Abstract
The use of probiotics is increasing in popularity for both the prevention and treatment of a variety of diseases. While a growing number of well-conducted, prospective, randomized, controlled, clinical trials are emerging and investigations of underlying mechanisms of action are being undertaken, questions remain with respect to the specific immune and physiological effects of probiotics in health and disease. This Review considers recent advances in clinical trials of probiotics for intestinal disorders in both adult and pediatric populations. An overview of recent in vitro and in vivo research related to potential mechanisms of action of various probiotic formulations is also considered.
Introduction
The human gut is a natural reservoir for numerous species of microorganisms. A mutualistic relationship between beneficial symbionts and commensals is important for the maintenance of health and wellbeing; alterations in this balance leads to dysbiosis and ultimately clinical disease expression.1 This ‘symbiotic ecosystem’ is very complex2 and the largest consortium of microorganisms is located in the lumen and outside mucus layer of the colon. This microbial ecosystem has the ability to function as a ‘virtual’ organ system3 that aids host nutrition and maintenance of homeostasis. The fact that the gut microbiome contains 150 times the genetic content of the human body4 is a testament of its importance and highlights the need to understand it better. This Review considers recent advances in clinical trials of probiotics for intestinal disorders in both adult and pediatric populations. In vitro and in vivo research related to potential mechanisms of action of various probiotic formulations is also discussed.
Colonization and diversity
Humans are naturally colonized at birth with microorganisms that inhabit the skin, oral cavity, vagina and gastrointestinal tract.5 Development of the microbiota occurs in early infancy, and is influenced by route of delivery (vaginal versus caesarean section), gestational age (prematurity versus full term), and use of antibiotics in the perinatal period, especially in the neonatal intensive care unit setting. For example, increased intestinal colonization with bifidobacteria, but not lactobacilli, is associated with vaginal births compared with caesarean section deliveries.6 By contrast, caesarean section births are associated with increased colonization by Klebsiella, Enterobacter and Clostridia,7 which are organisms prevalent in hospital settings. Infant feeding patterns can also affect microbial colonization. Breastfeeding reduces intestinal permeability in human infants compared with formula feeding.8 Breast milk contains antibodies, antimicrobial factors, and lactobacilli,9 each of which are important in mediating innate and adaptive immune defenses against microbial pathogens.10 Formula feeding is associated with an increased presence of both Clostridia and Bacteroides in the intestinal tract.7 Therefore, route of delivery and early life feeding patterns have an influence on intestinal colonization.
Early environmental exposure to microbes is fundamental in shaping an individual’s microbiota.11 In rodents the microbiota can be easily altered in early life, but becomes more resistant to change in adulthood.12 In humans, the microbiota evolves during different stages of life, from infancy to adulthood to old age.13 Decreased microbial diversity in infancy seems to be associated with an increased risk of atopic disease later in childhood.14 Decreased diversity of the gut microbiome is a recurring theme in a variety of conditions that are potentially related to dysbiosis, including chronic IBD, chronic diarrhea and necrotizing enterocolitis. Therefore, exposure to certain bacteria in early life and changes in the diversity of the gut microbiome can have a profound effect on health and wellbeing throughout an individual’s life.
Dysbiosis
The composition of the intestinal flora fluctuates over time: for example, with intercurrent infections15 and treatment with oral antibiotics.16 Various disease states also have profound influences on the presence and levels of various bacteria normally present in the human microbiome. Chronic autoimmune inflammatory diseases, including celiac disease,17 type 2 diabetes18 and obesity19 are associated with intestinal dysbiosis. IBD, in particular Crohn’s disease,20 and chronic diarrhea21 are conditions among those recognized to be associated with an altered microbial composition of the large bowel. A recent study in twins revealed that a reduced abundance of the commensal bacterium Fecalibacterium prausnitzii and an increased abundance of Escherichia coli species was associated with an ileal Crohn’s disease phenotype. This dysbiosis correlated better with disease phenotype than with the genotype of the patient.22 This finding is in contrast with a previous study, which found that Crohn’s-disease-associated ulceration was not linked to dysbiosis.23 Dysbiosis has been shown to be associated with protracted diarrhea resulting in a reduction in F. prausnitzii and Bacteroides in such patients.21 The levels of these bacteria return towards normal values following administration of the yeast probiotic Saccharomyces boulardii.21 Although dysbiosis is increasingly recognized in various intestinal diseases,20,21 it remains to be determined whether it is, in fact, a cause-and-effect relationship.
Probiotic organisms
Numerous organisms meet the criteria established by the WHO to define a probiotic: “a live organism, which provides a benefit to the host when provided in adequate quantities” (Table 1).24 S. boulardii, the Gram-negative E. coli strain Nissle 1917, various lactic-acid-producing lactobacilli strains, and a number of bifidobacteria strains are the primary microorganisms classified and studied as probiotic agents. However, not all probiotics are beneficial in all circumstances. The careful selection of specific organisms based on desired clinical outcome is an effective strategy to select appropriate therapy for an ailment.25
Table 1.
Selected organisms that are used as probiotic agents
| Probiotic | Human disease in which benefit is shown |
Animal model in which benefit is shown |
|---|---|---|
| Yeast | ||
| Saccharomyces boulardii | Clostridium difficile infection96,98 | Citrobacter rodentium-induced colitis57 |
| Gram-negative bacteria | ||
| Escherichia coli Nissle 1917 | NA | DSS-induced colitis59 |
| Gram-positive bacteria | ||
| Bifidobacteria bifidum | NA | Rat model of necrotizing enterocolitis91 |
| Bifidobacteria infantis | IBS29 | NA |
|
Lactobacillus rhamnosus GG (used with lactoferrin) |
Sepsis in very low birth weight infants88 | NA |
|
Lactococcus lactis (engineered to produce IL-10 or trefoil factors) |
Crohn’s disease121 | DSS-induced colitis and IL-10−/− mice (spontaneous IBD)120,123 |
| Lactobacillus plantarum 299v | Antibiotic-associated diarrhea100 | IL-10−/− mice (spontaneous IBD)71 |
| Lactobacillus acidophilus | NA | Visceral hyperalgesia40 and C. rodentium-induced colitis67 |
| Lactobacillus rhamnosus | Pediatric antibiotic-associated diarrhea101 |
– |
| Lactobacillus casei | NA | DNBS-induced colitis66 |
| Bacillus polyfermenticus | NA | DSS-induced colitis and TNBS-induced colitis68 |
| Combination regimens | ||
|
Lactobacillus rhamnosus GG combined with Bifidobacterium lactis |
Bacterial infections10 | NA |
|
Lactobacillus rhamnosus combined with Lactobacillus helveticus |
NA |
C. rodentium-induced colitis,56,118 chronic stress,39 and early life stress36 |
| VSL#3 (Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus Lactobacillus bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidocacterium infantis and Streptococcus thermophilus) |
Pouchitis and pediatric ulcerative colitiS76,77 |
DSS-induced colitis,60 IL-10−/− mice (spontaneous IBD; DNA only),72 and SAMP mouse model of spontaneous IBD73 |
Abbreviations: DNBS, dinitrobenzene sulfonic acid; DSS, dextran sodium sulfate; IL-10, interleukin 10; NA, not available; TNBS, trinitrobenzene sulfonic acid.
The inadequate identification in the literature of probiotic strains prevents the verification of published studies and continues to be an obstacle to both investigators and the general population. The merit of employing a single organism versus a combination of probiotic strains also remains a point of ongoing contention, even among experts in the field. Organisms may behave differently when administered in combinations than in isolation. The use of probiotics in combination regimens concerns some investigators, particularly with respect to attempts to define the precise underlying mechanisms of action of a therapy. Such preparations raise the question: which organism is having what influence?
Probiotics in disease
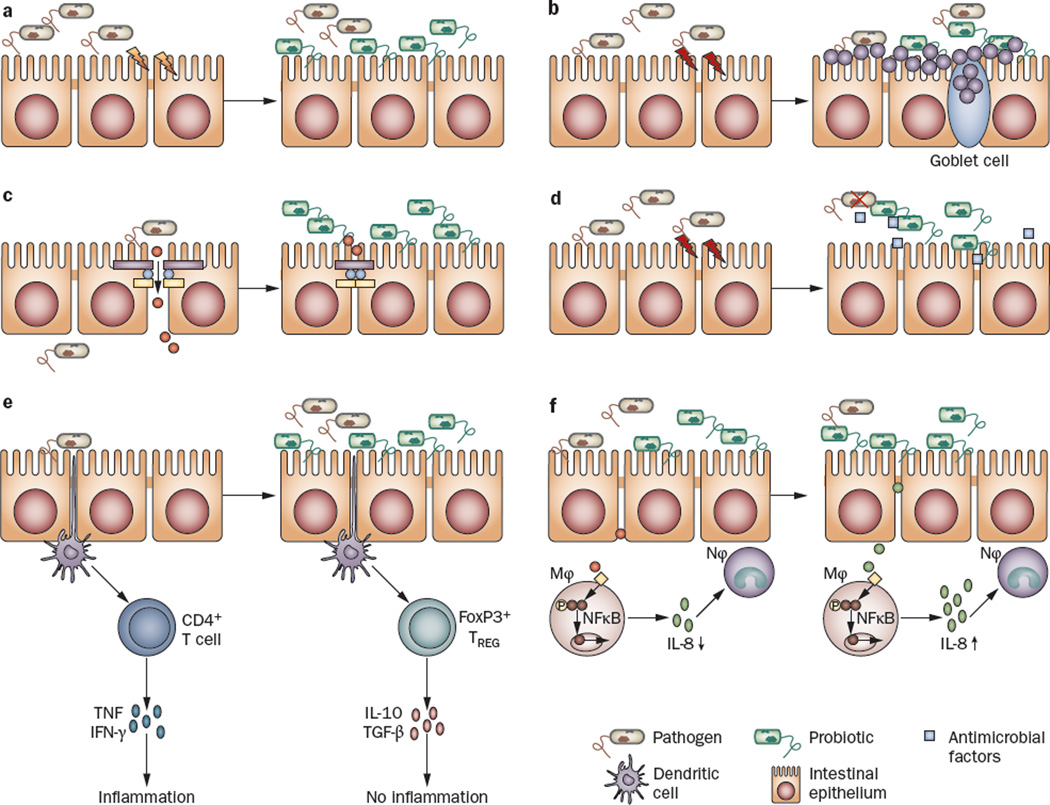
Given that the intestinal tract is the largest reservoir of microbes in the human body, it is not surprising that the use of probiotic organisms in disease has been investigated extensively in intestinal disorders (Table 2). Multiple different putative mechanisms for the reported beneficial effect of probiotics in intestinal disease exist. These mechanisms differ according to the specific strain of probiotic organism and disease model tested, and include maintenance of appropriate host-microbe interactions and pathogen exclusion (Figure 1a), mucus secretion from goblet cells (Figure 1b), modulation of epithelial barrier function (Figure 1c), production of antibacterial factors (Figure 1d) and activation of the host adaptive immune system (Figure 1e,f).26
Table 2.
Effects of probiotics in randomized, clinical trials of intestinal disease
| Disease | Effect |
|---|---|
| Crohn’s disease | Little or no benefit50 |
| Ulcerative colitis | Increased maintenance of remission in children76,77 |
| IBS | Better quality trials required to confirm benefit on clinical symptoms (abdominal pain, bloating, stool consistency)33,34 |
| Clostridium difficile infection | May prevent infection and recurrence of infection95 |
| Infectious diarrhea | Decreases the severity and duration of diarrhea103–105 |
| Antibiotic-associated diarrhea | Prevention of diarrhea101 and reduction of symptoms100 |
| Necrotizing enterocolitis | Reduces mortality84 |
Figure 1.
Potential mechanisms of action of probiotics. Probiotic organisms can provide a beneficial effect on intestinal epithelial cells in numerous ways, a | Some strains can block pathogen entry into the epithelial cell by providing a physical barrier, referred to as colonization resistance55–57 or b | create a mucus barrier by causing the release of mucus from goblet cells, c | Other probiotics maintain intestinal permeability by increasing the intercellular integrity of apical tight junctions, for example, by upregulating the expression of zona-occludens 1 (a tight junction protein),59 or by preventing tight junction protein redistribution60 thereby stopping the passage of molecules into the lamina propria, d | Some probiotic strains have been shown to produce antimicrobial factors, e | Still other strains stimulate the innate immune system by signaling dendritic cells, which then travel to mesenteric lymph nodes and lead to the induction of TREG cells and the production of anti-inflammatory cytokines, including IL-10 and TGF-β. f | Some probiotics (or their products) may also prevent (left-hand side) or trigger (right-hand side) an innate immune response by initiating TNF production by epithelial cells and inhibiting74 (or activitating) NFκB in Mφ and dampening (or priming) the host immune response by influencing the production of IL-8 and subsequent recruitment of Nφ to sites of intestinal injury. Abbreviations: Mφ, macrophage; Nφ, neutrophil; TREG cell, regulatory T cell.
IBS
IBS is a heterogeneous condition often diagnosed by exclusion of other underlying diseases, including IBD. Symptoms include abdominal pain, bloating and altered bowel habits (constipation, diarrhea, or alternating between both). IBS is associated with increased intestinal permeability, altered motility, visceral hypersensitivity, altered bacterial colonization, as well as extraintestinal manifestations, including concomitant anxiety and depression.27 A subgroup of individuals who develop IBS symptoms after an episode of acute enteric infection (commonly Campylobacter, nontyphoidal Salmonella and enterohemorrhagic E. coli O157:H7 [EHEC] infections) are categorized as having postinfectious IBS.28
The use of probiotics for the treatment of patients with IBS remains controversial. Patients with IBS treated with Bifidobacterium infantis, strain 35624, but not Lactobacillus salivarius, strain UCC4331, reported improved quality of life and reduced abdominal pain and bloating in the absence of changes in stool consistency. These improvements in clinical symptoms were associated with increased production of the immunomodulatory cytokine interleukin (IL)-IO in isolated peripheral blood mononuclear cells in vitro.29 A study in women with IBS revealed that treatment with B. infantis, strain 35624, reduced abdominal pain, bloating, bowel dysfunction, incomplete evacuation, straining, and gas, compared with placebo after 4 weeks of therapy30 Altered gut motility was ameliorated in another study of Bifidobacterium lactis, strain DN-173-010, in patients with IBS. Patients with constipation-predominant IBS who received this probiotic demonstrated less abdominal distension and increased colonic transit times compared with patients who received placebo.31 These findings are supported by a multicenter, randomized, double-blind, controlled trial using Bifidobacterium animalis, strain DN-173 010. This trial reported an improved health-related quality of life score in patients with constipation-predominant IBS.32 In a systematic review of the literature, various lactobacilli species, when taken either alone or co-administered with bifidobacteria, resulted in an improvement in symptoms in patients with IBS.33 However, it is important to acknowledge that the appropriateness of the design of studies has been criticized.34 Given the heterogeneity of IBS, research that focuses on the use of probiotics for specific IBS subgroups and for specific IBS-related symptoms is probably the best way forward.
Mechanisms of action in IBS
Epithelial barrier function
Early life stress in neonatal rats followed by acute psychological stress in adulthood is described as a rodent model of IBS.35 This early life stress, resulting from short periods of maternal separation, alters bacterial colonization in the colon of neonatal rats36 and this dysbiosis is sustained into adulthood.37 Oral administration of Lactobacillus helveticus, strain R0052, and Lactobacillus rhamnosus, strain R0011, to neonatal rats that have been exposed to early life stress restores the normal microbiota and prevents stress-induced dysfunctions in colonic epithelial barrier function in adult animals.36 This finding is attributable, in part, to normalization of the hypothalamus-pituitary-adrenal axis, although the precise mechanism is unknown at this time. Chronic psychological stress in rats, such as daily exposure to 1 h of water avoidance, is also shown to be detrimental with respect to gut epithelial barrier function and altered bacteria–host interactions.38 These changes can be ameliorated by the administration of a mixture of L. helveticus, strain R0052, and L. rhamnosus, strain R0011.39 The precise mechanisms by which these probiotics maintain gut epithelial barrier function and prevent intestinal dysbiosis remain to be determined.
Visceral hyperalgesia and motility
Another common symptom of IBS, abdominal pain, occurs due to altered visceral perception. A study conducted in a mouse model of chronic colonic hypersensitivity describes modulation of the murine microflora with Lactobacillus acidophilus, strain NCMF, to induce opioid and cannabinoid receptor expression on intestinal epithelial cells and restore normal perception of visceral pain.40 In a Trichinella spiralis mouse model of IBS, antibiotic-induced visceral hypersensitivity and postinfective muscle hypercontractility are reported to be prevented by treating the animals with Lactobacillus paracasein strain NCC2461.41,42 Furthermore, administration of Lactobacillus reuteri to rats following colorectal distension decreases visceral pain through effects on colonic afferent nerve fibers present in dorsal root ganglia, eliminating cardio-autonomic responses.43 In addition, stress-induced increased Fos protein expression following colorectal distension in rats is reduced after treatment with Lactobacillus farciminis, strain CIP 103136.44 Taken together, these studies highlight the fact that maintenance of epithelial barrier function, changes in gut motility and modulation of visceral pain sensitivity are potential underlying mechanisms of action of probiotics in the setting of stress and IBS.
IBD
In IBD, including Crohn’s disease and idiopathic ulcerative colitis, altered gut permeability, mucosal inflammation and ulceration are present45 Consequently, the use of anti-inflammatory agents and, more recently, biologic agents directed against proinflammatory mediators, such as tumor necrosis factor (TNF), are primary therapeutic options. The intestinal microbiota in patients with IBD seems to drive an overactive immune response, leading to disease expression and concurrent morbidity.46 The potential for probiotics to modulate the microbiota, provide beneficial immunomodulatory effectors, and restore epithelial barrier defects suggests that a probiotic strategy might prove a viable future treatment option for patients with IBD.
There is increasing evidence that an altered gut microbiota has an important role in the pathogenesis of IBD, particularly Crohn’s disease. For example, the abundance of F. prausnitzii, an anti-inflammatory commensal organism, is decreased in patients with Crohn’s disease compared with healthy individuals.47 Absence of this commensal organism in the colonic microflora is also associated with an increased risk of disease recurrence following surgical resection in these patients.47 In addition, adherent-invasive E. coli (AIEC) strains are identified with increased frequency in patients with ileal Crohn’s disease compared with healthy individuals.48 This finding is supported by increased AIEC receptor (carcinoembryonic antigen-related cell adhesion molecule 6, CEACAM6) expression in intestinal biopsy samples from patients with ileal Crohn’s disease compared with samples from healthy controls.49
In practice, however, studies of probiotics in patients with IBD have yielded mixed results with the selection of strains and disease phenotype studied varying considerably50 Despite this, a recent review article indicates a beneficial effect of probiotics for the treatment of ulcerative colitis with pouchitis, but not for the treatment of Crohn’s disease.50 The beneficial effect observed using a combination of eight probiotic bacteria, VSL#3 (Lactobacillus casei, L. plantarum, L. acidophilus, Lactobacillus bulgaricus, Bifidobacterium longum, Bifidobacterium breve, B. infantis and Streptococcus salivaris subspecies thermophilus), in this setting may be, at least in part, mediated by an increase in localized FoxP3 expression.51 This suggests probiotic stimulation of the regulatory T cell (TREG cell) adaptive immune response. In addition, VSL#3 administration is reported to induce remission in patients with ulcerative colitis, as measured by disease activity index.52 Additional studies are required to clearly define the precise roles and settings where the use of probiotics should be considered in patients with IBD.
Mechanisms of action in IBD
Preclinical studies to delineate the mechanisms responsible for the beneficial effects of probiotics in IBD have been undertaken both in vivo and in vitro. Animal studies have focused on models of colitis induced by a variety of compounds, including dextran sodium sulfate (DSS), dinitrobenzene sulfonic acid (DNBS), trinitrobenzene sulfonic acid (TNBS), or alternatively infection with Citrobacter rodentium. In addition, genetic animal models exist including the IL-10 gene knockout and SAMPl/YitFc (SAMP) mouse, which both spontaneously develop colitis.
Pathogen exclusion
Although no single enteric pathogen is associated with the development of disease,53 changes in the gut microbiota have been associated with IBD.54 AIEC strains, isolated from patients with Crohn’s disease, have the ability to bind to and invade intestinal epithelial cells. Such bacteria are significantly inhibited by L. casei, strain DN-114 001, which prevents interaction between bacteria and epithelial cells in vitro.55 Studies using the C. rodentium mouse model of infectious colitis indicate that pretreatment with L. helveticus, strain R0052, and L. rhamnosus, strain R0011, leads to the amelioration of disease activity, in part due to a reduced attachment of the pathogen to colonic epithelial cells.56 Treatment with the yeast probiotic S. boulardii also ameliorates C. rodentium-induced colitis in mice by maintaining intestinal epithelial barrier function and by preventing attachment of the pathogen to colonic epithelial cells.57
Epithelial barrier function
Maintenance of gut epithelial barrier function is important to prevent luminal antigens from entering the body and systemic circulation. Probiotics can be used to maintain normal intestinal epithelial barrier function. In some cases, conditioned medium, whereby secreted products from bacteria are filtered away from the live organisms and then applied in vitro or in vivo, is sufficient to demonstrate a beneficial effect. For example, conditioned culture medium derived from B. infantis is shown to increase transepithelial electrical resistance (TER) in polarized intestinal epithelial cells in vitro and prevents cytokine-induced drops in TER, indicating that this organism secretes a bioactive factor.58 TER is a measure of the integrity of the polarized epithelial barrier; an increase in TER correlates with improved integrity of the barrier. In a mouse model of DSS-induced colitis, E. coli, strain Nissle 1917 upregulates zona-occludens 1 expression in apical tight junctions in intestinal epithelial cells, resulting in reduced gut leakiness and enhanced mucosal integrity59 Similarly, administration of the probiotic mixture VSL#3 to mice maintains apical tight junction integrity in colonic epithelial cells by preventing decreased expression and redistribution of apical tight junction proteins in the setting of DSS-induced colitis.60 The beneficial effect of some probiotics does not require the presence of live bacteria. Heat-killed L. rhamnosus, strain OLL2838, has been shown to protect against mucosal barrier permeability defects in mice with DSS-induced colitis.61
Adaptive immunity
Some probiotics activate anti-inflammatory and regulatory immune effects in the settings of enteric infections and mucosal inflammation. In vitro studies with Shigella flexneri, an invasive Gram-negative bacterium that causes dysentery in humans, showed that L. casei, strain DN-114 001, attenuates the proinflammatory signal transduction cascade that is activated by the pathogen via inhibition of the NFκB signal transduction pathway62 The probiotic E. coli, strain Nissle 1917, induces and maintains anti-inflammatory effects on colonic epithelial cells by inhibiting TNF-induced CXCL8 secretion in a contact-independent manner.63 This feature is unique to this probiotic and was not observed with use of a laboratory E. coli, strain K12, which had no antiinflammatory properties in this setting.63 In contrast to the L. casei study, the inhibitive effects of E. coli, Nissile 1917 occurred in the absence of transactivation via NFκB signaling, which suggests an anti-inflammatory role for probiotics in the context of enteric infection and cytokine activation in epithelial cells. However, the exact role of NFκB signaling as a mediator of the effects of probiotics still remains to be precisely determined.
In mouse models of IBD, L. acidophilus, strain Bar 13, and B. longum, strain Bar 33, used in combination increase the expansion of TREG cells and reduce the number of intraepithelial lymphocytes in TNBS-induced colitis.64 In mice with DNBS-induced colitis, oral administration of L. casei, strain DN-114 001, alleviates disease severity, reflected by an increase in body weight and reduced mucosal inflammatory scores. These effects were attributed to the induction and expansion of colonic CD4+FoxP3+ TREG, cells present in the intestinal lamina propria.65 Some probiotic strains induce regulatory dendritic cells to promote CD4+FoxP3+ TREG cells to home to the site of inflammation, triggering an anti-inflammatory, regulatory immune response.66 Studies in neonatal mice demonstrate that modulation of the host immune response in early life by the oral administration of L. acidophilus, strain NCFM to prime dendritic cells can either prevent or attenuate C. rodentium-induced colitis in adult animals.67 Administration of Bacillus polyfermenticus as a probiotic agent ameliorates both DSS-induced and TNBS-induced colitis in mice, via the suppression of apoptosis and by enhancing epithelial cell proliferation in the colon.68 Similarly, L. casei, strain DN-114 001, has been shown to reduce T-cell apoptosis and cytokine production in vitro in specimens of intestinal tissue from patients with ileal Crohn’s disease.69,70
Administration of L. plantarum, strain 299V, prevents spontaneous colitis development in IL-10−/−mice.71 Treatment with probiotic DNA, derived from the probiotic combination VSL#3, is also able to ameliorate colonic inflammation and overall disease activity in IL-10−/− mice.72 A novel mechanism of action of probiotics in IBD has been suggested by use of the SAMP1/yit mouse model of IBD. Administration of VSL#3 resulted in improved intestinal barrier function, coupled with increased production of TNF and IκBα mRNA in isolated epithelial cells, indicating activation, rather than inhibition, of the NFκB signaling pathway73 The beneficial effect of the probiotic mixture was blocked by employing anti-TNF therapy. This finding is contradictory to the results of a previous study that used the same mixture of probiotics and reported a decrease in NFκB activation.74 The use of different mouse models of IBD in the two studies could explain the discrepant findings, and indicates that the underlying mechanisms of action of probiotics depend on the nature of the disease that is being treated (Figure 1f).
Probiotics in pediatrics
IBS and IBD
Similar to the results of studies in adults, children with IBD have been found to have a higher number of mucosa-associated facultative anaerobic and aerobic bacteria in the intestine and colon compared with healthy individuals.75 Two randomized, controlled trials, using the VSL#3 probiotic combination in pediatric patients with ulcerative colitis, have demonstrated the safety and efficacy of this preparation in maintaining disease remission compared with placebo.76,77 In children with IBS, a crossover trial reported that treatment with VSL#3 improved subjective symptoms and reduced abdominal pain and discomfort or bloating and gas compared with placebo.78 It is clear, however, that before probiotics can be considered as conventional therapy in children with IBS or IBD, more precise mechanisms of action for both the organism employed and the disease subtype being treated are required.79
Necrotizing enterocolitis and preterm infants
Necrotizing enterocolitis is a severe enterocolitis, which affects preterm infants and has both serious morbidity (intestinal perforation, intestinal stricture, and sepsis) and a high mortality rate.80 Although the precise underlying etiology of this disease remains unknown, altered microbial colonization, formula feeding, and neonatal stress are each thought to be involved in its pathogenesis.81 A retrospective study of very low birth weight premature infants showed that prolonged antibiotic treatment in the first days of life increases the risk of developing necrotizing enterocolitis and subsequent death.82 This finding highlights the role of an altered gut micro-biota in disease pathogenesis. 16S rRNA sequencing has shown a significant reduction in fecal microbial diversity in infants with necrotizing enterocolitis compared with unaffected preterm babies.83 Three meta-analyses84–86 indicate that probiotic supplementation in preterm infants significantly reduces the incidence of both severe necrotizing enterocolitis and mortality, but does not have any apparent long-term adverse effects on growth and neurodevelopmental outcomes; at least up to 3 years of age. These findings are supported by the results of a large multicenter trial conducted in Taiwan.87 Comparable studies undertaken in neonatal intensive care units in North America are now awaited with anticipation.
Premature newborns are susceptible to infectious complications, including bacteremia, sepsis, and meningitis. Preterm babies have delayed gut colonization and an increased risk of colonization with pathogens compared with full-term newborns;81 this finding may be due to the liberal use of antibiotics and the requirement for early medical interventions in preterm infants. Oral supplementation with lactoferrin (an innate immune defense compound present in breast milk) either alone or in combination with L. rhamnosus GG (LGG) significantly reduces the development of sepsis in preterm neonates.88 Treatment with probiotics also reduces the time to reach full enteral feeding in premature infants born weighing more than 1,000 g.89 Supplementation with probiotics in this high-risk group may also provide beneficial outcomes in terms of overall growth and in reducing the development of disease during a period of altered microbial colonization in hospitalized premature infants. Despite these promising findings, a recent retrospective study failed to show a beneficial effect of LGG in preventing necrotizing enterocolitis in Finnish babies.90 Taken together, these findings suggest that more research regarding modes of delivery of probiotics and specific probiotic strains is needed in premature infants at risk of developing necrotizing enterocolitis and its complications.
Mechanism of action in necrotizing enterocolitis
In a rat model of necrotizing enterocolitis, which involves early delivery by cesarean section, stress, and formula feeding, probiotics are reported to improve disease outcome.91 Supplementation with Bifidobacterium bifidum, strain OLB6378, is associated with reduced mucosal inflammation, improved intestinal integrity, and enhanced intestinal epithelial barrier function.91 Furthermore, LGG administration protects the immature intestine epithelium from undergoing apoptosis by upregulating cytoprotective gene expression.92 Taken together, these data indicate that reduced bacterial translocation, amelioration of intestinal epithelial barrier defects and decreased apoptosis in the face of exogenous stressors are underlying mechanisms of action of probiotics that prevent the occurrence of necrotizing enterocolitis in high-risk premature neonates.
Infectious and noninfectious diarrhea
Clostridium difficile infection
Clostridium difficile infection is a prevalent and increasingly severe nosocomial infection.93 While the organism can remain latent in many patients, those receiving antibiotics have an increased risk of developing diarrhea and pseudomembranous colitis. Probiotics have been used prophylactically in an effort to reduce C. difficile-associated diarrhea in elderly hospitalized patients.94 A meta-analysis has found that probiotics reduced the frequency of acute diarrhea in C. difficile-infected patients.95 S. boulardii, but not the Gram-positive probiotics LGG and L. plantarum, have been reported to protect against the development of C. difficile-associated diarrhea and prevent disease recurrence.96 Although controversial,97 treatment with S. boulardii seems to be particularly effective for secondary prevention of disease in specific patient populations at risk for recurrent C. difficile infection.98
Antibiotic-associated diarrhea
Treatment with antibiotics disrupts the host micro-biota,99 resulting in loose stools and diarrhea. To counter this imbalance, daily oral intake of L. plantarum, strain 299v, in patients treated with oral antibiotics for management of an intercurrent infection reduces the severity of intestinal symptoms compared with placebo.100 In a pediatric population, oral L. rhamnosus prevented diarrhea arising from antibiotic treatment.101 A recent meta-analysis of S. boulardii administration in adults also indicates therapeutic efficacy in the prevention of antibiotic-associated diarrhea.102
Infectious diarrhea
Acute enteritis and enterocolitis commonly occur following the consumption of contaminated foods or water. Administration of Bacillus mesentericus, Enterococcus fecalis, and Clostridium butyricum to children hospitalized with acute diarrhea has been shown to reduce both the severity and duration of diarrhea compared with placebo.103 Improvement in diarrheal disease in this study cohort was accompanied by increased fecal counts of Lactobacillus and bifidobacteria and an altered anti-inflammatory cytokine profile (increased IL-10 and decreased TNF-α) in serum samples.103 In healthy children, a multicenter trial of supplementation of yoghurt with L. casei, strain DN-114 001 demonstrated a beneficial effect of this organism on reducing the frequency and duration of diarrhea compared to the consumption of traditional yogurt.104,105 Meta-analyses, three using a variety of probiotics106–108 and two using single organisms,109,110 indicate a reduced risk and a decreased duration of diarrhea in children who have received probiotic treatment. Adherence of probiotic strains to the surface of intestinal epithelial cells can physically impede pathogen access to the apical surface, effectively reducing pathogenic burden. This competitive exclusion is supported by in vitro studies in which pretreatment of intestinal epithelial cells with L. helveticus, strain R0052, inhibits invasion by Campylobacter jejuni.111 Therefore, the use of probiotics following consumption of contaminated foodstuffs functions to limit intestinal disease by restoring the gut microbiota and reducing pathogen adherence.
Mechanisms of action in infectious diarrhea
Probiotic-produced antimicrobial factors
Certain probiotics produce antibacterial factors, which aid their ability to outcompete undesired organisms. Bacteriocins are one such factor; these small heat-stable peptides inhibit the growth of other bacteria, including enteric pathogens. Oral infection of mice with the invasive foodborne bacterial pathogen Listeria monocytogenes results in rapid colonization and activation of innate immunity with pathogen clearance via activation of T cells within 14 days. Mice pretreated with L. salivarius, strain UCC118, are protected from Listeria infection, as evidenced by reduced pathogen translocation to the liver and spleen in a manner that is dependent on the production of a bacteriocin.112
Surface-layer proteins are glycoproteins present on the cell surface of some lactobacilli. L. acidophilus, strain NCFM, has a surface-layer protein A, which, when mutated, results in loss of the ability of the probiotic to bind to the dendritic cell receptor, DC-SIGN, induce an immunoregulatory phenotype (TREG) and promote mucosal homeostasis.113 Surface-layer proteins on L. helveticus, strain R0052, prevent EHEC O157:H7 binding to epithelial cells in vitro.114 Other probiotics, including an E. coli preparation, induce the production of human beta defensin 2 and have potent antimicrobial activity in vitro.115
Epithelial barrier function and immunity
In cell culture experiments, probiotics prevent the detrimental effects of infection with various human pathogens on epithelial barrier function. For example, EHEC O157:H7 is an attaching and effacing, noninvasive bacterial pathogen that causes hemorrhagic colitis in humans by hijacking the host cell signal transduction machinery. L. helveticus, strains R0052 and LGG both prevent EHEC-induced increases in epithelial permeability (and decreases in TER), and maintain epithelial barrier function by reducing bacterial adhesion, preventing cytoskeleton rearrangements116 and preserving the architecture of intercellular apical tight junctions.117
Treatment with probiotics increases the survival of neonatal mice infected with C. rodentium.118 Reduced mortality in this model of infection in early life is mediated by T cells and their regulation of epithelial barrier function, although the specific subset of T cells involved in this process remains to be determined.118 Maintenance of epithelial barrier function by probiotics following pathogen infection, therefore, can prevent systemic infection and shorten disease duration. In a Salmonella typhimurium infection mouse model, treatment with B. infantis, strain 35624, protects mice against the effects of infection, resulting in improved disease score and reduced bacterial translocation.119 This protection was due to decreased NFκB activation in mice that received the probiotic compared with those that received placebo.
Designer probiotics
As probiotics are nonpathogenic organisms, they represent a mode of delivery for target compounds. Direct modulation of the mucosal immune system has been demonstrated by an L. lactis strain that was engineered to produce IL-10 locally in the colon. This modified probiotic organism has been shown to ameliorate disease in mice with DSS-induced colitis, and prevent disease development in IL-10−/− mouse.120 The potential for designer probiotics to serve as a credible therapeutic approach has been further demonstrated in a phase I clinical trial in patients with Crohn’s disease who were administered a strain of L. lactis that produced human IL-10.121 The genetically modified organism was well tolerated in all 10 patients, but whether this strategy has a beneficial impact on disease activity remains to be determined. A major concern for genetically modified probiotics is biologic containment, a feature that has been abrogated by genetic manipulation to ensure dependence of probiotic growth on the availability of exogenous thymidine. This strategy limits the potential for the organism to spread into the environment beyond the mammalian host. Phase II and phase III clinical trials with the L. lactis strain that produces human IL-10 are now underway and the results of these studies are eagerly awaited.
Similar genetic manipulation techniques have been employed to create a trefoil factor (TFF)-producing L. lactis microorganism. TFFs are cytoprotective compounds that promote wound healing in the intestinal tract and have been used in models of gastric ulceration, colitis and necrotizing enterocolitis.122 Instead of traditional orogastric administration of TFF, which sticks to the mucus layer in the small bowel preventing its localization in the colon, use of the recombinant bacterium enables delivery of TFF directly to the colonic mucosa and results in amelioration of DSS-induced disease in mice.123
The concept of designer probiotics has continued using other bacterial species, notably Lactobacillus gasseri. Biopsy specimens obtained from patients with IBD have been shown to have imbalances in the formation of reactive oxygen species (ROS) and antioxidant micronutrients, with decreases in the ROS-neutralizing enzyme superoxide dismutase (SOD). These findings suggest a rationale for the use of antioxidants as an alternative therapeutic option for patients with IBD.124 In a study of IL-10−/− mice, which spontaneously develop IBD, a manganese SOD gene from S. thermophilus was inserted into L. gasseri.125 After 4 weeks, inflammation was significantly improved in mice treated with this designer probiotic compared to mice treated with L. gasseri alone.125 A dietary trigger was recently developed to enable the control of a designer probiotic. Bacteroides ovatus, strain BOV975, which is a human commensal organism, was engineered to deliver human keratinocyte growth factor 2 in response to exposure to dietary xylan. This recombinant organism improved colitis in a DSS-induced mouse model of IBD.126 Taken together, these studies highlight the potential beneficial effects of designer probiotics.
Adverse effects of probiotics
Although generally considered safe, some studies have highlighted that probiotics may be ill advised in specific patient populations. For example, bacteremia,127 sepsis128 and meningitis129 have been described on rare occasions in children with short bowel syndrome and a central venous catheter on probiotics. These individuals are at increased risk of translocation of organisms, including viable bacteria and fungi strains that are employed in the clinical setting as probiotics.
A study has tested the safety and efficacy of intra-duodenal administration of a probiotic mixture of six bacteria in patients with severe pancreatitis who were cared for in an intensive care unit setting. Although initial animal studies indicated the potential for benefit,130 this clinical trial did not result in a reduced frequency of infectious complications of acute pancreatitis. Moreover, a significantly increased risk of death was reported in the population receiving the probiotic formulation.131 The patients who died had evidence of necrotizing jejunitis. This finding raises the possibility of an impaired splanchnic circulation that was compromised further by direct delivery of a high concentration of microorganisms into the proximal intestine. To date, this is the only study to associate probiotic use with an increased risk of death in the setting of a clinical trial. Nevertheless, it remains to be determined whether certain patient populations pose too great a risk for the development of serious complications, including sepsis, arising from the use of viable organisms in high concentrations. For the moment, patients with severe immune deficiencies (including congenital severe combined immune deficiency and HI V-induced acquired immunodeficiency syndromes) and those ill enough to require medical care in an intensive care unit should probably only receive probiotics in the setting of a carefully conducted clinical research protocol.
Probiotic selection
Testing for the probiotic potential of various microorganisms starts at the preclinical level and includes evaluations of antibiotic resistance, safety and potential efficacy132 Numerous studies, both in animal models and in human clinical trials, report success in reducing the severity of a number of diseases by use of certain probiotic strains, but not by use of others. Use of in vitro systems to initially test, for example, the proinflammatory capacity of different potential probiotic organisms, before testing the agent in vivo, could be used to predict the likelihood of the probiotic having efficacy in a specific condition. For example, testing three different Lactobacillus strains in vitro indicated that L. paracasei has the least stimulatory effects on dendritic cells compared with L. plantarum and LGG.25 These findings led to testing of the L. paracasei strain in mice with DSS-induced colitis.25 Therefore, care should be taken in the selection of probiotic strains depending on the desired outcome. This approach reinforces the need to further elucidate the underlying mechanisms of action of specific probiotics, which will assist in determining which specific organisms are most likely to provide a benefit for a specific disease condition. Such a mechanistic-based approach, coupled with carefully conducted multicenter, randomized, controlled trials will advance the knowledge base that is required to address the potential of probiotics as an alternative management option for various disease conditions that involve the gut.
Conclusions
There is a growing body of evidence to support the potential use of probiotics in the prevention and treatment of various human diseases, the caveat being that only specific organisms may be effective for certain disease manifestations. Determining whether live organisms, secreted products from live organisms (such as surface-layer proteins), or probiotic-derived products (such as bacteriocins) are sufficient to mediate a beneficial effect requires further study. In addition, establishing rules and regulations for the proper identification of organisms for specific uses and clearly demonstrating underlying mechanisms of action will shape the future of probiotic research with respect to various disease interventions. Carefully selected and fully tested probiotic strains will probably provide alterative options for individuals in whom conventional medical therapies have failed to promote health and perhaps, in the future, serve as a first-line choice of therapy for some patients.
Key points.
-
▪
Probiotics are increasingly being used for various digestive diseases, including IBS, IBD, necrotizing enterocolitis, acute infectious diarrhea and antibiotic-associated diarrhea
-
▪
Not all probiotic strains are appropriate for all ailments
-
▪
Depending on the strain, probiotics have different underlying mechanisms of action to provide a beneficial effect
-
▪
In addition to live organisms, probiotic-derived products, such as surface-layer proteins and bacteriocins may provide beneficial effects
-
▪
Probiotics are contraindicated in certain patient populations, including those with severe immune deficiencies
Acknowledgments
Work in the authors’ laboratories is supported by grants from the National Institutes of Health (WAW: R01 HD012437, R01 DK070260, P01 DK033506 and P30 DK040561), the Canadian Institutes of Health Research (PMS), and a Ray Shapiro-Cutler grant-in-aid from the Crohn’s and Colitis Foundation of Canada (MGG & PMS). MGG is the recipient of a CIHR/CCFC/Canadian Association of Gastroenterology fellowship. PMS is the recipient of a Canada Research Chair in Gastrointestinal Disease.
Footnotes
Competing interests
M. G. Gareau declares no competing interests. P M. Sherman declares associations with the following companies: Abbott, Institut Rosell Lallemand, Mead Johnson and Nestle.
W. A. Walker declares associations with the following companies: Dannon and Mead Johnson. See the article online for full details of the relationships.
Author contributions
M. G. Gareau researched data for the article and wrote the article. All three authors contributed equally to discussions of the content of the article and to review/editing of the manuscript before submission.
References
- 1.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav. Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Cai W, Feng Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin. Nutr. 2007;26:559–566. doi: 10.1016/j.clnu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Curr. Opin. Allergy Clin. Immunol. 2009;9:197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed. Med. 2009;4:11–15. doi: 10.1089/bfm.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin R, et al. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003;143:754–758. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy—a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2009;101:1722–1726. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J. Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friswell MK, et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS ONE. 2010;5:e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariat D, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vael C, Desager K. The importance of the development of the intestinal microbiota in infancy. Curr. Opin. Pediatr. 2009;21:794–800. doi: 10.1097/MOP.0b013e328332351b. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann C, et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 2009;77:4668–4678. doi: 10.1128/IAI.00493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill DA, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Palma G, et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen N, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannock GW. Molecular analysis of the intestinal microflora in IBD. Mucosal Immunol. 2008;1(Suppl. 1):S15–S18. doi: 10.1038/mi.2008.54. [DOI] [PubMed] [Google Scholar]
- 21.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Willing B, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm. Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- 23.Seksik P, et al. Search for localized dysbiosis in Crohn’s disease ulcerations by temporal temperature gradient gel electrophoresis of 16S rRNA. J. Clin. Microbiol. 2005;43:4654–4658. doi: 10.1128/JCM.43.9.4654-4658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Canada: FAO/WHO; 2002. [Google Scholar]
- 25.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS ONE. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr. Clin. Pract. 2009;24:10–14. doi: 10.1177/0884533608329231. [DOI] [PubMed] [Google Scholar]
- 27.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 28.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 29.O’Mahony L, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 30.Whorwell PJ, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal A, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173-010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2008;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 32.Guyonnet D, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults n primary care: a multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 33.Moayyedi P, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 34.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am. J. Gastroenterol. 2009;104:1033–1049. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 35.Soderholm JD, et al. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 36.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Mahony SM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Soderholm JD, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 39.Zareie M, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 41.Verdu EF, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdu EF, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826–837. doi: 10.1053/j.gastro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Kamiya T, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol. Motil. 2009;21:567–569. doi: 10.1111/j.1365-2982.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- 45.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology, clinical implications. Curr. Opin. Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 46.Shanahan F, Bernstein CN. The evolving epidemiology of inflammatory bowel disease. Curr. Opin. Gastroenterol. 2009;25:301–305. doi: 10.1097/MOG.0b013e32832b12ef. [DOI] [PubMed] [Google Scholar]
- 47.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darfeuille-Michaud A, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 49.Barnich N, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isaacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm. Bowel Dis. 2008;14:1597–1605. doi: 10.1002/ibd.20465. [DOI] [PubMed] [Google Scholar]
- 51.Pronio A, et al. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm. Bowel Dis. 2008;14:662–668. doi: 10.1002/ibd.20369. [DOI] [PubMed] [Google Scholar]
- 52.Bibiloni R, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 53.Hansen R, Thomson JM, El Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J. Gastroenterol. 2010;45:266–276. doi: 10.1007/s00535-009-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marteau P. Bacterial flora in inflammatory bowel disease. Dig. Dis. 2009;27(Suppl. 1):99–103. doi: 10.1159/000268128. [DOI] [PubMed] [Google Scholar]
- 55.Ingrassia I, Leplingard A, Darfeuille-Michaud A. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn’s disease patients to adhere to and to invade intestinal epithelial cells. Appl. Environ. Microbiol. 2005;71:2880–2887. doi: 10.1128/AEM.71.6.2880-2887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson-Henry KC, et al. Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J. Infect. Dis. 2005;191:2106–2117. doi: 10.1086/430318. [DOI] [PubMed] [Google Scholar]
- 57.Wu X, et al. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G295–G306. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- 58.Ewaschuk JB, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 59.Ukena SN, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLos ONE. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mennigen R, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 61.Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009;92:2400–2408. doi: 10.3168/jds.2008-1698. [DOI] [PubMed] [Google Scholar]
- 62.Tien MT, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human Intestinal epithelial cells. J. Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 63.Kamada N, et al. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun. 2008;76:214–220. doi: 10.1128/IAI.01193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roselli M, et al. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel Dis. 2009;15:1526–1536. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 65.Hacini-Rachinel F, et al. CD4+T cells and Lactobacillus casei control relapsing colitis mediated by CD8+ T cells. J. Immunol. 2009;183:5477–5486. doi: 10.4049/jimmunol.0804267. [DOI] [PubMed] [Google Scholar]
- 66.Kwon HK, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl Acad. Sci. USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr. Res. 2005;58:1185–1191. doi: 10.1203/01.pdr.0000183660.39116.83. [DOI] [PubMed] [Google Scholar]
- 68.Im E, Choi YJ, Pothoulakis C, Rhee SH. Bacillus polyfermenticus ameliorates colonic inflammation by promoting cytoprotective effects in colitic mice. J. Nutr. 2009;139:1848–1854. doi: 10.3945/jn.109.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carol M, et al. Modulation of apoptosis in intestinal lymphocytes by a probiotic bacteria in Crohn’s disease. J. Leukoc. Biol. 2006;79:917–922. doi: 10.1189/jlb.0405188. [DOI] [PubMed] [Google Scholar]
- 70.Llopis M, et al. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 2009;15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 71.Schultz M, et al. Lactobacillus plantarum 299V n the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm. Bowel Dis. 2002;8:71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Jijon H, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Pagnini C, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc. Natl Acad. Sci. USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madsen K, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 75.Conte MP, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760–1767. doi: 10.1136/gut.2005.078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miele E, et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 77.Huynh HQ, et al. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm. Bowel Dis. 2009;15:760–768. doi: 10.1002/ibd.20816. [DOI] [PubMed] [Google Scholar]
- 78.Guandalini S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J. Pediatr. Gastroenterol. Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 79.Fedorak RN. Understanding why probiotic therapies can be effective in treating IBD. J. Clin. Gastroenterol. 2008;42(Suppl. 3):S111–S115. doi: 10.1097/MCG.0b013e31816d922c. [DOI] [PubMed] [Google Scholar]
- 80.Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR. Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr. Surg. Int. 2009;25:309–318. doi: 10.1007/s00383-009-2344-8. [DOI] [PubMed] [Google Scholar]
- 81.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 82.Cotten CM, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISMEJ. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alfaleh K, Anabrees J, Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2009;97:93–99. doi: 10.1159/000235684. [DOI] [PubMed] [Google Scholar]
- 85.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 86.Chou IC, et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J. Pediatr. 2010;156:393–396. doi: 10.1016/j.jpeds.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 87.Lin HC, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 88.Manzoni P, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 89.Rouge C, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009;89:1828–1835. doi: 10.3945/ajcn.2008.26919. [DOI] [PubMed] [Google Scholar]
- 90.Luoto R, Matomaki J, Isolauri E, Lehtonen L. Incidence of necrotizing enterocolitis in very-low-birth-weight infants related to the use of Lactobacillus GG. Acta Paediatr. 2010;99:1135–1138. doi: 10.1111/j.1651-2227.2010.01795.x. [DOI] [PubMed] [Google Scholar]
- 91.Khailova L, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G940–G949. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr. Res. 2008;64:511–516. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008;5:40–48. doi: 10.1038/ncpgasthep1029. [DOI] [PubMed] [Google Scholar]
- 94.Leffler DA, Lamont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136:1899–1912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 95.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 96.Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD004611.pub2. Art no.: CD004611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller M. The fascination with probiotics for Clostridium difficile infection: lack of evidence for prophylactic or therapeutic efficacy. Anaerobe. 2009;15:281–284. doi: 10.1016/j.anaerobe.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Tung JM, Dolovich LR, Lee CH. Prevention of Clostridium difficile infection with Saccharomyces boulardii: A systematic review Can. J. Gastroenterol. 2009;23:817–821. doi: 10.1155/2009/915847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 100.Lonnermark E, et al. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J. Clin. Gastroenterol. 2010;44:106–112. doi: 10.1097/MCG.0b013e3181b2683f. [DOI] [PubMed] [Google Scholar]
- 101.Ruszczynski M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment. Pharmacol. Ther. 2008;28:154–161. doi: 10.1111/j.1365-2036.2008.03714.x. [DOI] [PubMed] [Google Scholar]
- 102.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010;16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen CC, et al. Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr. Infect. Dis. J. 2010;29:135–138. doi: 10.1097/inf.0b013e3181b530bf. [DOI] [PubMed] [Google Scholar]
- 104.Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000;54:568–571. [PubMed] [Google Scholar]
- 105.Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int. J. Clin. Pract. 1999;53:179–184. [PubMed] [Google Scholar]
- 106.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatr. Gastroenterol. Nutr. 2001;33(Suppl. 2):S17–S25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 107.Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- 108.Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig. Dis. Sci. 2002;47:2625–2634. doi: 10.1023/a:1020501202369. [DOI] [PubMed] [Google Scholar]
- 109.Szajewska H, Skorka A, Dylag M. Meta-analysis: Saccnaromyces boulardii for treating acute diarrhoea in children. Aliment. Pharmacol. Ther. 2007;25:257–264. doi: 10.1111/j.1365-2036.2006.03202.x. [DOI] [PubMed] [Google Scholar]
- 110.Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D. Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment. Pharmacol. Ther. 2007;25:871–881. doi: 10.1111/j.1365-2036.2007.03282.x. [DOI] [PubMed] [Google Scholar]
- 111.Wine E, Gareau MG, Johnson-Henry K, Sherman PM. Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 2009;300:146–152. doi: 10.1111/j.1574-6968.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- 112.Corr SC, et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl Acad. Sci. USA. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Konstantinov SR, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl Acad. Sci. USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9:356–367. doi: 10.1111/j.1462-5822.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 115.Mondel M, et al. Probiotic E.coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sherman PM, et al. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7-and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73:5183–5188. doi: 10.1128/IAI.73.8.5183-5188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun. 2008;76:1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J. Infect. Dis. 2010;201:81–91. doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 119.O’Mahony C, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steidler L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 121.Braat H, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 122.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vandenbroucke K, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–513. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 124.Lih-Brody L, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 125.Carroll IM, et al. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am. J. Physiol. Gastrointest Liver Physiol. 2007;293:G729–G738. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 126.Hamady ZZ, et al. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut. 2010;59:461–469. doi: 10.1136/gut.2008.176131. [DOI] [PubMed] [Google Scholar]
- 127.De Groote MA, Frank DN, Dowell E, Glode MR, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005;24:278–280. doi: 10.1097/01.inf.0000154588.79356.e6. [DOI] [PubMed] [Google Scholar]
- 128.Land MH, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 129.Barton LL, Rider ED, Coen RW. Bacteremic infection with Pediococcus: vancomycin-resistant opportunist. Pediatrics. 2001;107:775–776. doi: 10.1542/peds.107.4.775. [DOI] [PubMed] [Google Scholar]
- 130.van Minnen LP, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 131.Besselink MG, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 132.Sorokulova I. Preclinical testing in the development of probiotics: a regulatory perspective with Bacillus strains as an example. Clin. Infect. Dis. 2008;46(Suppl. 2):S92–S95. doi: 10.1086/523334. [DOI] [PubMed] [Google Scholar]