Arendt M, Rosenberg R, Foldager L, Sher L, Munk-Jørgensen P. Withdrawal symptoms do not predict relapse among subjects treated for cannabis dependence. The American Journal on Addictions. 2007;16:461–467. doi: 10.1080/10550490701640985. [DOI] [PubMed] [Google Scholar]
- Arnett JJ, Tanner JL, editors. Emerging adulthood: Understanding the new way of coming of age. American Psychological Association; Washington, DC: 2006. [Google Scholar]
- Buchan BJ, Dennis LM, Tims FM, Diamond GS. Cannabis use: Consistency and validity of self-report, on-site urine testing and laboratory testing. Addiction. 2002;97:98–108. doi: 10.1046/j.1360-0443.97.s01.1.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes J. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes J, Moore B, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahy-drocannabinol suppresses cannabis withdrawal symptoms. Drug and Alcohol Dependence. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Obert JL. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug and Alcohol Dependence. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103:787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: An Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Conrad K, Conrad K, Riley B, Funk R, Dennis M. Validation of the Current Withdrawal Scale (CWS) to the Rasch measurement model. Chestnut Health Systems; Chicago, IL: 2010. (GAIN methods report 1.0) [Google Scholar]
- Copeland J, Swift W. Cannabis use disorder: Epidemiology and management. International Review of Psychiatry. 2009;21:96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users*. The American Journal on Addictions. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive Behaviors. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug and Alcohol Dependence. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, GBD Illicit Drug Use Writing Group What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug and Alcohol Dependence. 2011;117:85–101. doi: 10.1016/j.drugalcdep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu W, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Huang Y. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO world mental health surveys. PLoS Medicine. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Funk R, Godley SH, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the global appraisal of individual needs (GAIN) and timeline followback (TLFB; form 90) among adolescents in substance abuse treatment. Addiction. 2004;99:120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Titus JC, White MK, Unsicker JI, Hodgkins D. Global appraisal of individual needs: Administration guide for the GAIN and related measures. Chestnut Health Systems; Bloomington, IL: 2003. [Google Scholar]
- Dennis ML, White M, Ives MI. Chapter 3. Individual characteristics and needs associated with substance misuse of adolescents and young adults in addiction treatment. In: Leukefeld C, Gullotta T, Tindall MS, editors. Handbook on adolescent substance abuse prevention and treatment: Evidence-based practice. Child and Family Agency Press; New London, CT: 2009. pp. 45–72. [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: Testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Swain-Campbell NR, Horwood LJ. Deviant peer affiliations, crime and substance use: A fixed effects regression analysis. Journal of Abnormal Child Psychology. 2002;30:419–430. doi: 10.1023/a:1015774125952. [DOI] [PubMed] [Google Scholar]
- Flórez-Salamanca L, Secades-Villa R, Budney AJ, García-Rodríguez O, Wang S, Blanco C. Probability and predictors of cannabis use disorders relapse: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug and Alcohol Dependence. 2013;132:127–133. doi: 10.1016/j.drugalcdep.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, The Methamphetamine Treatment Project Corporate Authors How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving: Craving and ongoing methamphetamine use. Substance Abuse: Research and Treatment. 2009;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- Garner BR, Godley SH, Funk RR. Evaluating admission alternatives in an outpatient substance abuse treatment program for adolescents. Evaluation and Program Planning. 2002;25:287–294. [Google Scholar]
- Gorelick DA, Levin KH, Copersino ML, Heishman SJ, Liu F, Boggs DL, Kelly DL. Diagnostic criteria for cannabis withdrawal syndrome. Drug and Alcohol Dependence. 2012;123(1):141–147. doi: 10.1016/j.drugalcdep.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Treacy S. Outcomes after methadone maintenance and methadone reduction treatments: Two-year follow-up results from the national treatment outcome research study. Drug and Alcohol Dependence. 2001;62:255–264. doi: 10.1016/s0376-8716(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: Protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents’ treatment response and outcomes: A 12-month prospective investigation. Journal of Addiction Medicine. 2014;8:359–367. doi: 10.1097/ADM.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grekin ER, Sher KJ, Wood PK. Personality and substance dependence symptoms: Modeling substance-specific traits. Psychology of Addictive Behaviors. 2006;20:415–424. doi: 10.1037/0893-164X.20.4.415. [DOI] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug and Alcohol Dependence. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halikas JA, Weller RA, Morse CL, Hoffmann RG. A longitudinal study of marijuana effects. Substance use & Misuse. 1985;20:701–711. doi: 10.3109/10826088509044290. [DOI] [PubMed] [Google Scholar]
- Hall W, Pacula RL. Cannabis use and dependence: Public health and public policy. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin R. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165:157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for selfreported use of cannabis and other illicit substances—Systematic review and meta-analysis. Addictive Behaviors. 2012;37:225–233. doi: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Irons JG, Babson KA, Bergeria CL, Bonn-Miller MO. Physical activity and cannabis cessation. The American Journal on Addictions. 2014;23:485–492. doi: 10.1111/j.1521-0391.2014.12135.x. [DOI] [PubMed] [Google Scholar]
- Kertesz SG, Horton NJ, Friedmann PD, Saitz R, Samet JH. Slowing the revolving door: Stabilization programs reduce homeless persons’ substance use after detoxification. Journal of Substance Abuse Treatment. 2003;24:197–207. doi: 10.1016/s0740-5472(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG., Jr. Abstinence symptoms during withdrawal from chronic marijuana use. Experimental and Clinical Psychopharmacology. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Lee D, Schroeder JR, Karschner EL, Goodwin RS, Hirvonen J, Gorelick DA, Huestis MA. Cannabis withdrawal in chronic, frequent cannabis smokers during sustained abstinence within a closed residential environment. The American Journal on Addictions. 2013;23:234–242. doi: 10.1111/j.1521-0391.2014.12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug and Alcohol Dependence. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Begovic A. A proof-of-concept randomized controlled study of gabapentin: Effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Luckey B. Young adults in alcohol-other drug treatment: An understudied population. Alcoholism Treatment Quarterly. 2003;21(1):17–32. [Google Scholar]
- McLachlan GJ, Krishnan T, Ng SK, editors. The EM algorithm and extensions. John Wiley; Hoboken, NJ: 2008. [Google Scholar]
- Mee-Lee D, editor. The ASAM criteria: Treatment criteria for addictive, substance-related and co-occurring conditions. 3rd The Change Companies; Carson City, NV: 2013. [Google Scholar]
- Moore BA, Budney AJ. Abstinence at intake for marijuana dependence treatment predicts response. Drug and Alcohol Dependence. 2002;67:249–257. doi: 10.1016/s0376-8716(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Watzke AB, Zimmermann J, Wong JWM, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug and Alcohol Dependence. 2010;106:133–141. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2012 national survey on drug use and health: Summary of national findings, NSDUH series H-46. Author; Rockville, MD: 2012. (HHS publication no. [SMA] 13-4795, No. NSDUH Series H-46) [Google Scholar]
- SAS Institute Inc. Missing data: Our view of the state of the art. In: Schafer JL, Graham JW, editors. Base SAS® 9.3 procedures guide. Psychological Methods. Vol. 7. Author; Cary, NC: 2011. 2011. 2002. pp. 147–177. [Version 9.3] [PubMed] [Google Scholar]
- Schaub MP, Haug S, Wenger A, Berg O, Sullivan R, Beck T, Stark L. Can reduce— The effects of chat-counseling and web-based self-help, web-based self-help alone and a waiting list control program on cannabis use in problematic cannabis users: A randomized controlled trial. BMC Psychiatry. 2013;13:305. doi: 10.1186/1471-244X-13-305. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Medicine Reviews. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schulenberg J, O’Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: Trajectories of frequent binge drinking during the transition to young adulthood. Journal of Studies on Alcohol and Drugs. 1996;57:289–304. doi: 10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: A prospective study. Journal of Consulting and Clinical Psychology. 2000;68:818–829. [PubMed] [Google Scholar]
- Singer JD, Willett JB. It’s about time: Using discrete-time survival analysis to study duration and the timing of events. Journal of Educational and Behavioral Statistics. 1993;18:155–195. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- Smith DC, Davis JP, Ureche DJ, Tabb KM. Normative feedback and adolescent readiness to change: A small randomized pilot trial. Research on Social Work Practice. 2015;25:801–814. doi: 10.1177/1049731514535851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Godley SH, Godley MD, Dennis ML. Adolescent community reinforcement approach outcomes differ among emerging adults and adolescents. Journal of Substance Abuse Treatment. 2011;41:422–430. doi: 10.1016/j.jsat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Ureche DJ, Davis JP, Walters ST. Motivational interviewing with and without normative feedback for adolescents with substance use problems: A preliminary study. Substance Abuse. 2015;36:350–358. doi: 10.1080/08897077.2014.988838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NT. A review of the published literature into cannabis withdrawal symptoms in human users. Addiction. 2002;97:621–632. doi: 10.1046/j.1360-0443.2002.00026.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Springer; New York, NY: 1992. pp. 41–72. [Google Scholar]
- Swift W, Hall W, Copeland J. One year follow-up of cannabis dependence among long-term users in Sydney, Australia. Drug and Alcohol Dependence. 2000;59:309–318. doi: 10.1016/s0376-8716(99)00131-3. [DOI] [PubMed] [Google Scholar]
- Titus JC, Smith DC, Dennis ML, Ives M, Twanow L, White MK. Impact of a training and certification program on the quality of interviewer-collected self-report assessment data. Journal of Substance Abuse Treatment. 2012;42:201–212. doi: 10.1016/j.jsat.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) World drug report. United Nations; Vienna, Austria: 2014. [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and Alcohol Dependence. 2005;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik PW, Carrello P, Tate SR, Field C. Progression of consequences among heavy-drinking college students. Psychology of Addictive Behaviors. 2000;14:91–101. [PubMed] [Google Scholar]
- Walters ST, Ondersma SJ, Ingersoll KS, Rodriguez M, Lerch J, Rossheim ME, Taxman FS. MAPIT: Development of a web-based intervention targeting substance abuse treatment in the criminal justice system. Journal of Substance Abuse Treatment. 2014;46:60–65. doi: 10.1016/j.jsat.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ST, Vader AM, Harris TR. A controlled trial of web-based feedback for heavy drinking college students. Prevention Science. 2007;8:83–88. doi: 10.1007/s11121-006-0059-9. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Nelson TF. Binge drinking and the American college students: What’s five drinks? Psychology of Addictive Behaviors. 2001;15:287–291. doi: 10.1037//0893-164x.15.4.287. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C, Muenz LR, Najavits LM, Jansson SB, Thompson HJ. Early prediction of initiation of abstinence from cocaine. The American Journal on Addictions. 1997;6:224–231. [PubMed] [Google Scholar]
- Weiss S. Cross-addiction on campus: More problems for student-athletes. Substance Use & Misuse. 2010;45:1525–1541. doi: 10.3109/10826081003682297. [DOI] [PubMed] [Google Scholar]