Abstract
Functional Electrical Stimulation (FES) has been used extensively over several decades to reverse muscle atrophy during rehabilitation for spinal cord injury patients. The benefits of the technology are being expanded into other areas, and FES has been recently utilized for injury rehabilitation and performance enhancement in horses. Six retired horses (age from 10 to 17 yrs) that had been previously used mainly for dressage riding were selected for this study. Clinical evaluation found epaxial muscle spasms in all horses with minimal to no pelvic extension when manually palpated. FES treatments were performed on the sacral/lumbar region 3 times per week for a period of 8 weeks, obtaining a total of 22 treatments per horse. The Modified Ashworth Scale for grading muscle spasms found a one grade improvement after approximately four FES treatments, indicating improved functional movement of the sacral/lumbar region, supporting the evidence by clinical palpations that a reduction in epaxial muscle spasms occurred. Skeletal muscle biopsies Pre and Post FES treatments were obtained from the longissimus lumborum muscle. Cryosections were stained with a Hemotoxylin-Eosin (H-E), and nicotinamide adenine dinucleotide tetrazolium reductase reaction (NADH-TR). The eventual size change of the muscle fibers were evaluated by morphometry in the H-E and NADH-TR stained cryosections, while in the NADH-TR slides the histochemical density and distribution of mitochondria were also determined. The main results of the morphometric analyses were: 1) As expected for the type of FES treatment used in this study, only a couple of horses showed significant increases in mean muscle fiber size when Pre- vs Post-FES biopsies were compared; 2) In the older horses, there were sparse (or many in one horse) very atrophic and angulated muscle fibers in both Pre- and Post-FES samples, whose attributes and distribution suggests that they were denervated due to a distal neuropathy; 3) The hypothesis of generalized FES-induced muscle fiber damage during epaxial muscle training is not supported by our data since: 3.1) Denervated muscle fibers were also present in the Pre-FES biopsies and 3.2) Only one horse presented with several long-term denervated muscles fibers Post-FES; 4) Preliminary data indicate an increased density and distribution of mitochondria in Post-FES biopsies, suggesting that the clinical improvements in the FES treated horses may be related to daily increased muscle contraction and perfusion induced by FES training. In conclusion, FES in horses is a safe treatment that provides clinical improvements in equine epaxial muscle spasms.
Key Words: equine, epaxial muscle spasms, rehabilitation, Functional Electrical Stimulation (FES), Hemotoxylin-Eosin (H-E), nicotinamide adeninedinucleotide tetrazolium reductase reaction (NADH-TR), mitochondrial density and distribution, subsarcolemmal mitochondrial patches
Functional Electrical Stimulation (FES) has been used extensively over several decades as an effective means to reduce or reverse muscle atrophy and to obtain some functional recovery by rehabilitation strategies for spinal cord injury patients,1-5 including those paraplegics with permanent and complete denervation of the legs (complete Conus and Cauda Equina Syndrome).6-23 The benefits of this technology are being expanded into other areas, and FES has been recently utilized for injury rehabilitation and performance enhancement in horses.24-27
The ability of FES to obtain precise, controlled functional movement, when compared to other electrotherapy approaches, is intriguing. FES has the flexibility to obtain minimal movement during the early stages of rehabilitation, as well as more aggressive movement during the later stages. Currently, research is being performed on the use of FES for inducing muscular exercise in populations that are either noncompliant to exercise, or are not physically able to exercise (e.g., balance disruption). In one study to determine the training effect of FES, healthy adults were placed on a training program (29 treatments over 6 wk) to obtain a cardiovascular exercise response without loading the limbs or joints. A treadmill test determined that a significant increase in peak aerobic capacity and quadriceps muscle strength occurred, suggesting that electrical muscle stimulation can be used in sedentary adults to improve physical fitness.28 An expanding list of studies are indicating that the application of FES on healthy muscle can elicit some of the same metabolic benefits as voluntary muscle active exercise.29-31
The purpose of this study was to confirm that FES is an effective and safe means to reduce chronic muscle spasms in the top line of horses. The present study will add objective histological evidence to previous clinical findings,27 through the evaluation of equine epaxial muscle biopsies harvested before and after 8 weeks of FES treatments.
Material and Methods
Horse demography
Six retired horses were selected for the study and ranged in age from 10-17 years of age (Table 1). The horses had been all clinically evaluated by veterinarians for axial musculoskeletal pathologies and none had been noted. The horses had no known myogenic or neurogenic disorders and had not been tested for those pathologies. The horses had not been ridden for at least 1 year and were not ridden during the study. The horses were placed in a free paddock for self exercise 1-6 hr daily, depending on weather conditions, and were stalled at night. The horses were used mainly for dressage riding and one horse had been used for some driving. All horses were evaluated by the owners and/or trainers as being uncomfortable and tight in the back muscles and difficult to ride, and therefore had been retired from riding. No nutritional or other management changes occurred during the period of the study.
Table 1.
Demography of the horses
| Number | Age | Discipline | Breed | Sex |
|---|---|---|---|---|
| 7003 | 10 | Dressage | Holsteiner/Arabian/Saddlebred | Male (Gelding) |
| 7004 | 13 | Dressage | Trakenhner | Female |
| 7005 | 15 | Dressage | Trakenhner | Female |
| 7006 | 12 | Driving/Dressage | Friesian/Arabian/Saddlebred | Female |
| 7007 | 14 | Dressage | Dutch Warmblood | Female |
| 7008 | 17 | Dressage | Trakenhner | Male (Gelding) |
Clinical examination of the horses
Clinical examination of the horses found epaxial muscle spasms in all horses, with minimal to no pelvic extension when manually palpated. The Modified Ashworth Scale (MAS) was used to determine the initial level of muscle spasm and to grade the changes observed during the FES treatments (Table 2).32 The MAS scale is widely used to objectively evaluate the rehabilitation progress for humans, and has been shown to have a 86.7% (p<.001) interrater reliability.33 The “catch” referred to in the MAS designates the “jerk” felt by the practitioner at the moment the muscle releases to the steady pressure applied to obtain joint movement. A “catch” is not desirable because the movement of the joint should be smooth.
Table 2.
Modified Ashworth Scale for grading muscle spasm. * ROM (range of motion)
| Modified Ashworth Scale | |
|---|---|
| Grade | Description |
| 0 | No increase in muscle tone |
| 1 | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension |
| 1+ | Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM* |
| 2 | More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved |
| 3 | Considerable increase in muscle tone, passive movement difficult |
| 4 | Affected part(s) rigid in flexion or extension |
Functional Electrical Stimulation Treatments
FES treatments were performed 3 times per week for a period of 8 weeks, yielding a total of 22 treatments. The first two treatments were given during the initial 24 hours and the remaining treatments were between 2-4 days apart to obtain 3 treatments per week. The first 2 treatments were given within 24 hours to better assist in obtaining an initial reduction in the muscle spasm. The FES system used was a 16-bit digital micro controller and provided a pulsed, biphasic, rectangular waveform at 60Hz, with a 0 net charge (FES310, EquiNew LLC, River Falls, WI, USA). The signal was pulsed at a rate of 2 seconds on and 2 seconds off.
Three channels, with 6 electrodes paired in an astrick design, were used to transfer the signal to the horse for a treatment time of 35 minutes. The electrodes were placed in a pad, which was centered over the biopsy site of each horse. The skin was sponged with water and ultrasound gel was used between the pad and the skin to reduce impedance. The voltage applied to elicit functional movement ranged from 7.6 to 15.8 volts. Twenty-two FES treatments were performed on the epaxial muscles of the horses including the superficial and middle gluteals and the dorsal edge of the biceps femoris muscle. During the FES treatments, the voltage was increased until pelvic extension was obtained. The pelvic extension ranged from approximately 5-15 degrees. Every 7-9 days, the grade of muscle spasm was determined thorough palpation of the epaxial muscles approximately 10 cm ventral to the dorsal spinal processes, together with palpation over the dorsal spinal processes. Three palpations were performed using the clinician’s fingers on both sides of the horse. The same clinician performed all of the palpations and performed all of the grading of the muscle spasms based on the Modified Ashworth Scale for consistency.
Muscle Biopsies
Muscle biopsies were harvested from the longissimus lumborum muscle at the beginning of the study and then approximately 8 weeks later (54 days) at the end of the study. The biopsies were taken approximately 72 hours prior to the first FES treatments and 72 hours after the last FES treatment. Biopsy specimens were obtained on the same side of the horse for both the Pre-FES and Post-FES samples. A 6 mm diameter Bergstrom biopsy needle was used at a depth of 3 cm to obtain the muscle specimens. Two cc of the local anesthetic lidocaine was given subcutaneous and a 1 cm incision was made over the right longissimus lumborum muscle. The pre treatment longissimus lumborum muscle specimens were obtained 20 cm cranial to the tuber sacrale and 3 cm lateral to the midline. The post treatment longissimus lumborum muscle specimens were obtained 18 cm cranial to the tuber sacrale and 3 cm lateral from the midline. Muscle specimens were approximately 2 cm long. One suture was used after the muscle sample was taken and the suture was removed at 10 days. Biopsy specimens were placed on saline moistened gauze in a plastic container and taken on ice to the laboratory within 2 hours of sampling. Fresh muscle samples were frozen in isopentane chilled in liquid nitrogen upon arrival at the laboratory.34-37 Thick sections of about 10 μm were stained with Hemotoxylin-Eosin (H-E) and nicotinamide adenine dinucleotide tetrazolium reductase reaction (NADH-TR).38 Hematoxilin-Eosin and NADH-TR stained slides were photographed and morphometry was performed on random-selected fields in the Translational Myology Lab of the Interdepartmental Research Center (CIR-Myo) of the University of Padova, Italy. Muscle fiber size before and after FES was determined in H-E and NADH-TR stained samples as described in Rossini et al. 2002.8 Quantitative analyses of mitochondrial density and distribution in the myofiber were determined on microphotographs taken at medium magnification (20x) of NADH-TR stained sections. The staining dots were defined well enough to discriminate larger muscle fibers with a low content of stain-dots (Type 2, glycolytic muscle fibers), from the smaller muscle fibers rich in staining (Type 1, oxidative muscle fibers). In the Type 1 oxidative muscle fibers, the stained dots are typically distributed in a central area with a relatively low-density of intermyofibrillar stain dots (similar to those found in the glycolytic muscle fibers), which are distributed at a higher density in a subsarcolemmal coronal area. Furthermore, some of the oxidative muscle fibers will display 3-9 patches of very high-density subsarcolemmal mitochondria. The intensity of the NADH-TR reaction and the presence or absence of the subsarcolemmal ring of high-density mitochondria, can also distinguish oxidative (Type 1) from glycolytic-oxidative (Type 2A) muscle fibers (Fig. 1).
Fig 1.
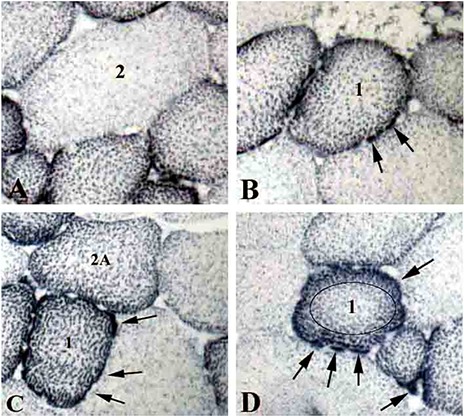
NADH-TR reaction identifies mitochondria distribution and density in horse muscle biopsies.
A, a typical Type 2, large, glycolytic muscle fiber, low-density mitochondria is depicted; B and D, typical Type 1, smaller, oxidative muscle fibers are depicted; C, beside a typical Type 1 muscle fiber, a typical Type 2A glycolytic-oxidative muscle fibers is present. Arrows point to subsarcolemmal mitochondrial patches. In panel D the circle defines the central intermyofibrillar mitochondrial area. Magnification = 20 X
Statistical Analysis
The observed percentage of change between pre- and post-FES for equine epaxial muscle spasms treatment were analyzed by two-sided t tests and significance was determined at p<0.05.39-41
Results
Clinical Analysis
Palpation by the same clinician was used to determine the grade of the muscle spasm during the initial observation before the first FES treatment (Table 3). The spasm grading was based on the Modified Ashworth Scale (Table 2). Improvements were defined as a change in the spasm scale to a lower grade, indicating reduced muscle spasms. These evaluations were performed by palpation of each horse’s back initially before the first FES treatment, and before each subsequent FES treatment.
Table 3.
Initial grading of horse muscle spasms based on Modified Ashworth Score (MAS), treatments required to obtain 1 grade change in MAS and average voltage of all FES treatments
| Number | Age | Sex | MAS Initial grade | Tx* to obtain MAS grade change of 1 | Average Voltage |
|---|---|---|---|---|---|
| 7003 | 10 | Male (Gelding) | 3 | 5 | 12.06 |
| 7006 | 12 | Female | 3 | 8 | 11.3 |
| 7004 | 13 | Female | 2 | 5 | 10.07 |
| 7007 | 14 | Female | 3 | 4 | 12.2 |
| 7005 | 15 | Female | 3 | 4 | 10.7 |
| 7008 | 17 | Male (Gelding) | 3 | 6 | 13.3 |
*Tx = treatments
The majority of the horses (5/6) were initially rated at Grade 3, indicating a high level of muscle spasm making spinal movement by hand manipulation impossible. A spasm grade of 2 was found in 1 out of the 6 horses, indicating that although muscle tone was greater than normal, some joint movement was possible with manipulation. The number of treatments necessary to change from a grade 3 or 2 to a grade 1 spasm, varied from 4 treatments in two horses, 5 treatments in two horses, 6 treatments in one horse and 8 treatments in one horse (Table 3). A comparison of the average voltage used during the 22-treatment period for each horse are found in Table 3. When the treatment notes were evaluated, there was a clear pattern of increasing acceptance by the horses of a higher voltage, as the treatment period progressed, together with an associated increase in the degree of pelvic movement. The higher the voltage the deeper the electrical field reaches into the muscle tissue.
Histological and histochemical analyses of muscle biopsies
While the majority of the cryosections of muscle biopsies, stained with H-E, presented with the normal aspects of mammal adult muscles, (Fig 2) some samples displayed a higher variability in size than normal. Table 4 shows changes in the overall muscle fiber diameter when Pre- vs Post-FES biopsies are compared. Two of the younger horses presented with an increased muscle fiber size (with one change being significant p<0.05), whereas the other 4 horses had a statistically significant decrease when muscle fiber size means were compared Pre- and Post-FES. The means of the pooled fiber data of all horses shows a 11% decrease in fiber size, which is also statistically significant (p<0.05).
Table 4.
Mean size (diameter, μm) and percentage of change Pre- and Post-FES of equine muscle fibers in cryosections stained by H-E. T test, significance set to p < 0.05. *SD = Standard deviation
| HORSE | AGE | Pre-FES Sample Mean Fiber Diameter |
Post-FES Sample Mean Fiber Diameter |
Δ% | |
|---|---|---|---|---|---|
| 7003 | 10 | 58.59 SD*=21.07 |
61.67 SD=22.83 |
5.26% (p=.1239) | |
| 7006 | 12 | 65.44 SD=18.91 |
54.18 SD=12.80 |
-17.20% (p<.001) | |
| 7004 | 13 | 57.18 SD=19.66 |
66.61 SD=15.41 |
16.48% (p<.001) | |
| 7007 | 14 | 59.57 SD=18.28 |
44.47 SD=15.22 |
-25.35% (p<.001) | |
| 7005 | 15 | 55.31 SD=17.17 |
40.41 SD=17.04 |
-26.95% (p<.001) | |
| 7008 | 17 | 63.25 SD=18.91 |
52.17 SD=15.41 |
-17.52% (p<.001) | |
| Total (pooled fibers) |
59.38 n=1461 SD=19.04 |
52.57 n=1643 SD=18.51 |
-11.46% (p<.001) |
Morphometry and topography of the NADH-TR stained cryosections were used to identify and count poor-reacting, large, glycolytic muscle fibers (fast-contracting, Type 2B) and highly-reacting, small, oxidative muscle fibers (slow contracting, Type 1) and the intermediate oxidative-glycolytic muscle fibers (fast-contracting Type 2A) (Table 5). The changes in mean muscle fiber size determined by NADH-TR stain (Table 5), when Pre-FES data are compared to Post-FES data, are in agreement with the values obtained in Table 4. As expected, the Type 2B (glycolytic) muscle fibers were substantially larger than the Type 1 (oxidative) muscle fibers. An evaluation of the Type 1 fibers, showed significant changes in mean muscle fiber size (either increases or decreases) in four horses. Type 2 fibers, showed significant changes in mean muscle fiber size (either increases or decreases) in 2 horses with a borderline significance in a third horse. The pooled data for both Type 1 and Type 2 muscle fibers showed significant decreases in mean muscle fiber sizes when Pre-FES fiber size means were compared to Post-FES fiber size means (Table 5). In all horses, and in both the H-E and NADH-TR stained cryosections, there were scanty very small muscle fibers found in the three younger horses, and focal groups of angulated small muscle fibers in the 3 older horses (encircled in Figs. 2, 3 and 4). In addition, the histogram in Fig. 5 shows that denervation is more pronounced in the three older horses as seen by the number of fibers 30 microns in size and smaller, with horse 7005 (15 yr) showing the largest number of Pre-and Post-FES very atrophic (i.e., denervated) muscle fibers. When evaluating the Pre-FES and Post-FES cryosections of horse 7005, these very small, angulated muscle fibers most likely contribute to the smaller Pre-and Post-FES mean muscle fiber sizes for this horse (Table 4). Based on the NADH-TR staining, the mean percentual content of Type 1, oxidative muscle fibers seemed to increase with age at the expense of Type 2, glycolytic muscle fibers (Table 6). When comparing the absolute changes in the percentage of fibers Pre- to Post-FES for all horses, there is no significant difference for both Type 1 or Type 2 muscle fibers (p=0.6442). When an increase in the percentage of Type 1 muscle fibers for one horse increases there is an associated decrease in the Type 2 muscle fibers for that same horse. The percentual content of oxidative muscle fibers, when comparing Pre-FES to post-FES, increases in 3 of the horses, however, one of those values is minimal (0.54%). When comparing the absolute changes in the percentage of fibers Pre- to Post-FES for all horses, there is no significant difference for both Type 1 or Type 2 muscle fibers (p=0.9853). Further, preliminary analyses of the NADH–TR stained cryosections (not shown) suggest a positive effect of FES on the mean mitochondrial density and distribution. The mean mitochondrial density seemed to increase for both NADH-TR high-positive fibers (Type 1, oxidative) and Low positive fibers (Type 2, glycolytic) in the Post-FES muscle fibers when compared to the Pre-FES muscle fibers (Barbara Ravara, personal observation).
Table 5.
Mean size (diameter, μm) and percentage of change Pre- and Post-FES of Type 1 (oxidative) and Type 2 (glycolytic) for equine muscle fibers in cryo sections stained by Mitochondrial NADH-TR. T test, significance set to p < 0.05. * SD = Standard deviation.
| Type 1 (oxidative) | ||||
|---|---|---|---|---|
| Horse | AGE | Pre-FES | Post-FES | Δ% |
| 7003 | 10 | 77.36 SD*=14.32 |
62.96 SD=17.98 |
-18.62% (p<0.001) |
| 7006 | 12 | 64.41 SD=13.17 |
64.89 SD=13.76 |
0.74% (p=0.830) |
| 7004 | 13 | 62.71 SD=16.00 |
72.82 SD=17.78 |
16.13% (p<0.001) |
| 7007 | 14 | 64.81 SD=22.66 |
48.86 SD=17.62 |
-24.61% (p<0.001) |
| 7005 | 15 | 69.51 SD=23.78 |
65.50 SD=14.68 |
-5.76% (p.223) |
| 7008 | 17 | 66.57 SD=19.00 |
55.26 SD=16.66 |
-16.99% (p<0.001) |
| Total (pooled fibers) |
67.06 n=482 SD=19.26 |
60.19 n=530 SD=18.55 |
-10.25% (p<0.001) |
|
| Type 2 (glycolitic) | AGE | Pre-FES | Post-FES | Δ% |
| 7003 | 10 | 108.10 SD=30.28 |
84.85 SD=21.48 |
-21.50% (p<0.001) |
| 7006 | 12 | 78.71 SD=15.72 |
85.94 SD=22.63 |
9.19% (p=0.0529) |
| 7004 | 13 | 83.31 SD=19.56 |
90.37 SD=23.53 |
8.47% (p=.084) |
| 7007 | 14 | 90.52 SD=22.65 |
76.10 SD=25.05 |
-15.93% (p=0.016 |
| 7005 | 15 | 83.33 SD=29.71 |
81.82 SD=22.54 |
-1.81% (p=.782) |
| 7008 | 17 | 101.09 SD=23.12 |
79.29 SD=16.32 |
-21.57% (p<0.001) |
| Total (pooled fibers) |
73.94 n=714 SD=23.81 |
67.69 n=778 SD=22.98 |
-8.45% (p<0.001) |
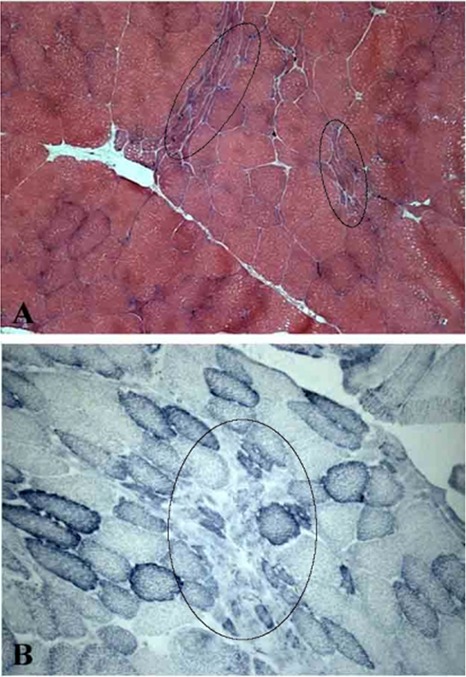
Fig 2.

H-E stain of Pre- (A) and Post- (B) FES muscle biopsies from horse 7003 (10 yr) and 7005 (15 yr). In A and B, arrows point to atrophic, angulated (denervated) muscle fibers. Arrowheads in B point to muscle fiber with central nuclei. Note that B is a magnified image. Bar, 100 μm
Fig 3.
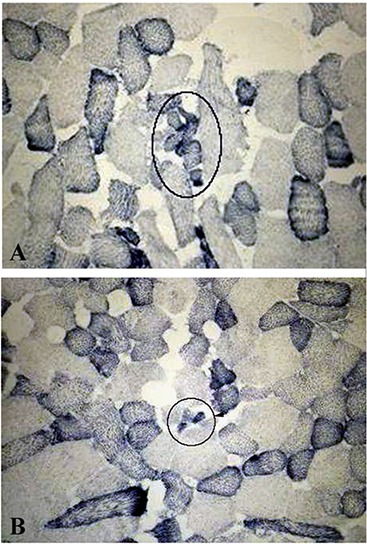
NADH-TR reaction of muscle fibers of Post- (A) and Pre- (B) FES. Circles indicate groups of very atrophic muscle fibers present in the horse 7005 (15 yr). Magnification = 20 x
Fig 4.
A, H-E stain Post-FES, B, NADH-TR reaction Post-FES of muscle fibers harvested from horse 7005 (15 yr). Groups of severely atrophic muscle fibers, whit distribution suggestive of an axonopathy. Magnification = 20 x
Fig 5.
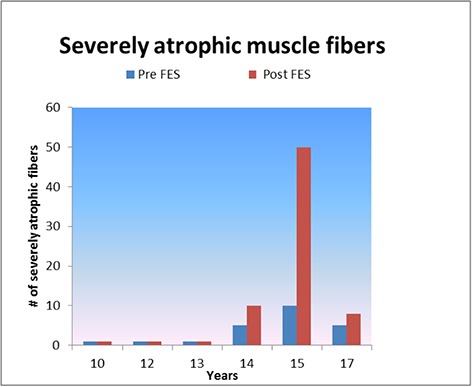
Histogram of contents of severely atrophic denervated muscle fibers versus the horse age
Table 6.
Percentual content of Type 1, oxidative and Type 2, glycolytic muscle fibers in equine muscle biopsies stained by NADH-TR reaction. z test of proportions, significance set to p < 0.05.
| Horse | Pre-FES % of Type 1 (oxidative) |
Post-FES % of Type 1 (oxidative) |
Absolute change in % | p value |
|---|---|---|---|---|
| 7003 | 58.65% | 51.82% | -6.83% | 0.3857 |
| 7006 | 60.48% | 61.02% | 0.54% | 1 |
| 7004 | 63.50% | 63.36% | -0.14% | 1 |
| 7007 | 67.69% | 73.96% | 6.27% | 0.2733 |
| 7005 | 62.20% | 64.08% | 1.88% | 0.8473 |
| 7008 | 71.32% | 62.96% | -8.36% | 0.1893 |
| Total (pooled fibers) | 64.18% | 64.01% | -0.17% | 0.9853 |
| Pre-FES % of Type 2 (glycolytic) |
Post-FES % of Type 2 (glycolytic) |
Absolute change in % | p value | |
| 7003 | 41.35% | 48.18% | 6.83% | 0.3857 |
| 7006 | 39.52% | 38.98% | -0.54% | 1 |
| 7004 | 36.50% | 36.64% | 0.14% | 1 |
| 7007 | 32.31% | 26.04% | -6.27% | 0.2733 |
| 7005 | 37.80% | 35.92% | -1.88% | 0.8473 |
| 7008 | 28.68% | 37.04% | 8.36% | 0.1893 |
| Total (pooled fibers) | 35.82% | 35.99% | 0.17% | 0.9853 |
Discussion.
In this study muscle biopsies were harvested from 6 horses before and after 22 FES treatments over 8 weeks. The horses that were sampled had all been retired from competition due to epaxial muscle spasms and had not been ridden for at least one year. All of the horses had been examined previously by veterinarians and no obvious neuromuscular pathologies were found. At the beginning of the study, all horses were clinically evaluated and the epaxial muscle spasms for each horse were graded based on the Modified Ashworth Score (Table 2). A previous study documenting the use of FES for epaxial muscle spasms, found that an improvement by one grade of muscle spasm happened quickly. Almost 80% (193) of the horses improved by one grade of spasm after 2 treatments, and an additional 14% (33) of the horses showed a change in one grade of spasm after 3 treatments.27 The horses in this study required an average of 4.3 treatments to achieve a one-grade improvement in muscle spasm. Therefore these horses appeared to have a somewhat higher level of epaxial muscle spasms than the typical population of horses that receive FES treatments.
An evaluation of the H-E stain cryosections found evidence of an overall decrease in the mean size of the muscle fibers Post-FES when compared to Pre-FES mean muscle fiber size (Table 4). Morphometry of the muscle fibers size and type was also performed for the cryosections stained for mitochondrial NADH-TR reaction. This evaluation analyzed the fiber sizes of the smaller Type 1 (oxidative) separate from the larger Type 2 (glycolytic) muscle fibers (Table 5). Quantitative analyses of the NADH-TR cryosections confirmed the H-E stain results showed that there was an overall significant decrease in the mean fiber size of both Type 1 and Type 2 muscle fibers in both Pre- and Post-FES biopsies. However, in both stainings, some of the younger horses in the study (10-13 yr) presented with an increase in muscle fiber diameters.
A decrease in size of muscle fibers are described as an apparently paradoxically effect of electrical muscle stimulation when endurance protocols are applied to the muscle.42 This reduction in muscle fiber size (physiological in nature) is related to amelioration of the oxygen diffusion from the capillaries to the core of the muscle fibers, when a fast to slow transformation of fiber types is wanted and obtained. FES treatments may, thus, produce in the muscle an increased resistance to fatigue accompanied with related fiber typing changes of the muscle seen initially by the decrease in muscle fiber size noted in this study.
Very small muscle fibers were found in both the Pre-FES and Post-FES muscle biopsies, in both the H-E and NADH-TR stained cryosections and in the majority of horses. These very small muscle fibers were absent or observed rarely in the three youngest horses, which have a mean age of 11 years (7003, 7004 and 7006). The mean age of 16 years of the group of horses (7005, 7007 and 7008) that displayed a large number of “denervated” muscle fibers is not that much older than the mean age of the group of younger horses. However, most sport horses begin to decline in sports performance after the age of 13. Only one horse (7005, 15 yr) had groups of very small muscle fibers Post-FES. In all samples, there were no inflammatory cells infiltrating the tissue surrounding the small, angulated muscle fibers (evidence of absence of inflammatory reaction or myositis). These observations are evidence that there are areas of muscle fiber denervation in both Pre- and Post-FES muscle biopsies. Therefore, the conclusion can be made that these very small muscle fibers are not the result of the FES, otherwise all post FES muscle fiber samples would show a similar level of very small, angulated (denervated) muscle fibers since all the horses were exposed to the same series of FES treatments. Further, in the horse 7005 the severely atrophic muscle fibers are also present in groups suggesting that they are the result of a peripheral nerve disorder. In addition the horse 7005 received one of the lowest average voltage levels of all 6 horses at 10.7 volts. The reason for the appearance of denervated muscle fibers in both Pre-and Post-FES muscle specimens could be a “subclinical” peripheral neuromuscular disorder that resulted in retirement of the horses from competitions due to “being difficult to ride” and/or being “sore in the back”. Indeed, all horses used in this study were retired due to the fact that they were difficult to ride and had consistently sore backs. Some horses had more denervated fibers than others, but all horses were found to have some very small muscle fibers in Pre- and/or Post-FES samples, which were harvested from different areas of the longissimus lumborum muscle. In summary, the fact that there is no generalized FES-induced muscle damage during epaxial muscle FES treatments is supported by our data since: 1) Only one horse (7005, 15 yr) presented with a high numbers of long-term denervated muscles Post-FES; 2) Individual muscle fibers or groups of small-diameter muscle fibers are also present in the Pre-FES biopsies and 3) Angulated myofibers with a diameter of less than 30 microns are taken as denervated myofibers that cannot respond to electrical stimulation, therefore it is not surprising that they remain atrophic.9,12,15-17 In addition, FES has also been shown to be safe in reducing atrophy and improving muscle strength in even the most fragile muscle tissue, the long-term denervated muscle.11
It seems that it is not by chance that the majority of the denervated muscle fibers are present in the Pre-FES biopsies from the older group of horses. Indeed, the histologic features of aging muscle in humans suggest that denervation contributes to atrophy, that immobility accelerates the process, and that routine exercise may protect against loss of motor units and muscle tissue.29 Based on the NADH-TR staining, the mean percentual content of oxidative muscle fibers seems to increase with age at the expense of glycolytic muscle fibers (Table 6). Most importantly though, when comparing the absolute changes in the percentage of fibers Pre- to Post-FES, there is no significant difference for either Type 1 or Type 2 for any of the horses. Therefore, the FES treatment protocol used in this study does not appear to change the percentage of Type 1 or Type 2 fibers when Pre- and Post-FES data is compared. This suggests, again, that the overall significant decrease in the mean muscle fiber size Post-FES, when compared to Pre-FES, which was noted in both the H-E and NADH-TR stainings, are not enough to change fiber typing. Preliminary data (not reported) suggest a positive effect of FES on mitochondrial density and distribution (Barbara Ravara, personal observation) by the NADH-TR reaction is interesting and worthy of confirmation by additional analyses. These changes in Post-FES muscle biopsies may be related to an increased number of contractions due to the FES stimulation,43 and to the related increase in blood perfusion in FES treated muscles. This seems to be a retained effect due to the fact that the muscle biopsies were harvested 72 hours after the last stimulation session. The increases in mitochondrial density and distribution could be the result of the adaptive mechanisms of the muscle fibers to the increased number of contractions per week and of the associated increase in muscle perfusion.6,7
In conclusion, the present data from the histological evaluation of the equine longissimus lumborum muscle pre and post 22 treatments of FES over a period of 8 weeks shows that: 1) As expected for the type of FES treatment used in this study, only a couple of horses showed significant increases in mean muscle fiber size when Pre- vs Post-FES biopsies were compared; 2) In the older horses, there were sparse (or many in one horse) very atrophic and angulated muscle fibers in both Pre- and Post-FES samples, whose attributes and distribution suggests that they were denervated due to a distal neuropathy; 3) The hypothesis of generalized FES-induced muscle fiber damage during epaxial muscle training is not supported by our data since: 3.1) Denervated muscle fibers were also present in the Pre-FES biopsies and 3.2) Only one horse presented many long-term denervated muscles fibers Post-FES; 4) Preliminary data indicate an increased density and distribution of mitochondria in Post-FES biopsies, suggesting that the clinical improvements in the FES treated horses may be related to daily increased muscle contraction and perfusion induced by FES training. Finally, we stress that FES treatment, as here provided, is a safe rehabilitation strategy in the management of equine epaxial muscle spasms and provides clinical improvements and some structural changes of the muscle tissue at the histological level.
Acknowledgement
U.C. thanks the Interdepartmental Research Center of Myology at the Department of Biomedical Sciences, University of Padova, Italy for collaboration and hospitality and the Ludwig Boltzmann Institute of Electrical Stimulation and Physical Rehabilitation of Vienna at the Department of Physical Medicine, Wilhelminenspital, Vienna, Austria for support and collaboration. In addition, thanks go out to Brian Knaeble for statistical assistance.
References
- 1.Kapadia N, Masani K, Catharine Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014;September;37(5):511-24. doi: 10.1179 /2045772314Y.0000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffell LD, Donaldson Nde N, Perkins TA, et al. Long-term intensive electrically stimulated cycling by spinal cord-injured people: effect on muscle properties and their relation to power output. Muscle Nerve 2008;38:1304-11. [DOI] [PubMed] [Google Scholar]
- 3.Krause P, Szecsi J, Straube A. FES cycling reduces spastic muscle tone in a patient with multiple sclerosis. Neurorehabilitation 2007;22:335-7. [PubMed] [Google Scholar]
- 4.Stefanovska A, Vodovnik L, Gros N, et al. FES and spasticity. IEEE Trans Biomed Eng 1989;July;36(7):738-45. [DOI] [PubMed] [Google Scholar]
- 5.Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng 2002;10:280-9. [DOI] [PubMed] [Google Scholar]
- 6.Kern H. Funktionelle Elektrostimulation Paraplegischer Patienten. ÖZPM, Österreichi sche Zeitschrift für Physikalische Medizin 1995;5:1-75. ISSN 1021-4348 [Google Scholar]
- 7.Kern H. Electrical Stimulation on Paraplegic Patients. Eur J Trans Myol/ Basic Appl Myol 2014;24:75-157. [Google Scholar]
- 8.Rossini K, Zanin ME, Carraro U. To stage and quantify regenerative myogenesis in human long-term permanent denervated muscle. Basic Appl Myol 2002;12:277-87. [Google Scholar]
- 9.Kern H, Boncompagni S, Rossini K, et al. Long-term denervation in humans causes degeneration of both contractile and excitation contraction coupling apparatus, which is reversible by functional electrical stimulation (FES). A role for myofiber regeneration? J Neuropathol Exp Neurol 2004;63:919-31. [DOI] [PubMed] [Google Scholar]
- 10.Kern H, Rossini K, Carraro U, et al. Muscle biopsies show that FES of denervated muscles reverses human muscle degeneration from permanent spinal motoneuron lesion. J Rehabil Res Dev 2005;42:43-53. [DOI] [PubMed] [Google Scholar]
- 11.Carraro U, Rossini K, Mayr W, Kern H. Muscle fiber regeneration in human permanent lower motoneuron denervation: relevance to safety and effectiveness of FES-training, which induces muscle recovery in SCI subjects. Artif Organs 2005;29:187-91. [DOI] [PubMed] [Google Scholar]
- 12.Boncompagni S, Kern H, Rossini K, et al. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci USA 2007;104:19339-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern H, Carraro U. Translational myology focus on clinical Challenges of functional electrical stimulation of denervated muscle. Eur J Transl Myol/ Basic Appl Myol 2008;18:37-100. [Google Scholar]
- 14.Kern H, Hofer C, Mayr W. Protocols for clinical work package of the European project RISE. Eur J Transl Myol/ Basic Appl Myol 2008;18:39-44. [Google Scholar]
- 15.Kern H, Hofer C, Mayr W, Carraro U. European Project RISE: Partners, protocols, demography. Eur J Transl Myol/ Basic Appl Myol 2009;19:211-6. [Google Scholar]
- 16.Kern H, Carraro U, Adami N, et al. One year of home-based Functional Electrical Stimulation (FES) in complete lower motor neuron paraplegia: Recovery of tetanic contractility drives the structural improvements of denervated muscle. Neurol Res 2010;32:5-12,doi: 10.1189/ 184313209 X385644. [DOI] [PubMed] [Google Scholar]
- 17.Kern H, Carraro U, Adami N, et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair 2010;24:709-21. doi: 10.1177/ 1545968310366129. Epub 2010 May 11. [DOI] [PubMed] [Google Scholar]
- 18.Gargiulo P, Reynisson PJ, Helgason B, et al. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res 2011;September:33(7):750-8. doi: 10.1179/174313 2811Y.0000000007. [DOI] [PubMed] [Google Scholar]
- 19.Gargiulo P, Carraro U, Mandl T, et al. Anthropometry of Human Muscle Using Segmentation Techniques and 3D Modelling: Applications to Lower Motor Neuron Denervated Muscle in Spinal Cord Injury. Handbook of Anthropometry. 2012, pp 323-54. [Google Scholar]
- 20.Gargiulo P, Helgason T, Reynisson PJ, et al. Monitoring of muscle and bone recovery in spinal cord injury patients treated with electrical stimulation using three-dimensional imaging and segmentation techniques: methodological assessment. Artif Organs 2011:35:275-81. doi: 10.1111/j.1525-1594.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 21.Zanato R, Stramare R, Boato N, et al. Dynamic Echomyography Shows That FES in Peripheral Denervation does not Hamper Muscle Reinnervation. Biomed Tech (Berl) 2013;September 7 pii:/j/bmte.2013.58.issue-s1-A/bmt-2013-4034 /bmt-2013-4034.xml. doi: 10.1515/bmt-2013-4034. [DOI] [PubMed] [Google Scholar]
- 22.Kern H, Carraro U. Home-based Functional Electrical Stimulation (h-b FES) for long-term denervated human muscle: History, basics, results and perspectives of the Vienna Rehabilitation Strategy. Eur J Transl Myol/Basic Appl Myol 2014;24:27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Sun T, Chen L, et al. Consensus of clinical neurorestorative progresses in patients with complete chronic spinal cord injury. Cell Transplant. 2014;23 Suppl 1:5-17. doi: 10.3727/096368914X684952. Epub 2014 Oct 9. [DOI] [PubMed] [Google Scholar]
- 24.Schils SJ. Review of electrotherapy devices for use in veterinary medicine, In: Proceedings of the 55th Annual Convention of the American Association of Equine Practitioners, Las Vegas, NV, December 5-9 2009;55:68-73. [Google Scholar]
- 25.Schils SJ. Functional electrical stimulation (FES) for use in equine medicine. Lindner A. (ed.) Performance diagnosis and purchase examination of elite sport horses. 2013; Wageningen Academic Publishers, Wageningen, the Netherlands: 2013;103-8. [Google Scholar]
- 26.Schils SJ, Turner TA. Review of early mobilization of muscle, tendon, and ligament after injury in equine rehabilitation. In: Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, December 4-8 2010;56: 374-80. [Google Scholar]
- 27.Schils SJ, Turner TA. Functional Electrical Stimulation for equine epaxial muscle spasms:retrospective study of 241 clinical cases. Comparative Exercise Physiology 2014;10:89-97. [Google Scholar]
- 28.Banerjee P, Caulfield B, Crowe L, Clark A. Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J Appl Physiol 2005;99:2307-11 DOI: 10.1152/japplphysiol. 00891.2004 [DOI] [PubMed] [Google Scholar]
- 29.Mosole S, Carraro U, Kern H, et al. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 2014;73:284-94. doi: 10.1097/NEN.0000000000 000032. [DOI] [PubMed] [Google Scholar]
- 30.Kern H, Barberi L, Löfler S, et al. Electrical stimulation counteracts muscle decline in seniors. Front Aging Neurosci 2014;July 24:6:189 doi: 10.3389/fnagi.2014.00189. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zampieri S, Pietrangelo L, Loefler S, et al. Lifelong Physical Exercise Delays Age-Associated Skeletal Muscle Decline. J Gerontol A Biol Sci Med Sci 2015;70:163-73. doi: 10.1093/gerona/glu006. Epub 2014 Feb 18. [DOI] [PubMed] [Google Scholar]
- 32.Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner 1964;192:540-542. [PubMed] [Google Scholar]
- 33.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206-7. [DOI] [PubMed] [Google Scholar]
- 34.Lindholm A and K Piehl. Fibre composition, enzyme activity and concentrations of metabolites and electrolytes in muscles of Standardbred horses. Acta Vet Scand 1974;15:287-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow DH and PS Guy. Muscle fibre composition of a number of limb muscles in different types of horses. Res Vet Sci 1980;28:134-144. [PubMed] [Google Scholar]
- 36.Rivero JL, Serrano AL, Barrey E, et al. Analysis of myosin heavy chains at the protein level in horse skeletal muscle. J Muscle Res Cell Motil 1999;20:211-21. [DOI] [PubMed] [Google Scholar]
- 37.Dubowitz V, Brooke MH. Muscle biopsy: A modern approach. London, W.B. Saunders; 1973. [Google Scholar]
- 38.Dubowitz V, Sewry CA, Fitzsimons RB. Muscle Biopsy: A Practical Approach. 2nd ed. London, Baillière Tindall; 1985. [Google Scholar]
- 39.Welch BL. The generalization of Student’s problem when several different population variances are involved. Biometrika 1947;34: 28-35. [DOI] [PubMed] [Google Scholar]
- 40.Guird V. Some Remarks on the Estimation of the Ratio of the Expectation Values of a Two-dimensional Normal Random Variable. Biom J 1989;31:681-97. [Google Scholar]
- 41.Howell DC. Statistical methods for psychology. 2nd ed. Boston: Duxbury Press:1987. [Google Scholar]
- 42.Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve 1981;4:94-105. [DOI] [PubMed] [Google Scholar]
- 43.Hood D. Plasticity in skeletal, cardiac, and smooth muscle: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 2001;90:1137-1157. [DOI] [PubMed] [Google Scholar]


