Abstract
Cellular senescence, a process that imposes permanent proliferative arrest on cells in response to various stressors, has emerged as a potentially important contributor to aging and age-related disease, and it is an attractive target for therapeutic exploitation. A wealth of information about senescence in cultured cells has been acquired over the past half century; however, senescence in living organisms is poorly understood, largely because of technical limitations relating to the identification and characterization of senescent cells in tissues and organs. Furthermore, newly recognized beneficial signaling functions of senescence suggest that indiscriminately targeting senescent cells or modulating their secretome for anti-aging therapy may have negative consequences. Here we discuss current progress and challenges in understanding the stressors that induce senescence in vivo, the cell types that are prone to senesce, and the autocrine and paracrine properties of senescent cells in the contexts of aging and age-related diseases as well as disease therapy.
Aging is the progressive loss of tissue and organ function over time1. The antagonistic pleiotropy theory of aging proposes that organismal fitness declines, at least in part, because natural selection favors genetic programs that have beneficial effects on reproductive fitness early in life without regard for negative impacts on health at later, post-reproductive ages2. One set of genes that is likely to qualify as antagonistically pleiotropic is the regulators of cellular senescence3, a potent anticancer mechanism that prevents malignancies by permanently withdrawing (pre-) neoplastic cells from the cell cycle4,5 but also has been implicated as a driver of aging and age-related disease6–8.
The emerging evidence suggests that the drawbacks of senescence are twofold. First, as one might expect, senescence causes a loss of tissue-repair capacity because of cell cycle arrest in progenitor cells. Second, senescent cells produce proinflammatory and matrix-degrading molecules in what is known as the senescence-associated secretory phenotype (SASP).
One reason that cellular senescence may have evolved alongside programmed cell death as an anticancer mechanism despite these downsides is that, in addition to being permanently arrested (a cell-autonomous effect), senescent cells may be cleared by immune cells that are recruited due to proinflammatory, chemotactic factors secreted as part of the SASP9–11. Preneoplastic lesions could thus be eliminated en masse by invading immune cells, which would generate non-cell-autonomous tumor-suppressive effect with broader impact12. Recently, evidence has pointed to beneficial effects of cellular senescence beyond tumor suppression, for instance in directing wound repair13 and in embryogenesis14,15. In these contexts, senescence serves a tissue remodeling role and the senescent cells produced have a relatively short half-life, presumably because they are efficiently cleared by immune cells15. In contrast, aged cells that chronically accumulate damage ultimately reach a threshold of cellular stress that prompts their permanent withdrawal from the cell cycle.
Because of their potential involvement in many aging and disease processes, eliminating senescent cells and attenuating the SASP have emerged as attractive therapeutic strategies; however, translation of these findings into relevant human applications is currently limited by our fragmentary understanding of both the basic molecular cell biology of in vivo senescent cells and the overall importance of senescence to age-related diseases. Here, we present a framework encompassing the causes and role of senescent cells in chronic disease as well as normal and pathological aging with a focus on new approaches to answering unresolved questions in aging research.
Lessons from the study of in vitro senescence
The seminal discovery of replicative senescence by Hayflick and Moorehead was the beginning of speculation that senescence and aging might be causally linked16. Their observation that primary human cells undergo a limited number of divisions in vitro immediately suggested a cell-autonomous theory of aging, whereby senescence depletes tissues of replication-competent cells required for homeostasis, repair and regeneration. Replicative arrest in culture has since become the model system for probing the molecular causes and effectors of the senescent state.
In proliferating human cells, progressive telomere erosion ultimately exposes an uncapped free double-stranded chromosome end, triggering a permanent DNA damage response (DDR). In this setting, the damage sensor ataxia telangiectasia mutated (ATM) is recruited to uncapped telomeres, leading to the stabilization of tumor suppressor protein 53 (p53) and upregulation of the p53 transcriptional target p21 (Fig. 1). In turn, p21 prevents cyclin-dependent kinase 2 (CDK2)-mediated inactivation of RB, subsequently preventing entry into the S phase of the cell cycle17–19. Other DNA-damaging stressors, such as ultraviolet (UV) or gamma irradiation20,21, chemotherapeutics22–24 and hyperproliferation caused by oncogenic Ras overexpression25, also engage the ATM-p53-p21 axis (Fig. 1). The rapidly acting p53-p21 pathway can also be engaged by DDR-independent expression of the p53 stabilizer p19Arf (p14 in humans)26, loss of the tumor suppressor PTEN26, overexpression of the S-phase transcription factor E2F3 (ref. 27) and, surprisingly, oncogenic Ras expression in human mammary epithelial cells28.
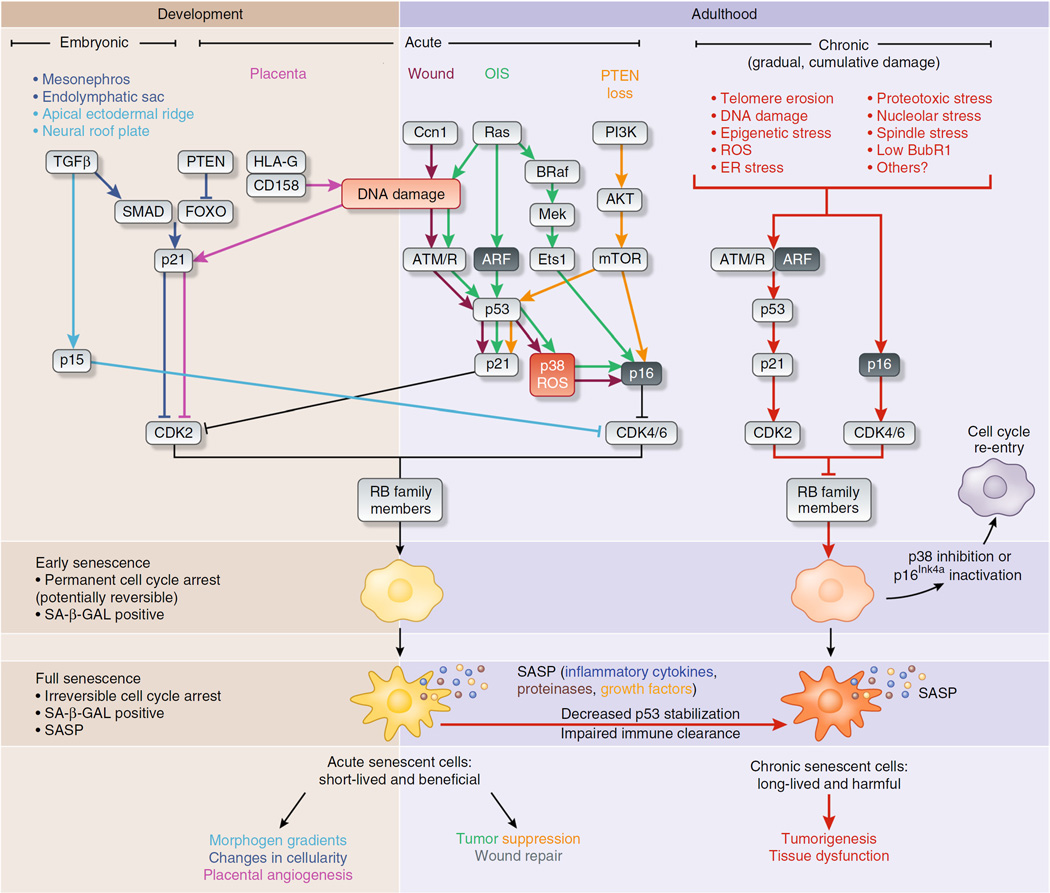
Figure 1.
Effector pathways of three senescent cell types. Stresses inducing senescence vary depending on the in vivo context, although there is substantial overlap in processing of the stress-response signal and activating effectors of senescence. For example, in all reported cases, rising levels of cyclin-dependent kinase inhibitors drive entry into senescence by activating RB to block cell cycle progression. In embryonic senescence, elevated TGFβ and reduced PTEN activity upregulate SMAD-FOXO transcriptional activation of the cyclin-dependent kinase inhibitor p21, as well as activation of p15Ink4b through unclear means. In contrast, acute senescence—in the placenta, after wounding or in response to oncogene activation or loss of the tumor suppressor PTEN—triggers DNA damage or p53 signaling to induce p21 and p16Ink4a. Both embryonic and acute senescence are beneficial, and presumably these cells are cleared rapidly by the immune system as part of their program. These two settings contrast with chronic senescence, which is a response to the slowly accumulating macromolecular damage of age, such as telomere erosion, proteotoxicity, DNA damage and likely many others. Effectors of chronic senescence probably include p21 and p16Ink4a, which are induced in aged tissues. Chronic senescence can also evolve from acute senescence if immune clearance is impaired with age, leading to prolonged arrest and possibly alterations in the SASP. In all senescence cases, cyclin-dependent kinase–mediated licensing of RB activity leads to an early senescent state where the arrest is permanent in vivo, but can be reversed with manipulation of single factors, such as p38 inhibition or inactivation of p16Ink4a. These early senescent cells are SA-β-GAL positive and may not have a SASP. Senescent cells may evolve further into a truly irreversible full senescence with SA-β-GAL positivity and a SASP. The cellular changes driving this phenotypic switch in vivo are unclear but are likely to include robust processes such as heterochomatinization of cell cycle genes and activation of an NF-κB–dependent transcriptional program generating the SASP.
As a second barrier to proliferation, p16Ink4a prevents CDK4- and CDK6-mediated inactivation of RB to block cell cycle progression (Fig. 1). This mechanism can act either alone or in combination with the p53-p21 pathway depending on stress or cell type. It seems that p21 is often upregulated first and p16Ink4a later, possibly representing distinct phases on the path from early to full senescence29. Interestingly, transition from a p21-mediated arrest to a fully senescent state may require an additional, forced round of cell division30. This results in aberrant cell cycle completion, failed mitosis and a 4N genome, as has been shown in multiple cell types including fibroblasts passaged extensively in vitro and p16Ink4a-expressing satellite cells collected from skeletal muscle of aged mice31–33.
In vitro senescent cells have several distinguishing characteristics, such as increased cell size, enzymatic activity of the lysosomal hydrolase senescence-associated β-galactosidase (SA-β-GAL)34, upregulation of prosurvival pathways to resist apoptosis35–39 and the development of a SASP, a distinctive secretome consisting of a various proinflammatory molecules, metalloproteases and growth factors (Fig. 1)40. The SASP is largely initiated by NF-κB and p38 MAPK signaling41 and is maintained in an autocrine fashion by the SASP factor interleukin Iα (IL-Iα; ref. 42). The discovery of the SASP suggested a mechanism by which senescent cells could affect tissue and organ function out of proportion to their numbers. Use of the above traits in combination is the current best practice for identifying senescent cells, as any individual feature can be found outside of senescence or may emerge at different times during the acquisition of senescence. For example, presenescent geriatric satellite cells upregulate p16Ink4a but lack detectable SA-β-GAL activity31.
Origin and identification of senescent cells in vivo
Identifying, cataloging and isolating senescent cells in vivo remains a major obstacle43. Testing for key senescent cell markers on a tissue level, such as increased SA-β-GAL activity34 and elevated transcript or protein levels of p16Ink4a and p21, has been effective at demonstrating the presence of senescence in bulk aged or pathological tissue. However, additional genetic strategies to tag and kill senescent cells have been essential for confirming cells’ identities and their role in biological processes. Senescent-cell reporter systems in mice using either a small senescence-responsive fragment of the p16Ink4a (CDKN2A) promoter to drive green fluorescent protein (GFP) expression (in the INK-ATTAC transgene)6 or 50 kilobases of the same promoter driving monomeric red fluorescent protein (mRFP) (p16-3MR)13 have been employed to isolate in vivo senescent cells by flow cytometry, confirming that key in vitro phenotypes such as SASP production and SA-β-GAL activity are conserved during senescence in vivo6,7. However, these approaches rely on enzymatic digestion of tissue followed by flow cytometry for the analysis of cells, thus carrying the potential for loss of vulnerable cells due to harsh processing. Alternatively, nondestructive in situ immunostaining for mRFP derived from p16-3MR identified senescent cells during wound repair as myofibroblasts13. Also noninvasively, a mouse with luciferase knocked into the endogenous CDKN2A locus has been used to show that senescence occurs in the desmoplastic response following tumor growth44. However, although p16Ink4a promoter activity is one of the best available markers of senescence, p16Ink4a is also expressed in non-senescent cells and cells that are transiently arrested, representing an unknown fraction of aging tissue43. In light of these considerations, we suggest an approach for identifying senescent cells in vivo in Box 1.
BOX 1 Pipeline for identifying senescent cells in vivo.
There is currently no universally accepted standard of evidence for identifying senescent cells and determining their functional significance in vivo. Drawing on our experience, we propose the following ‘best practices’ workflow for consideration.
The first step consists of asking whether senescence is present and in what cell type. SASP factor and CDKi expression should be assessed in tissue via RT-PCR or western blotting, keeping in mind that not all inflammation has its origin in senescence. Following this, the senescent cell type must be identified. SA-β-GAL–staining of whole tissue can, in our hands, be followed by immunofluorescence staining of cryosections for some cell type markers or by routine hematoxylin and eosin (H&E) staining. However, cell identification is best accomplished by in vivo mouse experiments using senescent-cell reporters, such as p16-LUC, p16-3MR or INK-ATTAC. p16-LUC consists of a luciferase knock-in at the p16Ink4a (CDKN2A) locus and permits whole-body imaging of p16Ink4a promoter activity. In contrast, p16-3MR and INK-ATTAC are transgenes that allow for the killing of senescent cells as well as their detection via fluorescence. p16-3MR uses a 50-kilobase fragment of the p16Ink4a promoter to drive expression of both mRFP and luciferase reporters as well viral thymidine kinase, which is lethal in the presence of the drug ganciclovir. INK-ATTAC expresses both GFP and a drug-activated FKBP–caspase-8 fusion protein under control of a 2.6-kilobase p16Ink4a promoter fragment. One of these systems should be used to isolate fluorescent senescent cells by flow cytometry to detect p16Ink4a, SASP-factor and cell-type markers by RT-PCR or western blotting, or for further biological characterization, such as single-cell RNA sequencing and assessment of proliferative capacity. This level of analysis will permit comparison of the secretory and other properties of senescent cells across biological contexts, addressing the key question of whether all senescent cells are created equally. The different proposed roles of senescence in various diseases may be explicable by the existence of senescent cell ‘subtypes’, resulting from differences in stressors, environment or cell type, revealed through this type of analysis.
The second step is testing the effect of senescence. Removing senescent cells reveals their indirect effects, such as consequences of the SASP, whereas breeding experimental mice onto a p16Ink4a-null background to prevent the senescence program tests both downstream effects and the consequences of the senescence arrest itself. Although this difference makes senescent cell killing a ‘cleaner’ experiment, INK-ATTAC and p16-3MR use different promoters and may be expressed differently depending on cell type. Furthermore, because these constructs trigger apoptosis through different means (effector caspase activation versus mitochondrial DNA damage), each may be more efficient at cell killing in some settings than in others. More concerning, though, than explaining inconsistencies that will arise between killing systems are the systematic errors that are likely to emerge through over-reliance on p16 as a biomarker.
The gold-standard definition of senescence remains permanent, irreversible exit from the cell cycle, and this should be tested directly in isolated cells, if possible. As p16Ink4a is an end effector of this arrest, its expression is still the most highly used surrogate marker of this arrest, together with SA-β-GAL enzymatic activity. However, both markers have well-known false positives, including T cells for p16 (ref. 167) and maturing tissue macrophages for SA-β-GAL168. One can in principle be used to validate the other: for example one can confirm p16 status of SA-β-GAL–positive cells by testing whether they can be killed by INK-ATTAC. Unfortunately, as both these false-positive cell types are potentially inflammatory, assays for SASP factor expression may also give false positives. Researchers in the field of senescence must take great pains to avoid these kinds of spurious results. The development of cell type–specific killing constructs, as well as ones operating under the control of other senescence-specific genes that are unknown at present, would help eliminate some of these sources of error.
Together, senescence reporter mice and marker analysis suggest that in vivo senescence can be broadly categorized into three classes: acute, embryonic and chronic (Fig. 1). Acute senescence occurs in response to discrete stresses that are required for tissue homeostasis, including CCN1-induced myofibroblast senescence in wound healing45, HLAG-induced neutrophil senescence in the placenta46 and oncogene-induced senescence (OIS) in neoplastic lesions47 (Fig. 1). Embryonic senescence is noncanonical, whereby an arrest maintained by p21 or the alternative CDK4 and CDK6 inhibitor p15Ink4b drives tissue patterning without involvement of p16Ink4a (refs. 14,15). Both acute and embryonic senescent cells appear to be beneficial, and their elimination seems programmed, presumably through involvement of the immune system12 (Fig. 1).
In contrast to these largely beneficial senescence processes, senescent cells generated through chronic or therapy-induced stimuli are more deleterious. Chronic senescence results from long-term, slow macromolecular damage due to stresses such as protein misfolding, protein aggregation, dysfunction of the nuclear lamina, epigenetic changes and various kinds of DNA damage including telomere shortening (Fig. 1). In contrast to chronic senescence resulting from normal ‘wear and tear’ and declining macromolecular repair mechanisms, therapy-induced senescence results from abrupt exogenous stresses placed on tissues during the course of disease management. Striking examples are irradiation or chemotherapy, both of which cause the substantial collateral macromolecular damage to non-neoplastic cells that are thought to be responsible for the early aging phenotypes frequently observed in cancer survivors48,49.
Given the wildly different contexts in which senescent cells arise, we predict that although the overall ‘life cycle’ of a senescent cell might be a common feature regardless of its classification as acute, embryonic or chronic, considerable phenotypic variation could exist. One such example is the SASP, which could vary depending on the time spent in senescence, the nature of the pro-senescent stress and the cell type. As the properties of a senescent cell change with time50, it is possible that chronic senescent cells persisting for extended periods may possess an ‘evolved’ SASP more damaging than that occurring in acute senescence29. However, the length of time for which a chronic senescent cell persists and whether this increases in advanced age are unknown. Similarly, the SASP is produced as a consequence of stress-response pathways51,52, which may be activated differently in acute versus chronic senescence. Therefore, the secretome of senescent cells in these contexts could be very different, as could the properties of acute senescent cells generated in youth versus old age. Although cultured senescent mouse cells exhibit similarities in SASP composition irrespective of the senescence-inducing stressor40, examination of ‘SASP factors’ in adipose tissue from different accelerated aging (progeroid) mouse models demonstrate that SASP profiles differ in vivo. We compared two progeroid mouse models in which prematurely aging tissues show increased cellular senescence as a result of low levels (hypomorphism) of BubR1 (a mitotic checkpoint protein) or Spartan (a component of the translesion DNA repair machinery53). Strikingly, BubR1-hypomorphic mice showed elevated IL-6 in prematurely aging adipose tissue, but Spartan-hypomorphic mice did not. This could indicate the existence of different SASPs or possibly a unique tissue response to the presence of senescent cells from different stressors53. Similarly, as several SASP factors are under control of DDR signaling54, one possibility is that other DNA-independent damage types coexisting in the same cell may also alter the SASP in vivo. For example, replicatively senescent cells were initially thought to undergo only telomere attrition but are now appreciated to also have a diminished unfolded protein response with increased proteotoxicity55, as well as heightened production of progerin, a lamin A splice variant56,57 that causes progeria.
Impact of senescence on human health
Persistent senescent cells in the adult are produced in at least three different contexts related to human health: normal aging, age-related disease and therapeutic interventions (Fig. 2). In normal ‘healthy’ aging, tissue dysfunction still occurs in all individuals, whereas specific age-related diseases strike only some. In aging individuals, processes required for tissue homeostasis inevitably cause damage, resulting in senescence (Fig. 2a). These chronic senescent cells may persist as a result of defects in the aging immune system or, potentially, because isolated senescent cells lack sufficient signaling to attract resident immune cells. Similarly, acute senescent cells from wound repair, tumor suppression or other unknown programmed processes may be incompletely disposed of by the aging immune system and persist. Collectively, lingering senescent cells arising from multiple mechanisms would make aged tissue less functional and simultaneously more susceptible to further deterioration when faced with other stressors.
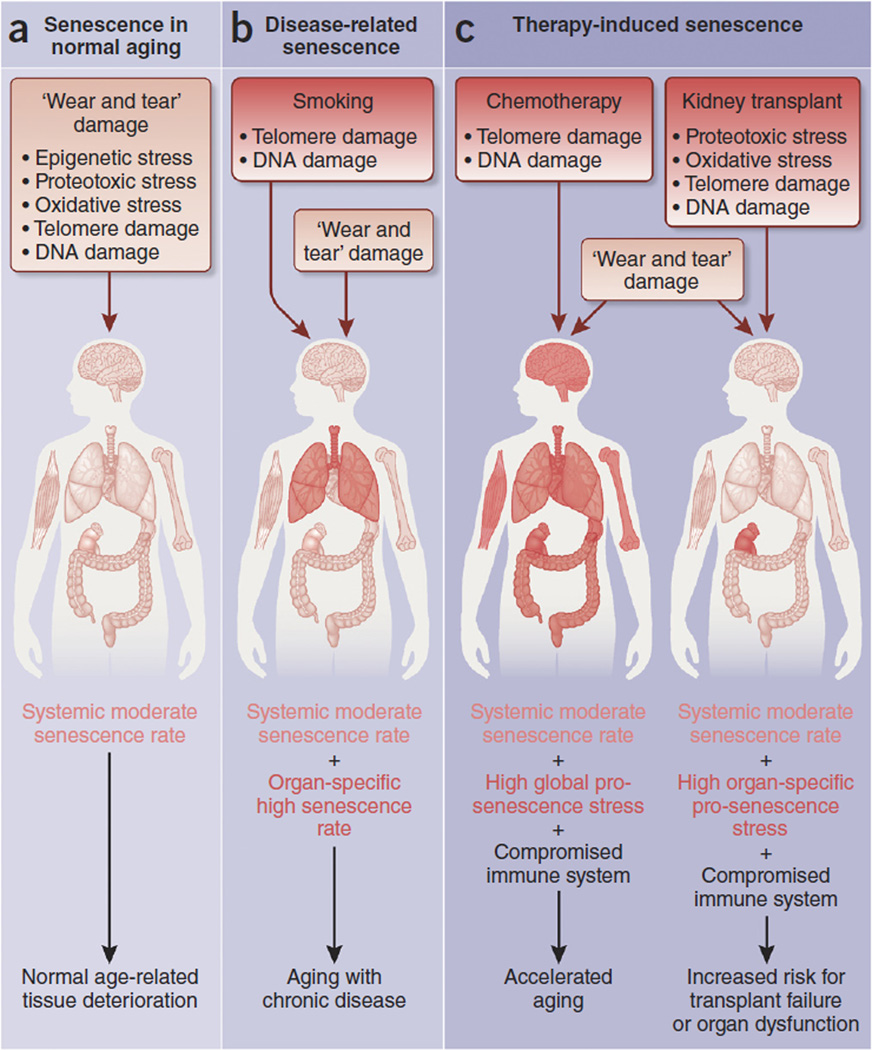
Figure 2.
Senescence in aging, age-related diseases and disease-related treatments. (a) Common cellular stresses yield senescent cells that accumulate in various tissues over time and may contribute to tissue dysfunction even within healthy aging. (b,c) In contrast, disease-related senescence (b) and therapy-induced senescence (c) generate an additional burden of senescent cells on top of the chronic senescence generated by aging itself. An example of disease-related senescence is chronic obstructive pulmonary disease (COPD) following cigarette smoking, an addiction that causes DNA damage from compounds present in cigarette smoke as well as telomere shortening due to increased demand for repair placed on the injured airway epithelium. Both DNA damage and telomere shortening occur during normal aging, but in smoking, the duration and intensity of the stress is much higher. In this case, although there are peripheral effects of smoking, much of the damage is concentrated in the lung, and a majority of smoking-induced senescent cells probably occur there. Therefore, disease-related senescence occurs at a high rate in one or a few target organs and may drive aging with chronic disease. Therapy-induced senescence is the result of stressors due to medical intervention and can be both a desired outcome of therapy or an undesirable side effect. Here we focus on two examples of undesirable therapy-induced senescence. Chemotherapy is known to cause accelerated aging. One way in which this is thought to occur is through organism-wide telomere erosion and DNA damage in non-neoplastic cells, leading to systemically high levels of senescence. Therapy-induced senescence can also be promoted in solitary organs, such as the kidney in the case of kidney transplant. Here, ischemia-reperfusion drives oxidative damage, DNA damage and proteotoxicity, and attempts by the donor kidney to replace lost cells following engraftment leads to telomere erosion. These stresses cause a high, organ-specific senescence burden, potentially leading to graft rejection or diminished excretory function.
On this background of lingering senescent cells, disease might emerge when additional stressors challenge vulnerable, senescent cell–rich tissue, such as insulin-resistant aged fat confronted with a high-fat diet58–60. Stress capable of causing disease may be unusual, such as DNA-damaging agents in cigarette smoke, or simply a more prolonged or more intense version of the same stresses operating in normal aging, such as telomere erosion following the repair of smoke-damaged lung epithelium61 (Fig. 2b). Since the stresses of disease have parallels in normal aging, it is not surprising that senescent cells are also produced by disease. Unlike normal aging, disease-induced senescence may be restricted to one or a few organs, and because disease-related senescence triggers are likely to be prolonged or more intense, the rate of accumulation of senescent cells is likely to be much higher than in normal aging.
Similarly, therapy-induced senescence is a response to potent exogenous stress. Therapy-induced senescence can be a deliberate goal or a side effect of medical treatments or procedures, or both, as with cytotoxic DNA-damaging chemotherapy and irradiation for cancer treatment, in which activation of the senescence program in tumor cells is the goal and collateral senescence of healthy cells the side effect (Fig. 2c).
Senescence in normal aging
Senescent cells are thought to accumulate in aged tissues based on the detection of cells with high SA-β-GAL activity and increased expression of the senescence master regulator, p16Ink4a (refs. 34,44,62–65). However, the rarity of these cells, the potential nonspecificity in both of these markers, and the lack of a good p16Ink4a antibody in the mouse have long precluded understanding of the causes of senescent arrest, the identity of the cells that undergo senescence and their contribution toward aging. Two tools have been instrumental in beginning to answer these questions: senescence-prone progeroid mouse models and transgenic mice that allow identification and killing of senescent cells.
Studies of progeroid mice expressing low levels of the mitotic checkpoint protein BubR1 have shown that although the damage causing senescence may be random, some cell types are more vulnerable to it than others6–8. For example, progenitor cells in fat tissue undergo senescence with predictable kinetics7,66. In this same model, fibroadipogenic progenitors in muscle also senesce prematurely, suggesting that progenitor cells may be uniquely susceptible to this stress type. Importantly, this model permitted rapid testing of the causal link between senescence and aging. Both abrogation of the senescence program by CDKN2A deletion and selective removal of p16Ink4a-expressing cells using INK-ATTAC, which functions as a senescent cell suicide transgene, in these animals blunted the aging process6,7. INK-ATTAC uses a fragment of the p16 promoter to drive expression of a caspase-8–FKBP fusion protein that is able to be dimerized and activated and to induce apoptosis when it encounters a synthetic drug, AP20187.
Though informative, these studies suffer the drawback that BubR1 deficiency is a non-physiological stress present from early development onward. The chronic senescent state in natural aging is likely to be more complex and probably induced by some combination of telomere attrition, (oxidative) DNA damage, ER stress and other slowly accumulating forms of macromolecular damage67–69. Further, the number of senescent cells in BubR1-hypomorphic animals is quite high compared with the senescent cell burden accumulating in normal aging7. Finally, whether these same cells are senescence prone in naturally aged mice and to what extent senescence drives aging in normal conditions remains to be seen.
Despite the current lack of evidence showing a causal link between senescence and natural aging, we can still speculate on a role for senescence in aging on the basis of the association of senescent cells with age. Evidence in vitro suggests that two key mechanisms are likely at play in senescence-driven tissue dysfunction, namely deterioration of tissue maintenance processes due to the SASP and removal of reparative stem and progenitor cells from the proliferative pool through senescence itself. Support for this two-part model includes the presence of SASP factors, as described in vitro, that are known to have in vivo functions in aging, such as the cytokines IL-6 and TNFα in aging tissues70,71.
Stem cell function can be affected by both cell-autonomous and paracrine functions of senescent cells. The cell-autonomous effects of senescence are most prominent in stem cells, where persistent growth arrest contributes to the overall decline in tissue regenerative potential. For example, in mouse skeletal muscle, the decline of the self-renewal capacity of muscle satellite cells impairs muscle regeneration72. Similarly, in the BubR1-hypomorphic progeroid mice, muscle and fat progenitor cells are highly prone to cellular senescence, driving loss of adipose tissue mass and profound sarcopenia6. Though one might expect tissues with rapid turnover, such as the gastrointestinal tract or the hematopoietic compartment73, to be senescence prone, this does not seem to be the case, suggesting that stresses other than those associated with replication drive chronic senescence. Rapidly dividing cells may also be more prone to apoptosis rather than senescence in response to stress, as evidenced by spermatogonial and crypt stem cells undergoing apoptosis in response to short telomeres74.
Optimal function of stem cells depends on their highly specialized microenvironment, or niche75,76, and, therefore, the SASP may deleteriously affect stem cells by altering this niche. For example, metalloproteinases in the SASP could destroy the polarized extracellular matrix. Removing these inhibiting factors may explain why killing of senescent progenitor cells in the BubR1 progeroid model ‘paradoxically’ improves sarcopenia6. The irreversibility of extracellular matrix damage is also consistent with the observation that senescent cell clearance halts, but does not reverse, tissue deterioration in this model6.
The SASP could also affect parenchymal cell function and tissue composition without influencing stem cells. Structural changes caused by the secretion of matrix metalloproteinases could damage surrounding extracellular matrix, potentially leading to effects such as loss of skin or lung elasticity77. Endocrine-responsive intracellular signaling cascades are also susceptible to known SASP factors. For example, senescent cells may directly affect the GH-IGF1 axis through TNFα, IL-1β and/or IL-6, secretion of which cause resistance to IGF1 signaling78. In combination with decreased circulating IGF1 caused by a decline in pituitary GH production79,80, peripheral IGF resistance in muscle can result in features of aging such as sarcopenia81 and reduced cardiac function82, though these studies should be interpreted with caution as they were all performed in mice. Components of the SASP can also lead to sterile inflammation, which is accompanied by macrophage and lymphocyte infiltration, apoptosis and fibrosis83.
When these results are taken together, a complex interaction between senescent cells and their local and systemic environments emerges. Over time, these effects develop into the classic age-related phenotypes of reduced tissue function and diminished stress resistance. Therefore, when a tissue that has been influenced by cellular senescence is challenged, it is more likely to become pathological.
Senescence in age-related disease
Numerous examples of senescence at sites of aging pathology have been reported, but whether they cause disease or are a consequence of pathology is unclear. In some cases, loss of proliferation-competent cells may be responsible for pathology, as in glaucoma84, cataracts6, the diabetic pancreas58 and osteoarthritis85. In others, inflammation from the SASP may play a causal role in disease, as in atherosclerosis86, diabetic fat58,59 and cancer87. Finally, SASP-mediated extracellular matrix remodeling may be key to disease progression or inhibition, as senescent cells drive pulmonary fibrosis88 but restrict liver fibrosis89.
These results lead us to propose a model of the interrelationship between chronic senescence and disease risk to guide further inquiry into these disease states. In youth and middle age, disease can occur when genetic or exogenous stressors overwhelm the normal capacity of a tissue to maintain function. For example, repetitive joint injuries can cause osteoarthritis in young adults when damage to the cartilage matrix exceeds the ability of this tissue to repair itself90. In this case, the short duration but intense stresses encountered in disease mirror those found during normal wear and tear that can produce senescent cells in aging. Supporting this, both injury-induced and age-related arthritic joints contain senescent chondrocytes91,92. Disease-causing stressors can also have no parallel during normative aging. For example, atherosclerosis is driven by the accumulation of oxidized low-density lipoprotein (LDL) in arterial walls as a consequence of high-fat diet93 despite LDL typically decreasing with age94.
In contrast to disease in youth, age-related disorders occur on a background of tissue already rendered dysfunctional by aging processes, including tissues that have senescent cell accumulation. We term these ‘primary’ senescent cells, because they result from normal wear-and-tear processes inherent to tissue maintenance. Senescence diminishes tissue resistance to disease-causing stresses via progenitor cell arrest, as well as through stem and parenchymal cell dysfunction via the SASP. As disease initiates and progresses, an additional wave of senescent cells is generated at sites of pathology. These ‘secondary’ senescent cells, like primary senescent cells, may amplify disease progression. This two-tiered model partially explaining why disease vulnerability rises with age is illustrated well by two case studies: the aging fat driving type 2 diabetes mellitus (T2DM) and the aging vasculature driving atherosclerosis (Fig. 3).
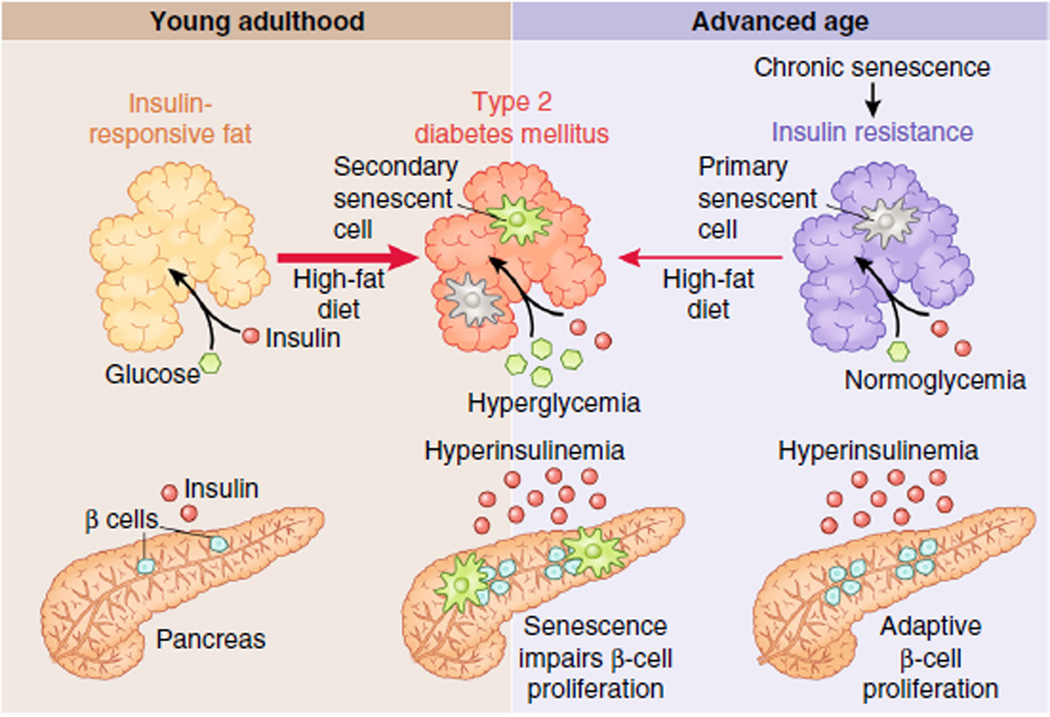
Figure 3.
Senescent cells as drivers and amplifiers of disease. The interrelationship of senescence-driven tissue dysfunction, susceptibility to disease-causing stress and senescence in disease is illustrated by the example of type 2 diabetes mellitus (T2DM). In young adulthood (shown at left), fat exposed to a healthy diet is insulin responsive and receives sufficient levels of insulin from the pancreas to take up glucose, maintaining normoglycemia. Chronic, high levels of free fatty acids in the circulation, due to obesity and a high-fat diet, can drive insulin resistance in peripheral adipose tissue by inducing proinflammatory senescent cells in fat. The pancreas can initially meet the increased demand for insulin through proliferation of insulin-producing β cells, maintaining normoglycemia with hyperinsulinemia. However, following telomere erosion, the capacity of β cells to expand production of insulin is limited by senescence. If insulin resistance worsens further, hyperglycemia and T2DM develop with accumulation of additional senescent cells in the fat. In contrast to T2DM development in youth, in advanced age (right), a form of peripheral insulin resistance already exists in the absence of overt dietary stresses that is due in part to the accumulation of senescent cells in aged fat. Therefore, the aged pancreas and fat can develop T2DM when stressed with a high-fat diet that is quite mild when compared to what is required for pathology to develop in youth.
Aging and T2DM
Circulating glucose triggers a release of insulin from the pancreas, permitting insulin-responsive peripheral tissues, such as fat and skeletal muscle, to take up glucose for cellular respiration95. In youth, chronic adipose tissue inflammation and high circulating free fatty acids can cause fat insulin resistance96,97, requiring the pancreas to generate more insulin to maintain normal glucose levels. Proliferation of insulin-secreting pancreatic β cells is required to meet demand. If peripheral tissue insulin demand exceeds supply, T2DM can result. Consistent with this, the regenerative capacity of β cells is a very important factor in the development of T2DM98. As in other diseases, increased replication of β cells lead to senescence through telomere attrition99, in principle restricting the adaptive response to insulin resistance and driving T2DM. Accordingly, nutrient-induced diabetic mice have increased SA-β-GAL–positive β cells and a reduction in the proliferation marker Ki67 in late-stage disease100. Further, deletion of CDKN2A rescues blood fasting glucose levels and improves β-cell proliferation in streptozocin-induced diabetes101.
Senescence may also predispose fat to becoming insulin resistant, as has been observed in fourth-generation Tert knockout mice, which have short telomeres, enhanced senescence in fat tissue and glucose intolerance59. Surgical removal of Tert−/− fat tissue attenuates glucose intolerance59, consistent with senescence in the fat driving insulin resistance. Additional support for a causal role of fat senescence in T2DM progression comes from disruption of the p53-p21 pathway. Global or adipose-specific deletion of TP53 improves glucose tolerance in obese mice, reduces adipose senescent cell burden and attenuates insulin resistance mediated by p53-dependent inflammation58,59.
Therefore, it appears that senescence is generated as a direct consequence of T2DM in both the fat and pancreas and drives progression of the disease. On the basis of this conclusion, we hypothesize that an increase in fat and β-cell senescence with age could explain the age-related increase in prevalence of T2DM. In the pancreas and fat, p16Ink4a levels increase with age in mice62 and humans101. This could restrict β-cell regenerative potential, limiting the adaptive response to insulin resistance101. In fat, levels of the SASP factors IL-6 and IL-1β increase with age, and these are known to cause insulin resistance when chronically high102–104. However, the enhanced glucose tolerance of naturally aged mice overexpressing p16Ink4a calls into question this model of a deleterious global role of senescence in T2DM105. This study identified enhanced insulin responsiveness in heart, liver and skeletal muscle, all tissues without established roles for senescence in glucose tolerance, suggesting that this may represent a tissue-specific effect. Furthermore, it is unclear what effect an extra copy of the CDKN2A locus has on senescent cell numbers in these tissues or fat.
Finally, the reduced ability to maintain glucose homeostasis in aging can lead to widespread glucose toxicity. This global stress could drive cellular senescence in various cell types such as fibroblasts, kidney tubular epithelial cells, endothelial cells and mesenchymal stem cells106–109. This potential of glucose to drive cellular senescence globally may contribute to other important age-related pathologies, such as vascular and kidney disease109.
Aging and atherosclerosis
In addition to causing T2DM, the stress of a high-fat diet can also drive atherosclerosis. Atherosclerosis is a disease of major arteries in which high levels of low-density lipoprotein bearing oxidative modifications accumulate in vessel walls, attracting phagocytic immune cells to form plaques110,111. Cardiovascular disease as a result of atherosclerosis is the primary cause of mortality in the Western world and is on the rise globally112. During plaque formation and expansion, smooth-muscle proliferation and declining levels of endothelial nitric oxide synthase can lead to telomere shortening and oxidative stress, respectively113,114. These are inducers of senescence, and it is not surprising that senescent vascular smooth muscle and endothelial cells have been reported in human and mouse atheromas115–117. Because of the complex signaling between these cell types and immune cells recruited to plaques, these findings raise the possibility of a multistep role of senescent cells in atherogenesis. First, plaque initiation could be driven by senescent endothelial cells, which, through the SASP and surface receptors, could mediate the initial invasion of circulating monocytes into the vessel wall86. Additionally, senescent endothelial cells are prone to apoptosis118, causing endothelial layer ‘leakiness’119 that would permit extravasation of oxidized LDL into the vessel wall. In turn, senescent endothelial cells cannot perform normal signaling tasks, such as the secretion of NO to restrain proliferation and prevent lipid peroxidation in smooth muscle cells120, and this could drive early intimal thickening, a key risk factor for atherosclerosis. Second, plaque progression could be mediated by chemoattractant factors in the SASP, including the cytokine MCP1 and interleukins with known proatherosclerotic functions121. Finally, senescent cells could contribute to plaque destabilization and formation of rupture-prone ‘vulnerable’ plaques that lead to acute complications such as stroke and myocardial infarction122. The transition from stable to unstable atheroma is driven in part by protease-mediated degradation of extracellular matrix in the smooth muscle–rich fibrous cap122. Elastase and matrix metalloproteases such as MMP1, MMP3 and MMP13 are known components of the vascular smooth muscle SASP and could potentially destabilize plaques by degrading extracellular matrix in the cap123–125.
The above scenario remains speculative and based on elevated p16Ink4a protein levels in atheromas alongside SA-β-GAL–positive cells, as well as in vitro proliferation assays of smooth muscle cells isolated from plaques as compared to healthy arteries93,126–128. Seemingly in contradiction to the model described above, some studies show that components of the senescence effector program, such as p53 (refs. 129,130) and p21 (ref. 131), actively protect against atherosclerosis. However, interpretation of these results is complicated by roles for these genes outside of senescence. As in cancer prevention, senescence in atherosclerosis may serve an initial protective role by restricting proliferation within developing lesions and minimizing plaque-disrupting apoptosis. However, at some threshold of senescence burden, the proinflammatory, matrix-degrading SASP may exacerbate disease, akin to how senescence may ultimately drive tumorigenesis via the SASP. It will therefore be critically important to test the causal contributions of senescence to atherogenesis at different stages of lesion development using new tools for the detection and killing of senescent cells in key model systems for human atherosclerosis.
Much as in T2DM, stresses of normal aging can induce senescence in cell types relevant to atherosclerotic disease. For example, senescent endothelial cells with short telomeres accumulate normally during aging in vascular beds with turbulent blood flow132. These same sites are atherosclerosis prone. It is therefore tempting to speculate that the age-related susceptibility to atherosclerosis arises in part from these chronic senescent cells and drives disease through the processes described above.
Therapy-induced senescence
Senescence as a therapeutic goal
Senescence as a therapeutic goal can be achieved either by applying systemic pro-senescent stress, such as ionizing radiation or DNA-damaging chemotherapy133, or by selectively restoring defective stress-response pathways (Fig. 2c). In cancer therapy, systemic treatments target cancer cells because their sustained engagement in the cell cycle makes them more susceptible to injury than nondividing cells. Additionally, cancer cells struggle to cope with further stress due to higher basal damage. These stresses proceed by activating a DDR or unfolded protein response. More recently, targeted therapies have been developed that inhibit senescence in order to ‘unmask’ the effects of stresses already at work within cancer cells and drive these cells into apoptosis134,135. One example in which senescence is an essential component of therapy is arsenic trioxide–retinoic acid therapy, which cures acute promyelocytic leukemia by blocking an oncogenic fusion protein and activating pro-senescent p53 signaling136. Solid tumors also benefit from senescence-inducing therapies, whereby inactivating crucial oncogenes or restoring tumor-suppressive signaling permits cancer cells to respond normally to intrinsic damage. This mechanism has been shown to occur when c-Myc overexpression137, PTEN insufficiency138 and Shp2 overexpression139 are corrected. Further, the CDK4 and CDK6 inhibitor palbociclib appears to restore senescence signaling in patients140, and derivatives of the p53 stabilizer Nutlin-3a are also being actively developed for the clinic. However, if such therapies generate persistent senescent cells that could damage or compromise surrounding tissue via the SASP, this may explain the long-term side effects of cancer treatment. Therefore, combining pro-senescence therapy with interventions that clear senescent cells could be beneficial for short- and long-term outcomes in cancer patients. Drugs that block pro-survival pathways in senescent cells, such as autophagy inhibitors, have shown promise for removing these lingering cells134.
Senescence as an undesirable side effect
Another informative example of therapy-induced senescence occurs in pediatric blood cancer therapy involving bone marrow transplantation. In this procedure, the patient’s immune system is ablated by ionizing radiation or chemotherapy, which, as an unwanted side effect, creates a senescent cell–rich milieu that might not only impair the engraftment and functionality of the hematopoietic system of the healthy donor141,142, but also accelerate tissue deterioration at a systemic level. Consistent with this, survivors of pediatric cancer who have gone through such treatments exhibit signs of premature aging143, including loss of cognitive function144 and heart failure145, with precocious elevation of p16Ink4a in skin49.
Finally, organ transplants may be a striking example of unwanted therapy-induced senescence. For example, transplanted kidneys are subject to ischemia-reperfusion injury146, a type of oxidative damage, as well as replicative stress as the shocked transplant has to repair inevitable tubular injury following engraftment147. Both of these stresses pose a risk for renal tubular epithelial senescence. Eliminating p16Ink4a-driven senescence in donor mice dramatically improves graft success148. Intriguingly, donor age and kidney p16Ink4a levels can predict graft success in humans149 and mice150. This might only indicate a lower stress tolerance or functionality of older donor organs that is not necessarily related to senescence151. However, a higher senescent cell burden could generate a proinflammatory environment in aged kidney and promote immune rejection. One other requirement of organ transplant is immunosuppression of the recipient. This could, unfortunately, lead to therapy-induced senescent cells remaining in the transplanted organ that may otherwise be clearable, reducing organ function via SASP effects.
Therapies targeting senescent cells: senotherapies
Clear healthspan benefits of preventing accumulation of p16Ink4a-expressing cells in BubR1-hypomorphic progeroid mice6,152, among other lines of evidence, have raised the possibility that therapeutic targeting of senescent cells is a promising strategy to overcome age-related disease and achieve healthy aging. There are several possible approaches, including inducing death of senescent cells or blocking the SASP, for targeting senescent cells and modulating their detrimental effects for therapeutic benefit. We refer to these strategies collectively as ‘senotherapies’. Each senotherapeutic modality possesses various advantages and disadvantages (Fig. 4).
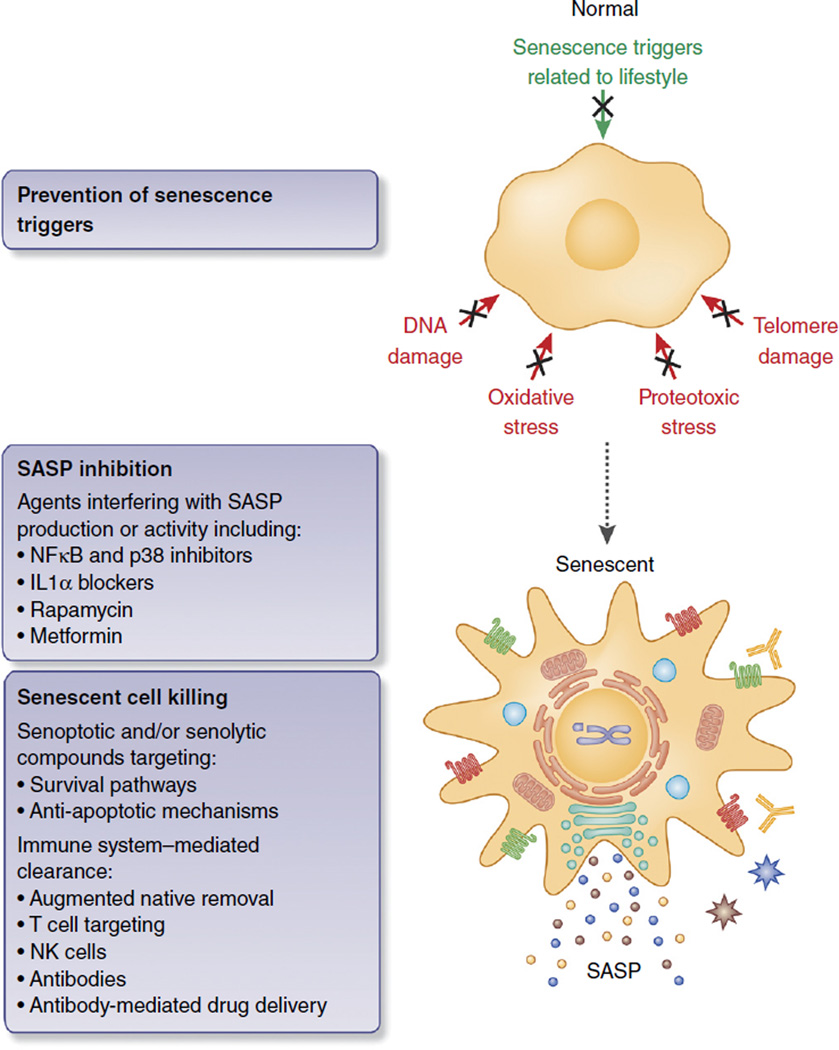
Figure 4.
Senotherapies to prevent disease and extend healthy life span. Outright killing of senescent cells by reprogrammed cytotoxic T cells, antibodies against abundant (selective) surface proteins of senescent cells, or small-molecule inhibitors (senolyic or senoptotic molecules) of, for instance, pro-survival pathways that senescent cells engage to avoid apoptosis. Alternatively, blocking p38 MAPK or IL-1α could inhibit the SASP itself, though this strategy would require continuous treatment and may therefore disrupt beneficial functions of senescence or other inflammatory processes. Inhibiting purely deleterious SASP factors or senescence-specific exocytosis processes, if they exist, would sidestep this problem. Finally, preventing the stresses leading to senescence, but not the senescence program itself, may promote healthy aging and prevent disease. Preventative steps include healthy diet, exercise and avoidance of lifestyle stresses such as smoking, but may also include ‘anti-aging’ drugs such as metformin and rapamycin.
The removal of senescent cells by direct killing, either by apoptotic (senoptosis) or nonapoptotic (senolysis)153 means, is probably the most straightforward option and offers clear advantages compared to other approaches. These advantages include permanent removal of the SASP source, permitting intermittent treatment, as well as elimination of senescent cells that may be preneoplastic, reducing cancer risk from senescence escape154. Therapeutics for killing senescent cells could take the form of senoptotic or senolytic small molecules or immune-based clearance (antibodies or cytotoxic T cells; Fig. 4). Like cancer cells, senescent cells undergo a chronic stress response due to persistent injuries such as eroded telomeres or aberrantly active mitogenic signaling. Whereas cancer cells are dependent on oncogenes, senescent cells rely on prosurvival stress response adaptations to avoid apoptosis155. This suggests that an attractive senescent cell killing approach would be to use small-molecule inhibitors to block cell death-resistance pathways, thereby using the endogenous stress to drive these long-lived cells into apoptosis. Existing inhibitors of prosurvival pathways used in cancer therapy may have utility for senescent cell killing, and could be even more effective for this use given that senescent cells, unlike cancer, do not proliferate. Therefore, no strong ‘selection pressure’ for drug resistance can develop.
One example of a cancer drug that may also target senescent cells is dasatinib153, a broad-spectrum kinase inhibitor that targets more than 30 different kinases43,156. In combination with quercetin, a plant flavonoid with lifespan-extending antioxidant and proteosome activating properties in worms157–160 and cytoprotective effects in mice161, dasatinib exhibits senolytic activity in cultured cells. In old mice, these compounds were shown to improve parameters of cardiac and vascular function, although the extent to which senescent cell killing occurred and contributed to these effects is unknown. Proof of causality would require showing that candidate senolytic or senoptotic compounds lack efficacy in an aged organism without the proposed target, senescent cells. For this reason, the greatest challenge to the emerging field of senotherapy research will be to directly attribute healthspan improvements to senescent cell killing rather than off-target effects. Reciprocally, whether known anti-aging compounds exert senotherapeutic activity as one of their healthspan-improving mechanisms is also an important consideration. Because of the multifaceted effects of these compounds, in instances where compounds reduce senescence, a crucial question to resolve is whether this observation is a cause of anti-aging effects or a consequence.
Minimizing off-target effects of senotherapeutics is an important consideration for both applied and basic science research. For example, senescence is beneficial in some contexts, such as wound repair, and these cells should be exempted from killing. Management of both types of off-target effects could be achieved by making clearance treatments periodic, thereby avoiding killing of beneficial senescent cells in the short term. On the other hand, periodic therapy must be weighed against our current ignorance of what fraction of senescent cells are needed to be removed to achieve clinical benefit. In the BubR1 hypomorphic mouse, the INK-ATTAC transgene prevented aging phenotypes through highly efficient senescent cell clearance. Furthermore, in this progeroid model, treatment begun in late life blunted further deterioration but did not reverse dysfunction6. One interpretation of this finding is that after a certain point, the SASP causes irreversible tissue deterioration. This observation raises the possibility that treatment in human aging must be done before a certain point to preserve function and also that the duration between treatment boluses may have an upper limit before damage is irreversible. These questions of what constitutes necessary and sufficient clearance could be answered by exploring senescent cell killing regimens in progeroid models or, ideally, in natural aging.
Minimizing off-target effects of senescent cell killing could be achieved in ways other than intermittent clearance. In the case of senescence in defined body compartments92,162, delivering drugs directly to the sites of pathology could avoid side effects. Antibodies raised against senescence-specific surface antigens, such as CD44 in the senescent endothelium163, could also be used to direct cytotoxic T cells for killing or to deliver cytotoxic nanoparticles. An intriguing possibility based on recent success in killing cancer cells is that senescent cell antigens could be used to raise T cells in vitro armed with artificial, chimeric antigen receptors (CARs) raised against senescent cell–specific surface antigens for infusion164. These CAR cells are emerging as highly efficient cancer therapy and may ultimately have a place for senescent cell clearance, once safety is demonstrated.
Another strategy that may complement senescent cell killing is blocking the paracrine effects of SASP (Fig. 4). This could be achieved by targeting signaling cascades upstream of the SASP, such as NF-κB or p38 MAPK. However, these signals are not exclusive to senescence, and thus intervention could affect communication between healthy cells, much as scavenging of ‘deleterious’ reactive oxygen species (ROS) has been shown to impair exercise performance in rats165. Making SASP inhibition into a viable therapy will require discovery of senescence-specific aspects of exocytosis in order to discriminate between the SASP and more ‘healthy’ types of inflammation. It is also possible that blocking the entire SASP is unnecessary and only senescence-specific SASP factors need to be removed to achieve therapeutic benefit.
Future directions
As our refinement of senescent cell identification and purification in vivo improves (see Box 1), we can begin to answer more interesting questions. One such great unknown is how long senescent cells live and why their quantity increases sharply with age. With the tools we have now, we can address such longstanding but simple questions as whether it is the rate of senescent cell production or the rate of clearance that is responsible for this effect. It is known that some senescent cells, such as premalignant hepatocytes, are killed by the immune system as a form of tumor suppression9,12. Senescent cells in fibrotic processes are similarly cleared10, whereas other senescent cells, such as senescent melanocytes, are spared this fate166. Understanding why some senescent cells are immune privileged and others are not could shed light on age-related senescent cell accumulation, which could be a type of similar immune privilege. Alternative explanations are a declining immune system, a reduced cellular tolerance for stress or a rise in certain types of stress with time.
A final, and perhaps the most important, unanswered question in the field is whether cellular senescence actually drives aging and reduces lifespan. Preventing the accumulation of senescent cells or removing them once they have arisen increases health span in the BubR1 hypomorphic mouse. However, lifespan is not thereby altered, and aging changes are preventable, but not reversible. Repeating this type of study in naturally aged mice is crucial to determine both whether the senescent cell clearance is practical in humans and the details of the approach. These questions can only be addressed by long-term natural aging studies in mice and, ultimately, in humans.
With the scientific community interested in senescent cell removal and a focused effort underway to identify compounds able to clear senescent cells, we may soon find ourselves testing pharmacological or biological therapies for senescent cell killing in vivo. The impact this will have on human health and disease is frankly unknown, but may reveal new biological phenomena. If senescent cells can be killed in humans, will this simply delay familiar aging dysfunction, or will new types of pathology emerge? Given the risks of all cytotoxic therapies and the long lifespan of humans, use of senescent cell killing compounds as a broad anti-aging therapy cannot be easily investigated in people. However, if approved for specific disease states, such compounds will inevitably end up being used chronically by adventurous patients ‘off label’. Much like people voluntarily performing calorie restriction, such ad hoc experimentation will probably be our first glimpse of the answer we all seek: is senotherapy a panacea for aging?
Acknowledgments
We are grateful to R. Naylor for reading the manuscript and providing helpful discussion. The US National Institutes of Health (J.M.v.D. R01CA96985 and AG41122-01P2), the Paul F. Glenn Foundation (D.J.B. and J.M.v.D.), the Ellison Medical Foundation (D.J.B.), and the Noaber Foundation (J.M.v.D.) provided financial support to the authors during the writing of the review.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Flatt T. A new definition of aging? Front. Genet. 2012;3:148. doi: 10.3389/fgene.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ungewitter E, Scrable H. Antagonistic pleiotropy and p53. Mech. Ageing Dev. 2009;130:10–17. doi: 10.1016/j.mad.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giaimo S, d’Adda di Fagagna F. Is cellular senescence an example of antagonistic pleiotropy? Aging Cell. 2012;11:378–383. doi: 10.1111/j.1474-9726.2012.00807.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 5.Sager R. Senescence as a mode of tumor suppression. Environ. Health Perspect. 1991;93:59–62. doi: 10.1289/ehp.919359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker DJ, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 9.Hoenicke L, Zender L. Immune surveillance of senescent cells—biological significance in cancer- and non-cancer pathologies. Carcinogenesis. 2012;33:1123–1126. doi: 10.1093/carcin/bgs124. [DOI] [PubMed] [Google Scholar]
- 10.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 13.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muñoz-Espín D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Storer M, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Alcorta DA, et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi A, et al. Mitogenic signalling and the p16Ink4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 19.Beauséjour CM, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaulian E, et al. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell. 2000;103:897–907. doi: 10.1016/s0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 21.Webley K, et al. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol. Cell. Biol. 2000;20:2803–2808. doi: 10.1128/mcb.20.8.2803-2808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu K, et al. [Cisplatin induces cell cycle arrest and senescence via upregulating P53 and P21 expression in HepG2 cells.] Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1253–1259. [PubMed] [Google Scholar]
- 23.Ge H, et al. Dexamethasone reduces sensitivity to cisplatin by blunting p53-dependent cellular senescence in non-small cell lung cancer. PLoS ONE. 2012;7:e51821. doi: 10.1371/journal.pone.0051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7:125–136. doi: 10.1111/j.1474-9726.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 25.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, et al. Differential p53-independent outcomes of p19(Arf) loss in oncogenesis. Sci. Signal. 2009;2:ra44. doi: 10.1126/scisignal.2000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 2005;25:2660–2672. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipriano R, et al. TGF-beta signaling engages an ATM-CHK2-p53-independent RAS-induced senescence and prevents malignant transformation in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 2011;108:8668–8673. doi: 10.1073/pnas.1015022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johmura Y, et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell. 2014;55:73–84. doi: 10.1016/j.molcel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 32.Martínez P, Blasco MA. Telomeric and extratelomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer. 2011;11:161–176. doi: 10.1038/nrc3025. [DOI] [PubMed] [Google Scholar]
- 33.Sousa-Victor P, Perdiguero E, Munoz-Canoves P. Geroconversion of aged muscle stem cells under regenerative pressure. Cell Cycle. 2014;13:3183–3190. doi: 10.4161/15384101.2014.965072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampel B, et al. Apoptosis resistance of senescent human fibroblasts is correlated with the absence of nuclear IGFBP-3. Aging Cell. 2005;4:325–330. doi: 10.1111/j.1474-9726.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- 36.Ryu SJ, Oh YS, Park SC. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007;14:1020–1028. doi: 10.1038/sj.cdd.4402091. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, et al. p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int. J. Mol. Med. 2008;21:645–653. [PubMed] [Google Scholar]
- 38.Pasillas MP, et al. Proteomic analysis reveals a role for Bcl2-associated athanogene 3 and major vault protein in resistance to apoptosis in senescent cells by regulating ERK1/2 activation. Mol. Cell. Proteomics. 2015;14:1–14. doi: 10.1074/mcp.M114.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gniadecki R, Hansen M, Wulf HC. Resistance of senescent keratinocytes to UV-induced apoptosis. Cell. Mol. Biol. 2000;46:121–127. [PubMed] [Google Scholar]
- 40.Coppé JP, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell. Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Nelson G, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 44.Burd CE, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajagopalan S. HLA-G-mediated NK cell senescence promotes vascular remodeling: implications for reproduction. Cell. Mol. Immunol. 2014;11:460–466. doi: 10.1038/cmi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhomen N, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Ness KK, et al. Frailty in childhood cancer survivors. Cancer. 2015;121:1540–1547. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcoux S, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat. Oncol. 2013;8:252. doi: 10.1186/1748-717X-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker DJ, Sedivy JM. Probing the depths of cellular senescence. J. Cell Biol. 2013;202:11–13. doi: 10.1083/jcb.201305155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohanna M, et al. Senescent cells develop a PARP-1 and nuclear factor-KB-associated secretome (PNAS) Genes Dev. 2011;25:1245–1261. doi: 10.1101/gad.625811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maskey RS, et al. Spartan deficiency causes genomic instability and progeroid phenotypes. Nat. Commun. 2014;5:5744. doi: 10.1038/ncomms6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim G, et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11:617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao K, et al. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 2011;121:2833–2844. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 2010;123:2605–2612. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu I, et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15:51–64. doi: 10.1016/j.cmet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Minamino T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 60.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 61.Walters MS, et al. Smoking accelerates aging of the small airway epithelium. Respir. Res. 2014;15:94. doi: 10.1186/s12931-014-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber HE, Ingram JA, Norton HJ, Hanley EN., Jr Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 64.Geng YQ, Guan JT, Xu XH, Fu YC. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem. Biophys. Res. Commun. 2010;396:866–869. doi: 10.1016/j.bbrc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Yang HC, et al. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J. Am. Soc. Nephrol. 2009;20:2380–2388. doi: 10.1681/ASN.2008111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep. 2013;3:1164–1174. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaszubowska L. Telomere shortening and ageing of the immune system. J. Physiol. Pharmacol. 2008;59(suppl. 9):169–186. [PubMed] [Google Scholar]
- 68.Titus S, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013;5:172ra121. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown MK, Naidoo N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012;3:263. doi: 10.3389/fphys.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morin CL, Pagliassotti MJ, Windmiller D, Eckel RH. Adipose tissue-derived tumor necrosis factor-alpha activity is elevated in older rats. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52:B190–B195. doi: 10.1093/gerona/52a.4.b190. [DOI] [PubMed] [Google Scholar]
- 71.Starr ME, Saito M, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation-II: the role of IL-1beta in age-dependent IL-6 upregulation in adipose tissue. J. Gerontol. A Biol. Sci. Med. Sci. 2014 Oct 24; doi: 10.1093/gerona/glu197. doi:10.1093/gerona/glu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernet JD, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 74.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 75.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang YC, Sinha M, Cerletti M, Dall’Osso C, Wagers AJ. Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb. Symp. Quant. Biol. 2011;76:101–111. doi: 10.1101/sqb.2011.76.010652. [DOI] [PubMed] [Google Scholar]
- 77.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 78.O’Connor JC, et al. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell. Immunol. 2008;252:91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong Z, et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown OA, Sosa YE, Dardenne M, Pleau J, Goya RG. Growth hormone-releasing activity of thymulin on pituitary somatotropes is age dependent. Neuroendocrinology. 1999;69:20–27. doi: 10.1159/000054399. [DOI] [PubMed] [Google Scholar]
- 81.Schaap LA, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moellendorf S, et al. IGF-IR signaling attenuates the age-related decline of diastolic cardiac function. Am. J. Physiol. Endocrinol. Metab. 2012;303:E213–E222. doi: 10.1152/ajpendo.00538.2011. [DOI] [PubMed] [Google Scholar]
- 83.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liton PB, et al. Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin. Orthop. Relat. Res. 2004;427(suppl.):S96–S103. doi: 10.1097/01.blo.0000143818.74887.b1. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X, Perez F, Han K, Jurivich DA. Clonal senescence alters endothelial ICAM-1 function. Mech. Ageing Dev. 2006;127:779–785. doi: 10.1016/j.mad.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Thangavel C, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl. Res. 2013;162:156–173. doi: 10.1016/j.trsl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Kong X, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr. Opin. Rheumatol. 2005;17:195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 91.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41:479–491. [PubMed] [Google Scholar]
- 93.Matthews C, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 94.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, HDL cholesterol decrease with age in older men, women The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 95.Wilcox G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 96.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costes S, Langen R, Gurlo T, Matveyenko AV, Butler PC. β-Cell failure in type 2 diabetes: a case of asking too much of too few? Diabetes. 2013;62:327–335. doi: 10.2337/db12-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo N, et al. Short telomeres compromise beta-cell signaling and survival. PLoS ONE. 2011;6:e17858. doi: 10.1371/journal.pone.0017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48:58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 101.Krishnamurthy J, et al. p16Ink4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 102.Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Harder-Lauridsen NM, et al. Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2014;306:E769–E778. doi: 10.1152/ajpendo.00571.2013. [DOI] [PubMed] [Google Scholar]
- 104.Gao D, et al. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014;307:E289–E304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.González-Navarro H, et al. Increased dosage of Ink4/Arf protects against glucose intolerance and insulin resistance associated with aging. Aging Cell. 2013;12:102–111. doi: 10.1111/acel.12023. [DOI] [PubMed] [Google Scholar]
- 106.Yang TK, et al. Davallialactone from mushroom reduced premature senescence and inflammation on glucose oxidative stress in human diploid fibroblast cells. J. Agric. Food Chem. 2013;61:7089–7095. doi: 10.1021/jf401691y. [DOI] [PubMed] [Google Scholar]
- 107.Liu J, et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell. Signal. 2014;26:110–121. doi: 10.1016/j.cellsig.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim YJ, et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev. 2012;21:1749–1760. doi: 10.1089/scd.2011.0429. [DOI] [PubMed] [Google Scholar]
- 110.Jialal I, Devaraj S. The role of oxidized low density lipoprotein in atherogenesis. J. Nutr. 1996;126:1053S–1057S. doi: 10.1093/jn/126.suppl_4.1053S. [DOI] [PubMed] [Google Scholar]
- 111.Ilhan F, Kalkanli ST. Atherosclerosis and the role of immune cells. World J. Clin. Cases. 2015;3:345–352. doi: 10.12998/wjcc.v3.i4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heidenreich PA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 113.Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209:35–38. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 115.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 116.Minamino T. [Contribution of vascular cell senescence to atherogenesis] Nippon Ronen Igakkai Zasshi. 2008;45:295–298. doi: 10.3143/geriatrics.45.295. [DOI] [PubMed] [Google Scholar]
- 117.Bürrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler. Thromb. 1991;11:1678–1689. [PubMed] [Google Scholar]
- 118.Zhang J, Patel JM, Block ER. Enhanced apoptosis in prolonged cultures of senescent porcine pulmonary artery endothelial cells. Mech. Ageing Dev. 2002;123:613–625. doi: 10.1016/s0047-6374(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 119.Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc. Cell. 2012;4:12. doi: 10.1186/2045-824X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hogg N, Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochim. Biophys. Acta. 1999;1411:378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 121.Rippe C, et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp. Gerontol. 2012;47:45–51. doi: 10.1016/j.exger.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 123.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robert L, Robert AM, Jacotot B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis. 1998;140:281–295. doi: 10.1016/s0021-9150(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 125.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Minamino T, Miyauchi H, Yoshida T, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomeres in endothelial dysfunction. J. Cardiol. 2003;41:39–40. [PubMed] [Google Scholar]
- 127.Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 128.Yamada N. Telomere shortening, atherosclerosis, and metabolic syndrome. Intern. Med. 2003;42:135–136. doi: 10.2169/internalmedicine.42.135. [DOI] [PubMed] [Google Scholar]
- 129.Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ. Res. 2005;96:667–674. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 130.van Vlijmen BJ, et al. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ. Res. 2001;88:780–786. doi: 10.1161/hh0801.089261. [DOI] [PubMed] [Google Scholar]
- 131.Khanna AK. Enhanced susceptibility of cyclin kinase inhibitor p21 knockout mice to high fat diet induced atherosclerosis. J. Biomed. Sci. 2009;16:66. doi: 10.1186/1423-0127-16-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsushita H, et al. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ. Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 133.Sabin RJ, Anderson RM. Cellular senescence—its role in cancer and the response to ionizing radiation. Genome Integr. 2011;2:7. doi: 10.1186/2041-9414-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dörr JR, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 135.Crescenzi E, Palumbo G, de Boer J, Brady HJ. Ataxia telangiectasia mutated and p21CIP1 modulate cell survival of drug-induced senescent tumor cells: implications for chemotherapy. Clin. Cancer Res. 2008;14:1877–1887. doi: 10.1158/1078-0432.CCR-07-4298. [DOI] [PubMed] [Google Scholar]
- 136.Ablain J, et al. Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat. Med. 2014;20:167–174. doi: 10.1038/nm.3441. [DOI] [PubMed] [Google Scholar]
- 137.Wu CH, et al. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc. Natl. Acad. Sci. USA. 2007;104:13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toso A, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9:75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 139.Serrano M. SHP2: a new target for pro-senescence cancer therapies. EMBO J. 2015;34:1439–1441. doi: 10.15252/embj.201591616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cadoo KA, Gucalp A, Traina TA. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer. 2014;6:123–133. doi: 10.2147/BCTT.S46725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang KJ, et al. [Mechanism of radiation induced premature senescence of bone marrow stromal cells: experiment with murine bone marrow stromal cells.] Zhonghua Yi Xue Za Zhi. 2006;86:3431–3434. [PubMed] [Google Scholar]
- 142.Cmielova J, et al. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int. J. Radiat. Biol. 2012;88:393–404. doi: 10.3109/09553002.2012.666001. [DOI] [PubMed] [Google Scholar]
- 143.Ness KK, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. J. Clin. Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brinkman TM, et al. Cognitive function social attainment in adult survivors of retinoblastoma: a report from the St. Jude Lifetime Cohort Study. Cancer. 2015;121:123–131. doi: 10.1002/cncr.28924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gudmundsdottir T, et al. Cardiovascular disease in Adult Life after Childhood Cancer in Scandinavia: a population-based cohort study of 32,308 one-year survivors. Int. J. Cancer. 2015;137:1176–1186. doi: 10.1002/ijc.29468. [DOI] [PubMed] [Google Scholar]
- 146.Chkhotua AB, Abendroth D, Froeba G, Schelzig H. Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl. Int. 2006;19:72–77. doi: 10.1111/j.1432-2277.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 147.Chkhotua A, et al. Replicative senescence in organ transplantation-mechanisms and significance. Transpl. Immunol. 2002;9:165–171. doi: 10.1016/s0966-3274(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 148.Braun H, et al. Cellular senescence limits regenerative capacity and allograft survival. J. Am. Soc. Nephrol. 2012;23:1467–1473. doi: 10.1681/ASN.2011100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Melk A, et al. Effects of donor age and cell senescence on kidney allograft survival. Am. J. Transplant. 2009;9:114–123. doi: 10.1111/j.1600-6143.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- 150.Koppelstaetter C, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell. 2008;7:491–497. doi: 10.1111/j.1474-9726.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 151.Gingell-Littlejohn M, et al. Pre-transplant CDKN2A expression in kidney biopsies predicts renal function and is a future component of donor scoring criteria. PLoS ONE. 2013;8:e68133. doi: 10.1371/journal.pone.0068133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baker DJ, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu W, Sharpless NE. Senescence-escape in melanoma. Pigment Cell Melanoma Res. 2012;25:408–409. doi: 10.1111/j.1755-148x.2012.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi H, Zhang CJ, Chen GYJ, Yao SQ. Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes. J. Am. Chem. Soc. 2012;134:3001–3014. doi: 10.1021/ja208518u. [DOI] [PubMed] [Google Scholar]
- 157.Saul N, Pietsch K, Menzel R, Steinberg CEW. Quercetin-mediated longevity in Caenorhabditis elegans: is DAF-16 involved? Mech. Ageing Dev. 2008;129:611–613. doi: 10.1016/j.mad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 158.Kampkötter A, et al. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 159.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 160.Chondrogianni N, et al. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010;45:763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 161.Lu J, et al. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J. Pathol. 2010;222:199–212. doi: 10.1002/path.2754. [DOI] [PubMed] [Google Scholar]
- 162.Price JS, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 163.Mun GI, Boo YC. Identification of CD44 as a senescence-induced cell adhesion gene responsible for the enhanced monocyte recruitment to senescent endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H2102–H2111. doi: 10.1152/ajpheart.00835.2009. [DOI] [PubMed] [Google Scholar]
- 164.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gomez-Cabrera MC, et al. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. (Lond.) 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 167.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 168.Bursuker I, Rhodes JM, Goldman R. Beta-galactosidase—an indicator of the maturational stage of mouse and human mononuclear phagocytes. J. Cell. Physiol. 1982;112:385–390. doi: 10.1002/jcp.1041120312. [DOI] [PubMed] [Google Scholar]