Abstract
Acousto-electric interaction signal (AEI signal) resulting from interaction of acoustic pressure wave and electrical current field has received recent attention in biomedical field for detection and registration of bioelectrical current. The signal is very of small value and brings about several challenges when detecting it. Several observations has been done in saline solution and on nerves and tissues under controlled condition that give optimistic indication about its utilization. Ultrasound Current Source Density Imaging (UCSDI) has been introduced, that uses the AEI signal to image the current distribution. This review provides an overview of the investigations on the AEI signal and USCDI imaging that has been made, their results and several considerations on the limitations and future possibilities on using the acousto-electric interaction signal.
Key Words: Acousto-electric interaction signal, Ultrasound Current Source Density Imaging, tissues, muscles
Acousto-electric signal is a result of interaction of pressure and current. As pressure wave propagates through a medium, it changes its conductivity locally. It causes an increase in conductivity when the pressure falls and a decrease when the pressure rises. Consequently if electrical current is flowing through the medium potential changes will take place. Periodical change in pressure is produced by an ultrasound wave traveling through a conductive medium. This in turn modifies the conductivity of the medium. This is called the acousto-electric effect and can be described by the Eq. 1:
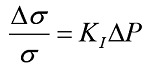 |
(1) |
where KI is the interaction coefficient characteristic for the medium, σ is the conductivity, Δσ conductivity change due to the ultrasound wave and ΔP the pressure change. If an electrical current is flowing through the medium at the same time a periodical voltage change will take place due to the change in conductivity. The resulting signal is called the aousto-electric interaction signal (AEI signal). Its origin is at the location of the pressure changes. This principle is visualized in Figure 1.
Fig 1.
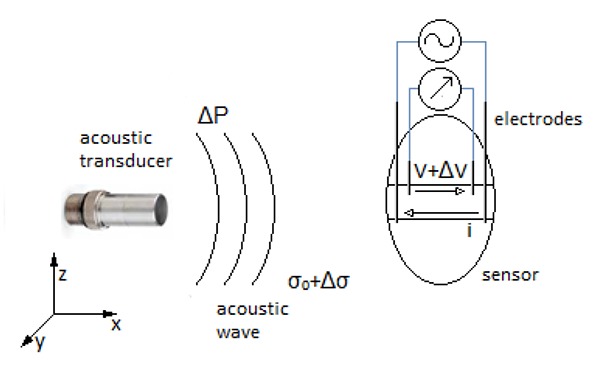
The principle of acousto-electric interaction signal. The pressure wave (ΔP) causes a local change in conductivity (Δσ) of the solution. When electrical current (i) flows between two electrodes, perpendicular to the propagation direction of the acoustic wave, it generates electrical field within specific area. Operating simultaneously, they give a rise to a signal generation (ΔV), measured between two recording electrodes, that is both proportional to the acoustic wave and the current flowing.
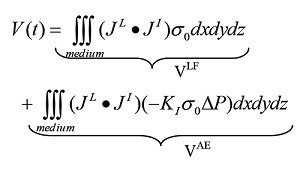
Using a focused ultrasound wave the strongest AEI signal will be generated in the focal point. The signal can be described by the Eq. 2:2
| (2) |
JL is the lead field of the recording electrodes and JI the distributed current source and V is the signal detected by electrodes. This equation can be rewritten:
 |
(3) |
The first term is a low frequency part of the injected current but the second term is the high frequency AEI signal. This frequency is the frequency of the ultrasound wave. The AEI signal can easily be filtered from other signals in living tissue, for example in muscle, as no natural signal has the frequency of an ultrasound wave.
Even though acousto-electrical effect has been observed since in the 1940s,3 it has only recently received interest in the biomedical field. As it is quite recent, relatively little has been written so far about it in this field, nevertheless giving some positive indications about the efficiency of its use in biomedical applications. In the late 1990s a group of French academics renewed the interest in acosto-electrical effect when they started to investigate and observe the AEI signal in a saline solution.1,3-5 About a decade later the a group from Canada also performed observations on it.6 Most subsequently Zhang and Wang used the acousto-electrical effect in an experiment for imaging the electric impedance properties of biological tissues in acousto-electric tomography.7 Influential in the research of acousto-electric interaction signal in the recent years, has been a group of American researchers at the University of Michigan, that have performed several investigations on the AEI signal and the possibility of using the signal to map biological current.2,8-17 Several more studies have been performed for further investigations and possible improvements of measurements and setup.18 Also our group has made some investigations and come up with a potentially useful application for denervated muscles.19
Measurements in saline solution
Acousto-electric interaction signal
All of the observations of acousto-electric interaction signal have been performed in saline solution. Saline solution has similar composition as blood and extracellular fluid, containing approximately 0.9% NaCl. Relatively simple measurement setup can be created, containing transducer transmitting a pressure wave, current generator and voltage recording. The dilemma is to control relevant variables and condition the signal, as the signal is of low magnitude.
This principle of setup is fundamental in the observations of acousto-electrical effect and is similar to all experiments (see Figure 2), though the geometry, derivation and values of distinctive parameters may differ. In principle, the pulser-receiver transmits an electrical pulse to the transducer that generates corresponding pressure wave. As the pressure wave propagates in the medium, the conductivity of the medium changes locally in the direction of the wave. A measurement cell, containing current injecting electrodes and recording electrodes is placed at the focal point of the transducer, where the maximum pressure occurs. The outer pair of electrodes injects the current, while the recording electrodes detecting the AEI signal are located within the current density field. These parameters may differ, as it is also possible to detect the AEI signal with only one recording electrode and a ground reference electrode. The cell has a defined geometry, which helps to control and evaluate the current density. The recorded signal is amplified before it is visualized on an oscilloscope. Hydrophone is often used to measure the pressure amplitude of the acoustic wave.
Jossinet, Lavandier and Cathignol investigated anew the acousto-electrical interaction signal at the end of last century. They reviewed the phenomena involved the generation of the AEI signal.3,4 The changes in conductivity arises from variations in pressure and temperature. Pressure is the primary cause, bringing about adiabatic process of conversion of the mechanical energy of pressure wave to heat, thus changing the temperature. This procedure is adiabatic process, as the time for heat transfer to take place is not enough within the period of acoustic wave. This procedure affects the properties of the solution in three ways: the bulk compressibility, the ionic mobility and the dissociation equilibrium. Bulk compressibility is caused by changes in the specific volume of the solutions and brings about changes in molarity; ionic mobility is affected by the change in viscosity; and dissociation coefficient of partially dissociated species varies both with temperature and pressure. For strong electrolytes, the dissociation coefficient is equal to unity and the electrolytes are totally dissociated. For a NaCl solution, bulk compressibility is responsible for about 47% of the effect, 18% are due to the effect of pressure upon the change in viscosity and changes of ionic mobility against temperature accounts for the residual 35%.
Special attention has to be paid measuring the AEI signal, as it is of small value. Jossinet et al. detected the acousto-electrical signal in saline solution, utilizing experimental setup comparable to the one described above.4 It was only several microvolts. A very small change in conductivity is caused by change in pressure, about 0.1% per MPa for NaCl solution,1,5 which contributes to small AEI signal. Additionally, a sinusoidal pressure wave, including both positive and negative pressure peaks, produces opposite conductivity changes within one wavelength. Over larger distance, as the distance between the recording electrodes, the opposite changes of conductivity tend to neutralize the influence of each other on the local resistance, making the acousto-electrical signal smaller. The use of unipolar pulses tends to avoid this problem.5 More effects resulting in a small signal include increased measured impedance because of the summation of the medium impedance and electrode contact impedance, increasing the overall impedance. The nonuniform sensitive distribution of the electrodes also plays a role, as well as imperfections in geometry and uncertainties in estimation of the interaction coefficient K.4,5 The acoustoelectric interaction constant K determines how much the electrical resistivity changes due to ultrasound pressure. Li et al. estimated the constant for different ionic salts and cardiac tissue.1,6 K appears to vary with concentration in a solution of many ions, such as CuSO4, because of the degree of dissociation of the ions in solution, whereas in monovalent salt, like NaCl, no such difference is observed. From the seven hearts observed, K in the heart was measured to be 0.041±0.012.%/MPa. The constant between different hearts varied slightly, which could be because of difference between the hearts and tissue preparation, but the interaction constant did not differ much with varying temperature. The K in hearts was of similar value as in 0.9% saline (0.034±0.003.%/MPa). That can be explained because of the composition of the extracellular fluid and blood that contain approximately 0.9% NaCl.
In order to evaluate the acoustic signal, the measured Ultrasonic Vibrational Potential (UVP) or Debye effect, has to be taken into account. Debye effect arises from periodical displacement of the ions due to pressure, causing unequal distribution of negative and positive charges and subsequently potential difference. Debye effect arises without applied current and has to be subtracted from the measured detected voltage in order to receive the AEI signal. Theoretically in order to get the greatest AEI signal, the current should be perpendicular to the ultrasound propagation. However at 90° the Debye effect component is so small and hard to detect. It would therefore induce some error and influence the measured signal. It is thus better to measure at a smaller angle, where it is easier to detect the Debye effect.1
The relationship between the AEI signal and current and pressure respectively has been investigated. Higher signal follows increased pressure and increased current density.3,5 The effect of pressure and current on the signal was measured, keeping one parameter constant while the other one was varied.1 The signal is linearly proportional to both, with an interaction factor of 5.3 µV mA-1MPa-1 between the voltage, pressure and current. The measured AEI signal in saline solution was in the order of several dozen microvolts under controlled condition. Gendron, Guardo and Bertrand achieved an AEI signal in order of hundreds of microvolts in their experimental setup, after eliminating the Debye effect by subtracting the AEI signal derived at the negative current peak from the one derived at the positive peak.6 Their setup resembled the previously used as described above. Additionally, it was observed that the signal was not directly proportional to the electric signal driving the transducer and a certain threshold needed to be reached in order to detect the AEI signal. This could be a result of cavitation that arises in the decompression phase of the ultrasound. These bubbles affect the conductivity of the solution and thus influence the AEI signal and can lead to incorrect measurement of the signal. Special attention should be in avoiding cavitation, by using pressure amplitude below cavitation formation threshold or by using unipolar pulses.
Ultrasound Current Source Density Imaging
The acousto-electrical interaction signal can be reconstructed and used in medical imaging. Zhang and Wang have developed the idea of acousto-electric tomography (AET) to image electric impedance.7 The measurement was performed in a bath of mineral oil, which promotes acoustic coupling and electrical isolation. The difference in conductivity could clearly be seen in 2D of rubber in saline solution and its size be identified. Fat on a chicken breast tissue was also imaged. As the tissue is not homogenous, like saline, several discontinuities were observed. The spatial resolution of acousto-electric tomography was of 2.3 mm, with a good contrast. The resolution was limited by the focal size of the ultrasound. Comparing this to electric impedance tomography (EIT), AET had better resolution and simpler process.
The team from Michigan has been focusing on electrical mapping using acousto-electric effect, a technique called Ultrasound Current Source Density Imaging (UCSDI). Electrical mapping has mainly been done in 2D but can be made in 3D using the same procedure. A current distribution in two dimensions was reconstructed.8 The injected current, resembling the current of the heart, with peak current density of 8 mA/cm2, was injected into a chamber filled with saline solution. The reconstructed signal represents relatively well the simulated ECG signal, though the some parts of the high frequency were missing. An accuracy of approximately 1 ± 2 mm was achieved in reconstruction of a 2D dipole field in a saline solution, by assuming that the current is coherent within a beamwidth.9 Likewise a current source and sink were successfully locate in 2D, with the precision accomplished to be within 1 mm, with previous estimation of resistivity distribution, and corresponded well with simulated data.2 The accuracy was limited by the sampling interval, which was of 2.2 mm.
This method can also be used for time dependent volume imaging, as shown using alternative current for depicting dipole field.10 The reconstruction was made in a saline solution, with ultrasound transducer scanned in a plane. At each time interval electrodes together with a ground reference depicted an image. Time-varying image of the current distribution was obtained, though the slow scan time necessary to obtain 4D data introduces some limitations, but more than an hour was required. 4D UCSDI simulation of a dipole field was developed for comparison and validation of the AEI signal.11 Multi-channel UCSDI could potentially improve the mapping and achieve more detailed information about the internal current distribution of the tissue.
UCSDI has a high spatial resolution and sensitivity. The resolution is determined by the ultrasound beam and sampling interval.2,9 The frequency and the shape of the beam also matters.12 By using higher ultrasound bandwidth, the spatial resolution was improved, but on the cost of sensitivity. Likewise, chirp excitation of the ultrasound improved the sensitivity, compared to square wave. Yang et al. from University of Minnesota proposed used unipolar pulses to the ultrasound to reconstruct an image of the current density distribution.18 By using unipolar pulses instead of bipolar the generated AEI signal turns out to be stronger, with an increase of more than 250 times when using pressure amplitude of 1 MPa. According to Yang, the unipolar pulses give thus a better estimation of the current density distribution, but the setup needs at least three recording electrodes. The AEI signal detected by unipolar pulse, showed more reliable variation within the measurement area, as it is not as sensitive to boundaries where strong current gradient exists, but is more sensitive instead to variation within the imaged object. It has stable performance for different parameter setups and noise is minimal. Further optimization of the ultrasound parameters, could improve the sensitivity and imaging of bioelectrical current even more.
By using the pulse echo signal generated by the ultrasound transducer the geometry measured by the traditional B-scan ultrasound can be co-registered along with the AEI signal.9 This procedure could offer valuable improvements, compared to existing examining methods, as it offers anatomical image simultaneously, during registration of the electrical activity within the body. This could offer possibilities in surgical guidance and during treatment of various diseases. Conventional methods use often computed tomography (CT) or magnetic resonance image (MRI) to map the anatomy and give a static image prior to intervention. But during the procedure, it is often not possible to review the circumstances. The registration error between the CT and/or MRI to the electroanatomical mapping has been reported to be about 2-10 cm.2 This method could facilitate corrective procedures for cardiac and neural abnormalities.17,15,11 These experiment use measurement setups in a saline solution under controlled condition. This demonstrates the optimum condition for detecting the small AEI signal. The geometry is controlled, the solution is homogenous and relatively little disturbances encountered. Furthermore in these setups it is assumed that the conductivitity distribution is assumed to be known. Within the body the resitivity distribution vary and is gernerally not know beforehand. It could be could be obtain using the images from electrical impedance tomography or tissue segmentation using CT or MRI.18 Several difficulties were met in recording the AEI signal for the UCSDI. The geometry brings about limitation, as it is not possible to register near to the injecting current wires.2 Also the detected AEI signal has to be appropriately recorded, filtered and conditioned. The scan time and acquisition rate is limited by scanning of the transducer. In theory, UCSDI should be able to perform as fast as traditional pulse-echo ultrasound, or even faster because it only demands one-way propagation of the acoustic wave and not returning echo as pulse-echo ultrasound.11
Observations in nerves
While most of the observations have been focused on the acousto-electric effect itself, relatively few observations have been made on these effects on biological nerves or tissues so far. The team from the University of Michigan has made observation on AEI in sciatic nerve of a rat, lobster nerve cord and tissues from chicken. Experimental system was well controlled. A piece of the nerve or the tissue was cut, isolated and immersed in mineral oil that serves as acoustic coupling and electrical insulation. Specific geometrical dimension of both the investigated objects and the measurement setup help to register the AEI signal and offers relatively simple software model. Acousto-electrical effect was used to image current distribution in a sciatic nerve that was obtained fresh from a rat.13 The nerve was isolated, immersed in mineral oil and placed on pairs of electrodes that either wise injected current or recorded the voltage within the current density field. In this simple geometry it was possible to determine electrical current spatially and temporally. Simultaneously a pulse-echo image was registered, that matched to the AEI signals. The signal was proportional to the current density, which was in relation to signal to noise ratio. Similar procedure was followed when imaging injected current in an abdominal segment of a fresh lobster nerve cord.14 Three-compartment chamber was used for this experiment, where the central part of the nerve was placed in middle chamber filled with mineral oil. The injecting electrodes were situated in the side chambers, giving electrical current through the nerve, while recording electrodes were located at the exterior of the nerve in the middle chamber. The AEI signal measured showed that the acoustoelectric signal was directly proportional to both the applied pressure and current density, with the slope of focal pressure being 23 µV/MPa at 75 mA/cm2, whereas the current density has the slope 0.7 µV/mA cm2 at 2 MPa. The current density and the pressure at physiological values were detected, where the current density was less than 10 mA/cm2 and pressure in the range of clinical ultrasound imaging.
Finally, two tissues from turkey were investigated simultaneously.15 Two distinctive slices from a tissue of a turkey were placed initially in saline solution before inserted into a chamber filled with mineral oil at different distance from the transducer. The current was injected to them in opposite directions. Recording electrodes were connected to each one and subsequently to a differential amplifier. The slices were located very close to each other, separated by a thin film of 0.2 mm. The recorded AE images appeared to be identical to the real tissues, in size and location, and the tissues could be successfully separated. The AEI signal was in relation to both pressure and current, with the detection levels in the order of 1 mA/cm2 at 258 kPa. The magnitude and direction of the current, the location of the current field within the ultrasound field and the geometry play all part in the construction of the image and sensitivity determined by the diameter of the acoustic beam.
Observations in muscles
Even fewer observations have been made on acoustoelectric interaction signal in muscles than with nerves and tissues. At the University of Michigan experiment has though been performed where a biopotential of a fresh rabbit heart was measured. The preparation and procedure resembled with previous experiments of the nerves and tissues, where the heart was decoupled, isolated and placed in specific holder that specifies the outlay of the heart.
UCSDI imaging was used to map biopotential in a fresh heart of a rabbit.17 The heart was injected with the excitation-contraction decoupler BDM to suppress the motion, avoiding motion artifacts. The heart was isolated and placed in a predetermined way in a tank that was surrounded by a bigger tank filled with deionized water.
Four stimulating electrodes were placed in the heart; two in the apax and in the right atrium another pair. The recording electrodes were inserted in the left ventricle approximately along the long axis of the heart. Under this controlled condition, it was possible to detect the spreading activation wave, spatially and temporally, that had a propagation velocity estimated to be 0.25 ± 0.05 mm/ms. The detection levels were in the order of 1 mA/cm2 at 258 kPa. This velocity is comparable to the results obtained from electrocardiogram (ECG).
A limitation of this study was the hardware that could not acquire all the electrodes simultaneously. Gudjonsdottir et al. has derivated that it is possible to use AEI signal to map the current distribution in denervated thigh muscle.19 The AEI signal that would be detected is of measurable dimensions and frequency that is easy to filter out. This can be practical during functional electrical stimulation (FES) on the thigh, to ensure that the whole muscle is stimulated during the treatment and estimating the efficiency of the treatment.
Discussion and Conclusions
These researches have shown that it is possible to detect the acousto-electric effect and reconstruct ultrasound current source density image (UCSDI) to model the distribution of current density. So far, the acousto-electrical effect or the AEI signal has only been observed in saline solution under controlled condition. It should be sensitive enough to detect biological current, by using safe levels of clinical ultrasound. It should be born in mind that the AEI signal is measured under optimal condition, where the geometry is controlled and defined in a clear way. Unlike physiological tissues, saline solution is homogenous and minimal disturbances were encountered. Conductivity distribution and sensitivity distribution are nonhomogenous in physiological condition and unknown. As the AEI signal is of very low amplitude, this could introduce specific problems encountering it within biological tissues. Critical is to use safe amount of current, so the patient would not perceive it and ultrasound within clinical limits, to avoid destruction of some tissues. Higher ultrasound frequencies do not penetrate as deep as lower one, but have better resolution. Also cavitation has to be avoided, as it can cause tissue damage. More research is needed in order to get sufficient in depth information about the signal and its possibilities.
A big advantage of UCSDI is that it is noninvasive and would thus not affect or destroy organs. UCSDI has a high spatial and temporal resolution, which is limited by the ultrasound beam geometry, pulse length and data recording. So far, it has mainly been recorded in 2D, but 3D dimensional mapping should also be possible, even though scan time and acquisition rate still are really time consuming. Optimizations are still required before it will be possible to utilize it.
This method could offer valuable improvements, compared to existing examining methods. It has the potential to offer anatomical image simultaneously with registration of electrical activity within the body. This could be useful during treatment or surgical intervention UCSDI could be used to record and register in the treatment of cardiac, muscular and neural abnormalities.
Few observations have been reported for nerves and tissues and only cardiac tissue of rabbits has been investigated in vitro. The specific dimensions of the tissues and the isolation from other tissues with the mineral oil make it easier to detect the acoustoelectrical effect, than it would in real tissues. It can though be expected that the AEI signal can be measured in skeletal muscles, though many more observations on the skeletal muscle are still needed. The AEI signal is of small amplitude and is sensitive to disturbances, noise and other currents, especially when the medium has larger dimension than the ultrasound wave length as within the human body. Precise measurement devices, performances and processing methods are needed in order to capture the desired signal and its direction. It is a promising field, that could lead to lot of possibilities for use in medicine or the biological field in general.
Fig 2.
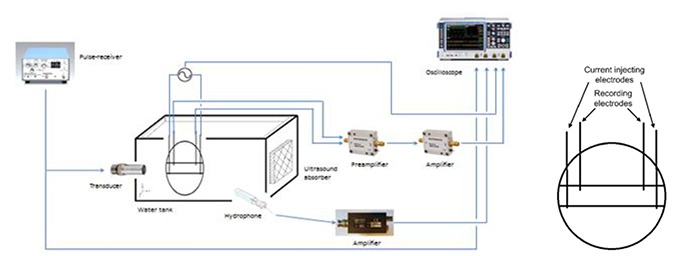
An example of experimental setup of measurement of acousto-electrical signal in saline solution, with more detailed view of the measurement cell. The recording electrodes of the measurement cell lay within the current density generated by the injecting current electrodes.
References
- 1.Lavandier B, Jossinet J, Cathignol D. Experimental measurement of the acousto-electric interaction signal in saline solution. Ultrasonics 2000;38:929–36. [DOI] [PubMed] [Google Scholar]
- 2.Olafsson R, Witte RS, Huang S-W, O’Donnell M. Ultrasound Current Source Density Imaging. IEEE Trans Biomed Eng 2008;55:1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jossinet J, Lavandier B, Cathignol D. The phemenology of acousto-electric interaction signal in aqueous solution of electrolytes. Ultrasonics 1998;36:607–13. [DOI] [PubMed] [Google Scholar]
- 4.Jossinet J, Lavandier B, Cathignol D. Impedance modulation by pulsed ultrasound. Ann N Y Acad Sci 1999;873:396–407. [Google Scholar]
- 5.Lavandier B, Jossinet J, Cathignol D. Quantitative assessment of ultrasound-induced resistance change in saline solution. Med Biol Eng Comput 2000;38:150–5. [DOI] [PubMed] [Google Scholar]
- 6.Gendron M, Guardo R, Bertrand M. Experimental setup for developing acousto-electric interaction imaging. Conf Proc IEEE Eng Med Biol Soc 2009;2009:2279–83. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Wang LV. Acousto-electric tomography. Proc SPIE Photons Plus Ultrasound Imag Sens 2004;5320:145–9. [Google Scholar]
- 8.Olafsson R, Witte RS, Kim K, Ashkenazi S, O’Donnell M. Electric current mapping using the acousto-electric effect. Proc SPIE Med Imag Ultrason Imag Signal Process 2006;6147:614701–1–614701–11. [Google Scholar]
- 9.Olafsson R, Witte RS, O’Donnell M. Measurement of a 2D electric dipole field using the acousto-electric effect. Proc SPIE 6513 Med Imaging 2007 Ultrason Imaging Signal Process 2007;6513:5130S. [Google Scholar]
- 10.Wang ZH, Olafsson R, Ingram P, Li Q, Qin Y, Witte RS. Four-dimensional ultrasound current source density imaging of a dipole field. Appl Phys Lett 2011;99:113701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Witte RS. Simulation-based validation for four- dimensional multi-channel ultrasound current source density imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Wang Z, Ingram P, Li Q, Witte RS. Optimizing frequency and pulse shape for ultrasound current source density imaging. Ultrason Ferroelectr Freq Control IEEE Trans On 2012;59:2149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olafsson R, Li Q, Wang Z, Ingram P, Witte RS. Ultrasound current source density imaging of a time-varying current field in a multielectrode nerve chamber. Ultrason Symp IUS 2009 IEEE Int 2009;:333–6. Z [Google Scholar]
- 14.Witte R, Olafsson R, Huang S-W, O’Donnell M. Imaging current flow in lobster nerve cord using the acoustoelectric effect. Appl Phys Lett 2007;90:163902. [Google Scholar]
- 15.Wang Z, Ingram P, Olafsson R, Li Q, Witte RS. Detection of multiple electrical sources in tissue using ultrasound current source density imaging. Proc SPIE Med Imaging 2010 2010;7629:76290H. [Google Scholar]
- 16.Li Q, Olafsson R, Ingram P, Wang Z, Witte R. Measuring the acoustoelectric interaction constant using ultrasound current source density imaging. Phys Med Biol 2012;57:5929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olafsson R, Witte RS, Jia C, Huang S-W, Kim K, O’Donnell M. Cardiac activation mapping using ultrasound current source density imaging (UCSDI). IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R, Li X, Liu J, He B. 3D current source density imaging based on the acoustoelectric effect: a simulation study using unipolar pulses. Phys Med Biol 2011;56:3825–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudjonsdottir B, Gargiulo P, Helgason T. Use of ultrasound current source density imaging (UCSDI) to monitor electrical stimulation of denervated muscles and fiber activity, some theoretical considerations. 10th Vienna Int Workshop Funct Electr Stimul and 15th IFESS Annu Conf Proceedings 2010;384–7. [Google Scholar]


